Abstract
C16H15ClN2O2, triclinic,
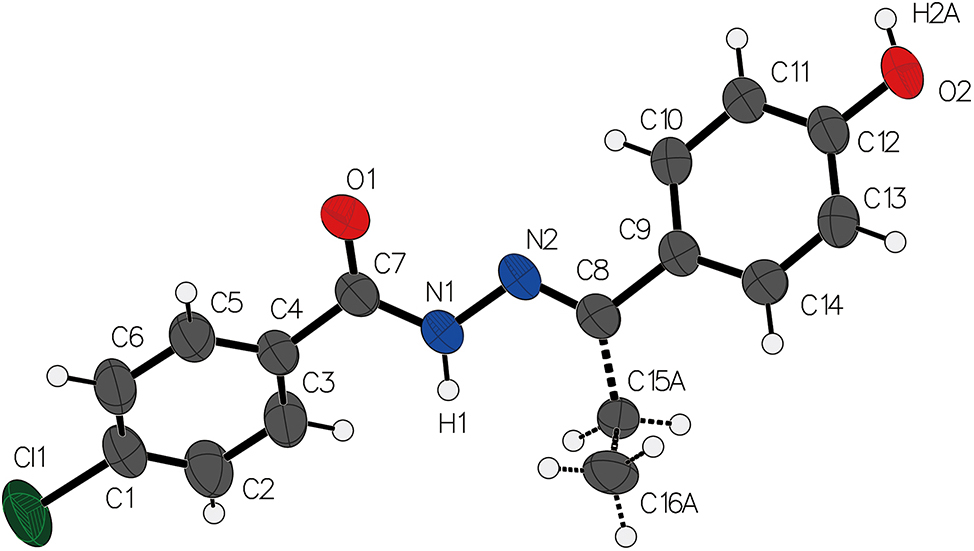
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Yellow block |
| Size: | 0.52 × 0.47 × 0.38 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.27 mm−1 |
| Diffractometer, scan mode: | SuperNova, ω |
| θmax, completeness: | 29.1°, >99 % |
| N(hkl)measured, N(hkl)unique, Rint: | 6184, 3328, 0.016 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2σ(Iobs), 2347 |
| N(param)refined: | 213 |
| Programs: | CrysAlisPRO [1], SHELX [2, 3], OLEX2 [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1 | 0.1653 (3) | 0.5014 (3) | −0.12017 (18) | 0.0553 (5) |
| C2 | 0.1862 (3) | 0.4207 (3) | −0.01679 (19) | 0.0643 (6) |
| H2 | 0.131639 | 0.309692 | −0.014083 | 0.077* |
| C3 | 0.2897 (3) | 0.5072 (3) | 0.08354 (18) | 0.0569 (6) |
| H3 | 0.304740 | 0.453870 | 0.154370 | 0.068* |
| C4 | 0.3714 (2) | 0.6725 (2) | 0.07970 (16) | 0.0448 (5) |
| C5 | 0.3473 (3) | 0.7502 (3) | −0.02581 (18) | 0.0564 (6) |
| H5 | 0.401373 | 0.861240 | −0.029234 | 0.068* |
| C6 | 0.2435 (3) | 0.6649 (3) | −0.12654 (18) | 0.0599 (6) |
| H6 | 0.227133 | 0.717784 | −0.197494 | 0.072* |
| C7 | 0.4901 (3) | 0.7712 (3) | 0.18380 (17) | 0.0461 (5) |
| C8 | 0.5360 (3) | 0.7686 (4) | 0.49176 (19) | 0.0699 (7) |
| C9 | 0.6754 (3) | 0.8313 (3) | 0.59582 (17) | 0.0513 (5) |
| C10 | 0.8494 (3) | 0.8680 (3) | 0.58385 (17) | 0.0512 (5) |
| H10 | 0.877142 | 0.849096 | 0.510680 | 0.061* |
| C11 | 0.9819 (3) | 0.9317 (3) | 0.67768 (17) | 0.0522 (5) |
| H11 | 1.097618 | 0.956207 | 0.667338 | 0.063* |
| C12 | 0.9431 (3) | 0.9593 (2) | 0.78736 (16) | 0.0461 (5) |
| C13 | 0.7721 (3) | 0.9224 (3) | 0.80196 (18) | 0.0550 (5) |
| H13 | 0.745269 | 0.940042 | 0.875636 | 0.066* |
| C14 | 0.6397 (3) | 0.8589 (3) | 0.70719 (18) | 0.0606 (6) |
| H14 | 0.524246 | 0.834232 | 0.718083 | 0.073* |
| Cl1 | 0.03559 (10) | 0.39387 (9) | −0.24769 (5) | 0.0860 (3) |
| N1 | 0.4591 (2) | 0.7232 (2) | 0.29109 (14) | 0.0536 (5) |
| H1 | 0.365342 | 0.651790 | 0.297269 | 0.064* |
| N2 | 0.5802 (2) | 0.7903 (2) | 0.39134 (14) | 0.0496 (4) |
| O1 | 0.60672 (19) | 0.88890 (19) | 0.17172 (13) | 0.0618 (4) |
| O2 | 1.0701 (2) | 1.0209 (2) | 0.88231 (12) | 0.0619 (4) |
| H2A | 1.163935 | 1.051780 | 0.860674 | 0.093* |
| C15Ba | 0.3316 (12) | 0.7607 (7) | 0.5011 (4) | 0.066 (2) |
| H15Aa | 0.322814 | 0.835653 | 0.563693 | 0.079* |
| H15Ba | 0.264445 | 0.785526 | 0.426897 | 0.079* |
| C16Bb | 0.2766 (15) | 0.5886 (9) | 0.5287 (6) | 0.094 (2) |
| H16Ab | 0.283431 | 0.518051 | 0.464046 | 0.141* |
| H16Bb | 0.158146 | 0.567705 | 0.541290 | 0.141* |
| H16Cb | 0.351784 | 0.566065 | 0.599034 | 0.141* |
| C15Aa | 0.3743 (7) | 0.6419 (6) | 0.5145 (4) | 0.0405 (14) |
| H15Ca | 0.399185 | 0.593965 | 0.590733 | 0.049* |
| H15Da | 0.338337 | 0.553412 | 0.453427 | 0.049* |
| C16Ab | 0.2284 (9) | 0.7423 (7) | 0.5120 (5) | 0.0588 (16) |
| H16Db | 0.201640 | 0.784176 | 0.434796 | 0.088* |
| H16Eb | 0.269202 | 0.833272 | 0.569701 | 0.088* |
| H16Fb | 0.125093 | 0.671045 | 0.530067 | 0.088* |
-
aOccupancy: 0.507 (10),boccupancy: 0.493 (10).
1 Source of materials
In a 100 mL round-bottom flask, 1 mmol of 4–chlorobenzohydrazide and 1 mmol of 4–hydroxypropiophenone were added, followed by the addition of 25 mL of absolute alcohol and 2 drops of glacial acetic acid. The resulting solution was stirred at 80 °C for 5 h. Subsequently, the ethanol solvent was completely evaporated using a rotary evaporator, yielding the crude product. The crude product (0.05 g) was dissolved in 15 mL of ethanol, and the solvent was allowed to slowly evaporate at room temperature. After a period of 3 days, a crystal was obtained.
2 Experimental details
The crystal structure was solved with SHELXT [2], and further refined using the SHELXL program [3]. All hydrogen atoms were positioned at calculated coordinates and refined isotropically. The crystal structure visualization was generated using the OLEX2 software package.
3 Comment
Acylhydrazones demonstrate remarkable structural flexibility, which renders them highly adaptable in both chemical and pharmaceutical contexts [5]. In order to investigate the structure of acylhydrazone derivatives, numerous related structures have been reported [6], [7], [8], [9], [10].
The molecules in the titled compound adopt non-planar conformations, with the two aromatic rings positioned in different planes, separated by a dihedral angle of 66.6°. Moreover, the chlorophenyl group and the amide group form a dihedral angle of 36.4°, while the chlorophenyl group and the amine group form a dihedral angle of 3.64°.
Within the crystal structure, the molecular assembly is primarily stabilized by intermolecular hydrogen bonding interactions involving the hydroxyl and oxygen atoms of the acyl hydrazone moiety. These hydrogen bonds create a network of intermolecular interactions, resulting in stable packing arrangements. The hydrogen bond length is measured to be 1.889(3) Å, while the bond angle is determined to be 174.47(16)°. The presence of such hydrogen bonding in the crystal structure is distinctive when compared to similar N-acyl hydrazone derivatives lacking these intermolecular interactions. The structure is similar with other reports [11], [12], [13], [14].
In addition to the hydrogen bonding network, the crystal structure also exhibits π–π stacking interactions between adjacent molecules containing the chlorophenyl groups.
Funding source: Projects of Key Laboratory of Molecular Imaging and Drug Synthesis of Xianyang City
Award Identifier / Grant number: (2021QXNL-PT-0008)
Funding source: Scientific Research Plan Project of Shaanxi Provincial Department of Education (23JK0323)
Award Identifier / Grant number: (23JK0323)
Funding source: Key Research Project of Shaanxi Provincial Department of Education – the Collaborative Innovation Center
Award Identifier / Grant number: (23JY006)
-
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
-
Research funding: Projects of Key Laboratory of Molecular Imaging and Drug Synthesis of Xianyang City (2021QXNL-PT-0008), the Scientific Research Plan Project of Shaanxi Provincial Department of Education (23JK0323) and the Key Research Project of Shaanxi Provincial Department of Education – the Collaborative Innovation Center (23JY006).
References
1. Rigaku, O. D. CrysAlisPRO; Rigaku Oxford Diffraction Ltd: Yarnton, Oxfordshire, England, 2017.Search in Google Scholar
2. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8.10.1107/S2053273314026370Search in Google Scholar PubMed PubMed Central
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
5. Socea, L.-I., Barbuceanu, S.-F., Pahontu, E. M., Dumitru, A.-C., Nitulescu, G. M., Sfetea, R. C., Apostol, T.-V. Acylhydrazones and their biological activity: a review. Molecules 2022, 27, 8719; https://doi.org/10.3390/molecules27248719.Search in Google Scholar PubMed PubMed Central
6. Arsanious, M. H. N., Boulos, L. S. The behavior of phosphorus reagents towards substituted diazine and hydrazine derivatives. Monatsh. Chem. 2006, 137, 1177–1184; https://doi.org/10.1007/s00706-006-0517-x.Search in Google Scholar
7. Song, M. N′-(2–hydroxy-1,2-diphenylethylidene) benzohydrazide. Acta Crystallogr. 2010, E66, o2692.10.1107/S1600536810038365Search in Google Scholar PubMed PubMed Central
8. Ni, C., Zhang, L., Hu, J. Nucleophilic fluoroalkylation of α,β-enones, arynes, and activated alkynes with fluorinated sulfones: probing the hard/soft nature of fluorinated carbanions. J. Org. Chem. 2008, 73, 5699–5713; https://doi.org/10.1021/jo702479z.Search in Google Scholar PubMed
9. Ianelli, S., Carcelli, M. Syntheses and structural characterization of two novel α-diketone bisacylhydrazones. J. Chem. Crystallogr. 2001, 31, 123–127; https://doi.org/10.1023/a:1014362502709.10.1023/A:1014362502709Search in Google Scholar
10. Mishra, M., Tiwari, K., Singh, A. K., Singh, V. P. Synthesis, structural and corrosion inhibition studies on Mn(II), Cu(II) and Zn(II) complexes with a Schiff base derived from 2-hydroxypropiophenone. Polyhedron 2014, 77, 57–65; https://doi.org/10.1016/j.poly.2014.04.003.Search in Google Scholar
11. He, G.-F., Dou, J.-M., Chang, J.-G. 2-hydroxy-N′-[(1E)-1-(2-hydroxy-5-methylphenyl)-2-phenylethylidene]benzohydrazide. Acta Crystallogr. 2006, E62, o5527–o5528.10.1107/S1600536806046757Search in Google Scholar
12. Chang, J.-G., Zhao, R.-G. 2-hydroxy-N′-[(1Z)-1-(2-hydroxy-5-methylphenyl)-2-methylpropylidene] benzohydrazide. Acta Crystallogr. 2008, E64, o197.10.1107/S160053680706357XSearch in Google Scholar PubMed PubMed Central
13. Hao, Y.-M. Crystal structure of 2-chloro-N′-(2-hydroxy-3,5-diiodobenzylidene)benzohydrazide, C14H9ClI2N2O2. Z. Kristallogr. N. Cryst. Struct. 2011, 226, 85–86; https://doi.org/10.1524/ncrs.2011.0041.Search in Google Scholar
14. Zhu, N., Zhao, J., Zhang, L. Crystal structure of E-2-chloro-N′-(1-(5-chloro-2-hydroxyphenyl)propylidene) benzohydrazide, C16H14Cl2N2O2. Z. Kristallogr. N. Cryst. Struct. 2022, 237, 1065–1066; https://doi.org/10.1515/ncrs-2022-0413.Search in Google Scholar
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of poly[diaqua-(μ4-3,3′-di(1H-1,2,4-triazol-1-yl)-[1,1′-biphenyl]-4,4′-dicarboxylate-N:N′:O:O′)cadmium(II)], C18H14N6O6Cd
- Crystal structure of (8R,8′S,13S,13′R)-8,8′-bis(hydroxymethyl)-9,9′,10,10′-tetramethoxy-5,5′,6,6′,8,8′,13,13′-octahydro-[13,13′-bi[1,3]dioxolo[4,5-g]isoquinolino[3,2-a]isoquinoline]-7,7′-diium chloride-methanol (1/2), C46H58N2O14Cl2
- The crystal structure of 8-methoxy-2,2-diphenyl-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C29H25BN2O3S
- Crystal structure of aqua-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N″,N‴)copper(II) 5-carboxyisophthalate tetrahydrate, C25H50N4CuO11
- The crystal structure of 1-(naphthalen-2-ylsulfonyl)-2,2-diphenyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ij]quinoline, C31H23BN2O2S
- Crystal structure of iodido-(η6-benzene) (1-(pyridin-2-yl)-N-(p-fluoro-methanamine)-κ2N,Nʹ)ruthenium(II) hexaflourophosphate, (C18H15F7IN2RuP)
- The crystal structure of 1-(3-oxo-1-phenyl-3-(p-tolyl) propylidene)-1,3-dihydro-2H-inden-2-one, C25H20O2
- Crystal structure of tricyclohexyl[4-(4H-1,2,4-triazol-4-yl)-benzoato-κO]tin(IV), C27H39N3O2Sn
- Crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ4N,O,O,O)cadmium(II)]monohydrate, C12H15NO9Cd
- Crystal structure of ethyl 2-((4-(3,5-dimethylisoxazol-4-yl)-2,6-difluorophenyl)amino)benzoate, C20H18F2N2O3
- The crystal structure of 2-(hydroxymethyl)-2-(4H-1,2,4-triazol-4-yl)propane-1,3-diol, C6H11N3O3
- The crystal structure of 1,2-bis(2,4-dinitrophenyl) hydrazine, C12H8N6O8
- Crystal structure of 1-(2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)-1,2-dihydro-4H-benzo[d][1,3]oxazin-4-one, C19H14Cl2N2O3
- The crystal structure of 5-amino-5-oxo-4-(1-oxo-4-(2-oxopyrrolidin-1-yl)isoindolin-2-yl)pentanoic acid, C17H19N3O5
- Crystal structure of N2,N6-bis(2-(((Z)-5-bromo-2-hydroxybenzylidene)amino) phenyl)pyridine-2,6-dicarboxamide, C33H23Br2N5O4
- The crystal structure of (E)-2-methoxy-6-(((5-methyl-1,3,4-thiadiazol-2-yl)imino)methyl)phenol, C11H11N3O2S
- The crystal structure of 3-((tert-butyldiphenylsilyl)methyl)-5,5-diphenyl-6-(p-tolyl) tetrahydro-2H-pyran-2-one, C41H42O2Si
- Crystal structure of 9-fluoro-4-(6-methoxypyridin-3-yl)-5,6-dihydrobenzo[h]quinazolin-2-amine, C18H15FN4O
- The crystal structure of 2-bromo-5-(4-cyanophenoxy)benzyl 1-methyl-1,2,5,6-tetrahydropyridine-3-carboxylate, C21H19BrN2O3
- Crystal structure of 3,3′-(1,4-phenylenebis(methylene))bis(1-isopropyl-1H-imidazol-3-ium) bis(hexafluorophosphate(V)), C10H14F6N2P
- The crystal structure of 2,2-di(thiophen-3-yl)-1-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C24H19BN2O2S3
- Crystal structure of 5-bromo-1-(2-iodobenzoyl)-1H-indole-3-carbaldehyde, C16H9BrINO2
- The crystal structure of monocarbonyl-2-carboxypyridinato-κ2N,O-triphenylphosphine-rhodium(I) acetonitrile solvate, C26H20.50N1.50O3PRh
- Crystal structure of dichlorido-tetrakis(1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ1N)manganese(II), C60H68O4N12Cl10Mn
- Crystal structure of 3-(tert-butyldiphenylsilyl)-1-(2,6-dichlorophenyl)-2,2-diphenylpropan-1-ol, C37H36Cl2OSi
- Crystal structure of langite from Mine du Pradet (France)
- The crystal structure of 5′-(furan-2-yl)-3′-((4-methylphenyl)sulfonamido)-3′,4′,5′,6′-tetrahydro-[1,1′:3′,1″-terphenyl]-4′-carboxylic acid, C30H27NO5S
- Synthesis and crystal structure of bis{2-(((4-acetophenone)imino)methyl)-4-fluorophenolato-κ2N,O}zinc(II), C30H22F2N2O4Zn
- The crystal structure of poly[(tripyridine-κ3N,N′,N″) μ3-(pyridine-3,4-dicarboxylate-κ3N:O:O′) manganese(II)], C22H22N4O8Mn
- The crystal structure of (E)-4-chloro-N′-(1-(4-hydroxyphenyl)propylidene)benzohydrazide, C16H15ClN2O2
- Synthesis and crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino) ethyl)phenyl)imino)methyl)phenolato-κ2N,O}cobalt(II), C40H46CoN4O4
- Crystal structure of tetraaqua-[(1-(carboxymethyl)-1H-pyrazole-3-carboxylato-κ2N,O)cobalt(II)], C6H12CoN2O8
- (6R,7S)-2,3,13-trimethoxy-6,7-dimethyl-5,6,7,8-tetrahydrobenzo[3′,4′]cycloocta [1′,2′:4,5]benzo[1,2-d][1,3]dioxol-1-ol, C22H26O6
- Crystal structure of 2-((2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)amino)benzoic acid, C18H14Cl2N2O3
- Crystal structure of (5aS,6aS,8aR,9R,11aS, 11bS,13R,13aS)-1,1,8a,11a-tetramethyl-9-((S)-1-((S)-5-methyl-6-oxo-3,6-dihydro-2H-pyran-2-yl)ethyl)-3-oxo-1,7,8,8a,9,10,11,11a,11b,12,13,13a-dodecahydro-3H,6H-cyclopenta[5,6]cyclopropa[1,8a]naphtho[2,1-c]oxepin-13-yl acetate, C32H44O6
- Crystal structure of catena-poly[triaqua-(μ2-1-(4-carboxylatophenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylato-O,O′:O″)cobalt(II)], C12H12N2O8Co
- Crystal structure of 3-[(furan-2-ylmethyl)-amino]-2-(2,3,4,5-tetrafluoro-benzoyl)-acrylic acid ethyl ester, C17H13F4NO4
- Crystal structure of methyl 4-(2-ethoxy-2-oxoethoxy)-3-methoxybenzoate, C13H16O6
- Crystal structure of 4-bromo-2-(4-chlorophenyl)-1-methyl-5-(trifluoromethyl)-1H-pyrrole-3-carbonitrile, C13H7BrClF3N2
- The crystal structure of triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ3N,O,O)nickel(II) monohydrate, C12H15NO9Ni
- Crystal structure of dihydroxy(2,4,6-triisopro-pylphenyl)telluronium trifluoromethanesulfonate, C16H25F3O5STe
- The crystal structure of 1-(carboxymethyl)-1H-imidazole 3-oxide
- The crystal structure of 1,3,5-tris(dibromomethyl)benzene, C9H6Br6
- Crystal structure of (Z)-3-(4-methoxyphenyl)-4-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-N-phenylthiazol-2(3H)-imine, C25H21N5OS
- Crystal structure of (Z)-3-(3-(4-hydroxyphenyl)-2-(phenylimino)-2,3-dihydrothiazol-4-yl)-2H-chromen-2-one, C24H16N2O3S
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of poly[diaqua-(μ4-3,3′-di(1H-1,2,4-triazol-1-yl)-[1,1′-biphenyl]-4,4′-dicarboxylate-N:N′:O:O′)cadmium(II)], C18H14N6O6Cd
- Crystal structure of (8R,8′S,13S,13′R)-8,8′-bis(hydroxymethyl)-9,9′,10,10′-tetramethoxy-5,5′,6,6′,8,8′,13,13′-octahydro-[13,13′-bi[1,3]dioxolo[4,5-g]isoquinolino[3,2-a]isoquinoline]-7,7′-diium chloride-methanol (1/2), C46H58N2O14Cl2
- The crystal structure of 8-methoxy-2,2-diphenyl-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C29H25BN2O3S
- Crystal structure of aqua-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N″,N‴)copper(II) 5-carboxyisophthalate tetrahydrate, C25H50N4CuO11
- The crystal structure of 1-(naphthalen-2-ylsulfonyl)-2,2-diphenyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ij]quinoline, C31H23BN2O2S
- Crystal structure of iodido-(η6-benzene) (1-(pyridin-2-yl)-N-(p-fluoro-methanamine)-κ2N,Nʹ)ruthenium(II) hexaflourophosphate, (C18H15F7IN2RuP)
- The crystal structure of 1-(3-oxo-1-phenyl-3-(p-tolyl) propylidene)-1,3-dihydro-2H-inden-2-one, C25H20O2
- Crystal structure of tricyclohexyl[4-(4H-1,2,4-triazol-4-yl)-benzoato-κO]tin(IV), C27H39N3O2Sn
- Crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ4N,O,O,O)cadmium(II)]monohydrate, C12H15NO9Cd
- Crystal structure of ethyl 2-((4-(3,5-dimethylisoxazol-4-yl)-2,6-difluorophenyl)amino)benzoate, C20H18F2N2O3
- The crystal structure of 2-(hydroxymethyl)-2-(4H-1,2,4-triazol-4-yl)propane-1,3-diol, C6H11N3O3
- The crystal structure of 1,2-bis(2,4-dinitrophenyl) hydrazine, C12H8N6O8
- Crystal structure of 1-(2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)-1,2-dihydro-4H-benzo[d][1,3]oxazin-4-one, C19H14Cl2N2O3
- The crystal structure of 5-amino-5-oxo-4-(1-oxo-4-(2-oxopyrrolidin-1-yl)isoindolin-2-yl)pentanoic acid, C17H19N3O5
- Crystal structure of N2,N6-bis(2-(((Z)-5-bromo-2-hydroxybenzylidene)amino) phenyl)pyridine-2,6-dicarboxamide, C33H23Br2N5O4
- The crystal structure of (E)-2-methoxy-6-(((5-methyl-1,3,4-thiadiazol-2-yl)imino)methyl)phenol, C11H11N3O2S
- The crystal structure of 3-((tert-butyldiphenylsilyl)methyl)-5,5-diphenyl-6-(p-tolyl) tetrahydro-2H-pyran-2-one, C41H42O2Si
- Crystal structure of 9-fluoro-4-(6-methoxypyridin-3-yl)-5,6-dihydrobenzo[h]quinazolin-2-amine, C18H15FN4O
- The crystal structure of 2-bromo-5-(4-cyanophenoxy)benzyl 1-methyl-1,2,5,6-tetrahydropyridine-3-carboxylate, C21H19BrN2O3
- Crystal structure of 3,3′-(1,4-phenylenebis(methylene))bis(1-isopropyl-1H-imidazol-3-ium) bis(hexafluorophosphate(V)), C10H14F6N2P
- The crystal structure of 2,2-di(thiophen-3-yl)-1-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C24H19BN2O2S3
- Crystal structure of 5-bromo-1-(2-iodobenzoyl)-1H-indole-3-carbaldehyde, C16H9BrINO2
- The crystal structure of monocarbonyl-2-carboxypyridinato-κ2N,O-triphenylphosphine-rhodium(I) acetonitrile solvate, C26H20.50N1.50O3PRh
- Crystal structure of dichlorido-tetrakis(1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ1N)manganese(II), C60H68O4N12Cl10Mn
- Crystal structure of 3-(tert-butyldiphenylsilyl)-1-(2,6-dichlorophenyl)-2,2-diphenylpropan-1-ol, C37H36Cl2OSi
- Crystal structure of langite from Mine du Pradet (France)
- The crystal structure of 5′-(furan-2-yl)-3′-((4-methylphenyl)sulfonamido)-3′,4′,5′,6′-tetrahydro-[1,1′:3′,1″-terphenyl]-4′-carboxylic acid, C30H27NO5S
- Synthesis and crystal structure of bis{2-(((4-acetophenone)imino)methyl)-4-fluorophenolato-κ2N,O}zinc(II), C30H22F2N2O4Zn
- The crystal structure of poly[(tripyridine-κ3N,N′,N″) μ3-(pyridine-3,4-dicarboxylate-κ3N:O:O′) manganese(II)], C22H22N4O8Mn
- The crystal structure of (E)-4-chloro-N′-(1-(4-hydroxyphenyl)propylidene)benzohydrazide, C16H15ClN2O2
- Synthesis and crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino) ethyl)phenyl)imino)methyl)phenolato-κ2N,O}cobalt(II), C40H46CoN4O4
- Crystal structure of tetraaqua-[(1-(carboxymethyl)-1H-pyrazole-3-carboxylato-κ2N,O)cobalt(II)], C6H12CoN2O8
- (6R,7S)-2,3,13-trimethoxy-6,7-dimethyl-5,6,7,8-tetrahydrobenzo[3′,4′]cycloocta [1′,2′:4,5]benzo[1,2-d][1,3]dioxol-1-ol, C22H26O6
- Crystal structure of 2-((2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)amino)benzoic acid, C18H14Cl2N2O3
- Crystal structure of (5aS,6aS,8aR,9R,11aS, 11bS,13R,13aS)-1,1,8a,11a-tetramethyl-9-((S)-1-((S)-5-methyl-6-oxo-3,6-dihydro-2H-pyran-2-yl)ethyl)-3-oxo-1,7,8,8a,9,10,11,11a,11b,12,13,13a-dodecahydro-3H,6H-cyclopenta[5,6]cyclopropa[1,8a]naphtho[2,1-c]oxepin-13-yl acetate, C32H44O6
- Crystal structure of catena-poly[triaqua-(μ2-1-(4-carboxylatophenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylato-O,O′:O″)cobalt(II)], C12H12N2O8Co
- Crystal structure of 3-[(furan-2-ylmethyl)-amino]-2-(2,3,4,5-tetrafluoro-benzoyl)-acrylic acid ethyl ester, C17H13F4NO4
- Crystal structure of methyl 4-(2-ethoxy-2-oxoethoxy)-3-methoxybenzoate, C13H16O6
- Crystal structure of 4-bromo-2-(4-chlorophenyl)-1-methyl-5-(trifluoromethyl)-1H-pyrrole-3-carbonitrile, C13H7BrClF3N2
- The crystal structure of triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ3N,O,O)nickel(II) monohydrate, C12H15NO9Ni
- Crystal structure of dihydroxy(2,4,6-triisopro-pylphenyl)telluronium trifluoromethanesulfonate, C16H25F3O5STe
- The crystal structure of 1-(carboxymethyl)-1H-imidazole 3-oxide
- The crystal structure of 1,3,5-tris(dibromomethyl)benzene, C9H6Br6
- Crystal structure of (Z)-3-(4-methoxyphenyl)-4-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-N-phenylthiazol-2(3H)-imine, C25H21N5OS
- Crystal structure of (Z)-3-(3-(4-hydroxyphenyl)-2-(phenylimino)-2,3-dihydrothiazol-4-yl)-2H-chromen-2-one, C24H16N2O3S

