Abstract
C22H26O6, orthorhombic, P212121 (no. 19), a = 7.6648(5) Å, b = 11.2663(6) Å, c = 22.7321(7) Å, V = 1963.0(2) Å3, Z = 4, Rgt (F) = 0.0652, wRref (F 2) = 0.1399, T = 273(2) K.
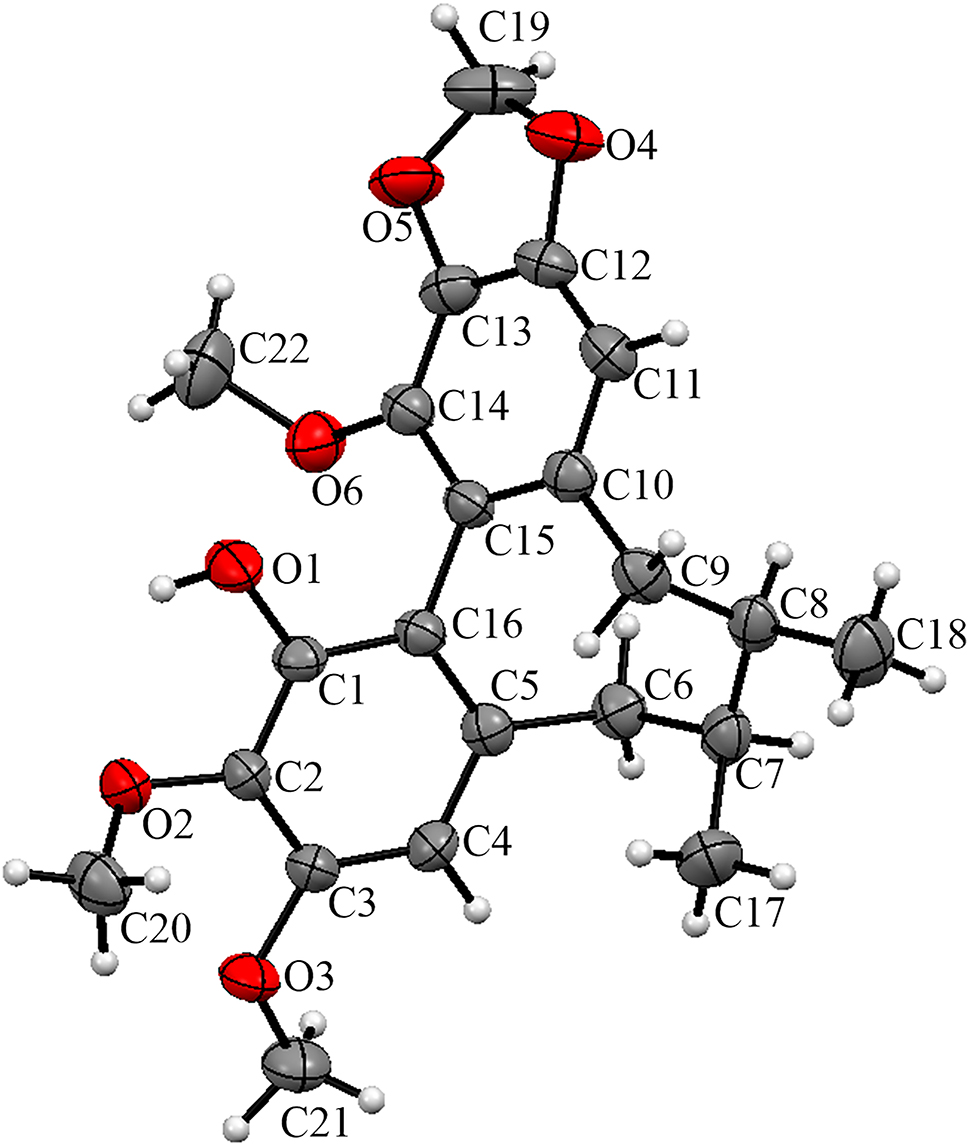
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.21 × 0.19 × 0.18 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.10 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| θ max, completeness: | 33.7°, >99 % |
| N(hkl)measured, N(hkl)unique, R int: | 35,325, 7783, 0.092 |
| Criterion for I obs, N(hkl)gt: | I obs > 2σ(I obs), 3580 |
| N(param)refined: | 259 |
| Programs: | Olex2 [1], Bruker [2], SHELX [3], Diamond [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| C1 | 0.4047 (4) | 0.4586 (2) | 0.44400 (13) | 0.0336 (7) |
| C2 | 0.3225 (4) | 0.3503 (2) | 0.45150 (12) | 0.0337 (7) |
| C3 | 0.2930 (4) | 0.2791 (2) | 0.40290 (13) | 0.0345 (7) |
| C4 | 0.3537 (4) | 0.3135 (2) | 0.34835 (13) | 0.0367 (7) |
| C5 | 0.4404 (4) | 0.4216 (2) | 0.34069 (13) | 0.0334 (6) |
| C6 | 0.5027 (4) | 0.4544 (2) | 0.27973 (13) | 0.0378 (7) |
| C7 | 0.6896 (4) | 0.4183 (3) | 0.26219 (13) | 0.0418 (8) |
| C8 | 0.8326 (4) | 0.5048 (2) | 0.28439 (13) | 0.0386 (7) |
| C9 | 0.8332 (4) | 0.5285 (2) | 0.35109 (13) | 0.0392 (7) |
| C10 | 0.7196 (4) | 0.6319 (2) | 0.36874 (13) | 0.0349 (7) |
| C11 | 0.7935 (4) | 0.7455 (2) | 0.36757 (13) | 0.0401 (7) |
| C12 | 0.6880 (4) | 0.8395 (2) | 0.38108 (14) | 0.0400 (7) |
| C13 | 0.5158 (4) | 0.8266 (2) | 0.39381 (14) | 0.0401 (7) |
| C14 | 0.4378 (4) | 0.7166 (2) | 0.39621 (13) | 0.0359 (7) |
| C15 | 0.5433 (4) | 0.6173 (2) | 0.38354 (12) | 0.0333 (7) |
| C16 | 0.4633 (4) | 0.4971 (2) | 0.38862 (12) | 0.0315 (6) |
| C17 | 0.7258 (5) | 0.2881 (3) | 0.27694 (16) | 0.0573 (10) |
| C18 | 1.0139 (5) | 0.4675 (3) | 0.26351 (17) | 0.0579 (10) |
| C19 | 0.5723 (5) | 1.0186 (3) | 0.39071 (19) | 0.0647 (11) |
| C20 | 0.3162 (5) | 0.2161 (3) | 0.53307 (15) | 0.0520 (9) |
| C21 | 0.1255 (5) | 0.1163 (3) | 0.36574 (16) | 0.0523 (9) |
| C22 | 0.1920 (5) | 0.7603 (3) | 0.45637 (16) | 0.0649 (11) |
| H1A | 0.380035 | 0.503031 | 0.520284 | 0.074* |
| H2 | 0.216526 | 0.080274 | 0.342883 | 0.078* |
| H3 | 0.061660 | 0.171128 | 0.341612 | 0.078* |
| H4 | 0.047938 | 0.055934 | 0.380018 | 0.078* |
| H5 | 0.693227 | 0.423330 | 0.219163 | 0.050* |
| H6 | 0.634738 | 0.239378 | 0.260880 | 0.086* |
| H7 | 0.729467 | 0.278302 | 0.318876 | 0.086* |
| H8 | 0.835776 | 0.264943 | 0.260334 | 0.086* |
| H9 | 0.808498 | 0.581293 | 0.265573 | 0.046* |
| H10 | 0.951926 | 0.543973 | 0.363672 | 0.047* |
| H11 | 0.793062 | 0.457820 | 0.371362 | 0.047* |
| H12 | 0.910326 | 0.756657 | 0.357935 | 0.048* |
| H13 | 0.264071 | 0.746754 | 0.490241 | 0.097* |
| H14 | 0.187388 | 0.843801 | 0.448123 | 0.097* |
| H15 | 0.076301 | 0.731433 | 0.463979 | 0.097* |
| H17 | 1.096012 | 0.529407 | 0.272168 | 0.087* |
| H18 | 1.011402 | 0.453642 | 0.221849 | 0.087* |
| H19 | 1.048152 | 0.396030 | 0.283388 | 0.087* |
| H19A | 0.580572 | 1.078027 | 0.421483 | 0.078* |
| H20 | 0.493240 | 0.539896 | 0.275649 | 0.045* |
| H21 | 0.422994 | 0.419264 | 0.251538 | 0.045* |
| H22 | 0.336724 | 0.263929 | 0.316142 | 0.044* |
| H23 | 0.285660 | 0.215581 | 0.574031 | 0.078* |
| H24 | 0.259655 | 0.150968 | 0.513586 | 0.078* |
| H25 | 0.440312 | 0.208260 | 0.529048 | 0.078* |
| H196 | 0.544316 | 1.058553 | 0.354102 | 0.078* |
| O1 | 0.4318 (3) | 0.53025 (17) | 0.49175 (9) | 0.0492 (6) |
| O2 | 0.2605 (3) | 0.32603 (16) | 0.50692 (9) | 0.0454 (6) |
| O3 | 0.1995 (3) | 0.17743 (17) | 0.41377 (9) | 0.0480 (6) |
| O4 | 0.7335 (3) | 0.95823 (17) | 0.38514 (11) | 0.0582 (7) |
| O5 | 0.4406 (3) | 0.93643 (17) | 0.40461 (12) | 0.0575 (7) |
| O6 | 0.2639 (3) | 0.69888 (18) | 0.40673 (10) | 0.0469 (6) |
1 Source of material
The rhizome of Schisandra sphenanthera produced in Guizhou was dried and crushed to obtain 7.5 kg of coarse powder, which was extracted three times (7 days/time) with 100 %, 90 % and 70 % methanol (MeOH) respectively at room temperature. The combined extract was then concentrated under reduced pressure to obtain a total extract of 1.02 kg. After suspending the total extract in warm water, petroleum ether, ethyl acetate and n-butanol were used for further extraction to afford three fractions: the petroleum ether (PE) extract (209 g), ethyl acetate (EtOAc) extract (423 g) and n-butanol extraction extract (201 g). Further, the EtOAc extract was applied to a MCI column (MeOH/H2O 30:70 to 100:0) to obtain 6 fractions (H1–H6). Fr. H4 was subjected to a silica gel column, eluting with the a gradient solvent system (PE:EtOAc 50:1 to 0:1) to give 4 subfractions (H4-1–H4-4). Fr. H4-2 was recrystallized with methanol to obtain the title compound which was suitable for X-ray single crystal diffraction. The compound name was determined as: (6R,7S)-2,3,13-trimethoxy-6,7-dimethyl-5,6,7,8-tetrahydrobenzo [3′,4′]cycloocta[1′,2′:4,5]benzo[1,2-d][1,3]dioxol-1-ol.
2 Experimental details
The carbon-bound hydrogen atoms were placed in their geometrically idealized positions and constrained to ride on their parent atoms with d(C–H) = 0.93–1.43 Å, U iso(H) = 1.5 times U eq(C) and 1.2 times U eq(O).
3 Comment
S. sphenanthera belongs to the Schisandra genus in the Schisandracea family, commonly known as “South Schisandra” [5]. This species is widely distributed in Asia and North America, especially in the southwest and southeast areas of China [6]. S. sphenanthera was used as a Chinese medicine, which was found to possess astringing effect that could nourish kidney yin and relieve mental stress, supplement qi and promote the production of body fluid. It is reported that people in Guizhou traditionally used S. sphenanthera as a medicinal herb and called the stem of S. sphenanthera purple gold bark, wind vine, etc., which was used to dispel wind, dehumidify system, and promote blood circulation to arrest pain. Previous studies have shown that the chemical components of S. sphenanthera are rich, mainly including lignans, triterpenes, sesquiterpenes, volatile oils, polysaccharides, flavonoids, organic acids, and amino acids [7]. The main chemical components are lignans, which are also the main active ingredients. Lignans have significant effects in anti-tumor [8], anti-inflammatory [9], liver protective, sedative, and hypnotic aspects [10], and can effectively improve the learning and memory abilities of model animals, as well as elderly dementia [11]. Our research group has conducted a phytochemical study on S. sphenanthera produced in Guizhou and isolated a variety of new compounds with novel structures and interesting bioactivities. This finding has encouraged us to further conduct an in-depth study on this species. As a result, a lignan was isolated from this plant. The compound, as shown in Figure, consists of an octacillar, 2 benzene rings, 2 ether bonds, 2 methyl groups, 3 methoxy groups and one hydroxyl group. The ether bonds were confirmed by the distances of 1.387(3) Å (C13–O5) and 1.385(3) Å (C12–O4). And the methoxyl groups were confirmed by the distance of 1.374(3) Å (C2–O2), 1.374(3) Å (C3–O3) and 1.369(4) Å (C14–O6), respectively. The hydroxyl was confirmed by the distance of 1.369(3) Å (C1–O1) [12–14].
Funding source: General Projects Guizhou Science and Technology Department
Award Identifier / Grant number: Qiankehejichu [2021] general 0017
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: General Projects Guizhou Science and Technology Department [grant number Qiankehejichu [2021] general 0017].
References
1. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Suche in Google Scholar
2. Bruker. APEX2, SAINT and SADABS; Bruker AXS Inc.: Madison, Wisconsin, USA, 2012.Suche in Google Scholar
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar
4. Brandenburg, K. DIAMOND. Visual Crystal Structure Information System. Ver. 4.0; Crystal Impact: Bonn, Germany, 2015.Suche in Google Scholar
5. Chen, R. S., Ye, J. H., Luo, Y. L., Xue, C. Y., Zhou, J., Zhang, J. J. Crystal structure of 1,2,3,5,13-pentamethoxy-6,7-dimethyl-1,2,3,4,4a,5,6,7,8,13b-decahydrobenzo [3′,4′] cycloocta [1′,2′:4,5] benzo [1,2-d] [1,3] dioxole, C24H30O7. Z. Kristallogr. N. Crystallogr. Struct. 2023, 238, 349–351, https://doi.org/10.1515/ncrs-2022-0580.Suche in Google Scholar
6. Yang, K., Qiu, J., Huang, Z., Yu, Z., Wang, W., Hu, H., You, Y. A comprehensive review of ethnopharmacology, phytochemistry, pharmacology, and pharmacokinetics of Schisandra chinensis (Turcz.) Baill. and Schisandra sphenanthera Rehd. et Wils. J. Ethnopharmacol. 2022, 284, 114759; https://doi.org/10.1016/j.jep.2021.114759.Suche in Google Scholar PubMed
7. Huang, S., Zhang, D. D., Li, Y., Fan, H., Liu, Y. Y., Huang, W. L., Deng, C., Wang, W., Song, X. M. Schisandra sphenanthera: a comprehensive review of its botany, phytochemistry, pharmacology, and clinical applications. Am. J. Chin. Med. 2021, 49, 1577–1622; https://doi.org/10.1142/s0192415x21500749.Suche in Google Scholar PubMed
8. Qu, H. M., Liu, S. J., Zhang, C. Y. Antitumor and antiangiogenic activity of Schisandra chinensis polysaccharide in a renal cell carcinoma model. Int. J. Biol. Macromol. 2014, 66, 52–66; https://doi.org/10.1016/j.ijbiomac.2014.02.025.Suche in Google Scholar PubMed
9. Guo, L. Y., Hung, T. M., Bae, K. H., Shin, E. M., Zhou, H. Y., Hong, Y. N., Kang, H. P., Kim, Y. S. Anti-inflammatory effects of schisandrin isolated from the fruit of Schisandra chinensis Baill. Eur. J. Pharmacol. 2008, 591, 293–299; https://doi.org/10.1016/j.ejphar.2008.06.074.Suche in Google Scholar PubMed
10. Zeng, H., Li, D., Qin, X., Chen, P., Tan, H., Zeng, X. Z., Li, X., Fan, X. M., Jiang, Y. M., Zhou, Y. W., Chen, Y. X., Wang, Y., Huang, M., Bi, H. C. Hepatoprotective effects of Schisandra sphenanthera extract against lithocholic acid-induced cholestasis in male mice are associated with activation of the pregnane X receptor pathway and promotion of liver regeneration. Drug Metab. Dispos. 2016, 44, 337–342; https://doi.org/10.1124/dmd.115.066969.Suche in Google Scholar PubMed
11. Xu, M. J., Yan, T. X., Fan, K. Y., Wang, M. S., Qi, Y., Xiao, F., Bi, K. S., Jia, K. Polysaccharide of Schisandra chinensis Fructus ameliorates cognitive decline in a mouse model of Alzheimer’s disease. J. Ethnopharmacol. 2019, 237, 354–365; https://doi.org/10.1016/j.jep.2019.02.046.Suche in Google Scholar PubMed
12. Chen, R. S., Zhang, J. J., Zhang, Y., Xu, C. Y., Ye, J. H. Synthesis and crystal structure of (4aR,7S)-7-hydroxy-7-isopropyl-1,1-dimethyldecahydro-2H, 6H-8a, 4a-(epoxymethano) phenanthren-12-one, C20H32O3. Z. Kristallogr. N. Crystallogr. Struct. 2023, 238, 417–419; https://doi.org/10.1515/ncrs-2022-0546.Suche in Google Scholar
13. Ye, J. H., Chen, R. S., Xu, C. Y., Zhou, J., Zhang, J. J. Crystal structure of (3a7R,13bR)-3-((1R)-1-hydroxy-1-(5-methyl-6-oxo-3,6-dihydro-2H-pyran-2-yl)ethyl)-3a,11,11,13b-tetramethyl-2,3,3a,4,5,11,11a,12,13,13b-decahydroindeno [5′,4′:4,5] cyclohepta [1,2-c] oxepin-9 (1H)-one, C30H40O5. Z. Kristallogr. N. Crystallogr. Struct. 2023, 238, 314–315.10.1515/ncrs-2022-0564Suche in Google Scholar
14. Changfu, X., Guanghong, B., Cunheng, H. Kexue Tongbao 1982, 27, 1081.Suche in Google Scholar
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of poly[diaqua-(μ4-3,3′-di(1H-1,2,4-triazol-1-yl)-[1,1′-biphenyl]-4,4′-dicarboxylate-N:N′:O:O′)cadmium(II)], C18H14N6O6Cd
- Crystal structure of (8R,8′S,13S,13′R)-8,8′-bis(hydroxymethyl)-9,9′,10,10′-tetramethoxy-5,5′,6,6′,8,8′,13,13′-octahydro-[13,13′-bi[1,3]dioxolo[4,5-g]isoquinolino[3,2-a]isoquinoline]-7,7′-diium chloride-methanol (1/2), C46H58N2O14Cl2
- The crystal structure of 8-methoxy-2,2-diphenyl-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C29H25BN2O3S
- Crystal structure of aqua-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N″,N‴)copper(II) 5-carboxyisophthalate tetrahydrate, C25H50N4CuO11
- The crystal structure of 1-(naphthalen-2-ylsulfonyl)-2,2-diphenyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ij]quinoline, C31H23BN2O2S
- Crystal structure of iodido-(η6-benzene) (1-(pyridin-2-yl)-N-(p-fluoro-methanamine)-κ2N,Nʹ)ruthenium(II) hexaflourophosphate, (C18H15F7IN2RuP)
- The crystal structure of 1-(3-oxo-1-phenyl-3-(p-tolyl) propylidene)-1,3-dihydro-2H-inden-2-one, C25H20O2
- Crystal structure of tricyclohexyl[4-(4H-1,2,4-triazol-4-yl)-benzoato-κO]tin(IV), C27H39N3O2Sn
- Crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ4N,O,O,O)cadmium(II)]monohydrate, C12H15NO9Cd
- Crystal structure of ethyl 2-((4-(3,5-dimethylisoxazol-4-yl)-2,6-difluorophenyl)amino)benzoate, C20H18F2N2O3
- The crystal structure of 2-(hydroxymethyl)-2-(4H-1,2,4-triazol-4-yl)propane-1,3-diol, C6H11N3O3
- The crystal structure of 1,2-bis(2,4-dinitrophenyl) hydrazine, C12H8N6O8
- Crystal structure of 1-(2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)-1,2-dihydro-4H-benzo[d][1,3]oxazin-4-one, C19H14Cl2N2O3
- The crystal structure of 5-amino-5-oxo-4-(1-oxo-4-(2-oxopyrrolidin-1-yl)isoindolin-2-yl)pentanoic acid, C17H19N3O5
- Crystal structure of N2,N6-bis(2-(((Z)-5-bromo-2-hydroxybenzylidene)amino) phenyl)pyridine-2,6-dicarboxamide, C33H23Br2N5O4
- The crystal structure of (E)-2-methoxy-6-(((5-methyl-1,3,4-thiadiazol-2-yl)imino)methyl)phenol, C11H11N3O2S
- The crystal structure of 3-((tert-butyldiphenylsilyl)methyl)-5,5-diphenyl-6-(p-tolyl) tetrahydro-2H-pyran-2-one, C41H42O2Si
- Crystal structure of 9-fluoro-4-(6-methoxypyridin-3-yl)-5,6-dihydrobenzo[h]quinazolin-2-amine, C18H15FN4O
- The crystal structure of 2-bromo-5-(4-cyanophenoxy)benzyl 1-methyl-1,2,5,6-tetrahydropyridine-3-carboxylate, C21H19BrN2O3
- Crystal structure of 3,3′-(1,4-phenylenebis(methylene))bis(1-isopropyl-1H-imidazol-3-ium) bis(hexafluorophosphate(V)), C10H14F6N2P
- The crystal structure of 2,2-di(thiophen-3-yl)-1-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C24H19BN2O2S3
- Crystal structure of 5-bromo-1-(2-iodobenzoyl)-1H-indole-3-carbaldehyde, C16H9BrINO2
- The crystal structure of monocarbonyl-2-carboxypyridinato-κ2N,O-triphenylphosphine-rhodium(I) acetonitrile solvate, C26H20.50N1.50O3PRh
- Crystal structure of dichlorido-tetrakis(1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ1N)manganese(II), C60H68O4N12Cl10Mn
- Crystal structure of 3-(tert-butyldiphenylsilyl)-1-(2,6-dichlorophenyl)-2,2-diphenylpropan-1-ol, C37H36Cl2OSi
- Crystal structure of langite from Mine du Pradet (France)
- The crystal structure of 5′-(furan-2-yl)-3′-((4-methylphenyl)sulfonamido)-3′,4′,5′,6′-tetrahydro-[1,1′:3′,1″-terphenyl]-4′-carboxylic acid, C30H27NO5S
- Synthesis and crystal structure of bis{2-(((4-acetophenone)imino)methyl)-4-fluorophenolato-κ2N,O}zinc(II), C30H22F2N2O4Zn
- The crystal structure of poly[(tripyridine-κ3N,N′,N″) μ3-(pyridine-3,4-dicarboxylate-κ3N:O:O′) manganese(II)], C22H22N4O8Mn
- The crystal structure of (E)-4-chloro-N′-(1-(4-hydroxyphenyl)propylidene)benzohydrazide, C16H15ClN2O2
- Synthesis and crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino) ethyl)phenyl)imino)methyl)phenolato-κ2N,O}cobalt(II), C40H46CoN4O4
- Crystal structure of tetraaqua-[(1-(carboxymethyl)-1H-pyrazole-3-carboxylato-κ2N,O)cobalt(II)], C6H12CoN2O8
- (6R,7S)-2,3,13-trimethoxy-6,7-dimethyl-5,6,7,8-tetrahydrobenzo[3′,4′]cycloocta [1′,2′:4,5]benzo[1,2-d][1,3]dioxol-1-ol, C22H26O6
- Crystal structure of 2-((2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)amino)benzoic acid, C18H14Cl2N2O3
- Crystal structure of (5aS,6aS,8aR,9R,11aS, 11bS,13R,13aS)-1,1,8a,11a-tetramethyl-9-((S)-1-((S)-5-methyl-6-oxo-3,6-dihydro-2H-pyran-2-yl)ethyl)-3-oxo-1,7,8,8a,9,10,11,11a,11b,12,13,13a-dodecahydro-3H,6H-cyclopenta[5,6]cyclopropa[1,8a]naphtho[2,1-c]oxepin-13-yl acetate, C32H44O6
- Crystal structure of catena-poly[triaqua-(μ2-1-(4-carboxylatophenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylato-O,O′:O″)cobalt(II)], C12H12N2O8Co
- Crystal structure of 3-[(furan-2-ylmethyl)-amino]-2-(2,3,4,5-tetrafluoro-benzoyl)-acrylic acid ethyl ester, C17H13F4NO4
- Crystal structure of methyl 4-(2-ethoxy-2-oxoethoxy)-3-methoxybenzoate, C13H16O6
- Crystal structure of 4-bromo-2-(4-chlorophenyl)-1-methyl-5-(trifluoromethyl)-1H-pyrrole-3-carbonitrile, C13H7BrClF3N2
- The crystal structure of triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ3N,O,O)nickel(II) monohydrate, C12H15NO9Ni
- Crystal structure of dihydroxy(2,4,6-triisopro-pylphenyl)telluronium trifluoromethanesulfonate, C16H25F3O5STe
- The crystal structure of 1-(carboxymethyl)-1H-imidazole 3-oxide
- The crystal structure of 1,3,5-tris(dibromomethyl)benzene, C9H6Br6
- Crystal structure of (Z)-3-(4-methoxyphenyl)-4-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-N-phenylthiazol-2(3H)-imine, C25H21N5OS
- Crystal structure of (Z)-3-(3-(4-hydroxyphenyl)-2-(phenylimino)-2,3-dihydrothiazol-4-yl)-2H-chromen-2-one, C24H16N2O3S
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of poly[diaqua-(μ4-3,3′-di(1H-1,2,4-triazol-1-yl)-[1,1′-biphenyl]-4,4′-dicarboxylate-N:N′:O:O′)cadmium(II)], C18H14N6O6Cd
- Crystal structure of (8R,8′S,13S,13′R)-8,8′-bis(hydroxymethyl)-9,9′,10,10′-tetramethoxy-5,5′,6,6′,8,8′,13,13′-octahydro-[13,13′-bi[1,3]dioxolo[4,5-g]isoquinolino[3,2-a]isoquinoline]-7,7′-diium chloride-methanol (1/2), C46H58N2O14Cl2
- The crystal structure of 8-methoxy-2,2-diphenyl-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C29H25BN2O3S
- Crystal structure of aqua-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N″,N‴)copper(II) 5-carboxyisophthalate tetrahydrate, C25H50N4CuO11
- The crystal structure of 1-(naphthalen-2-ylsulfonyl)-2,2-diphenyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ij]quinoline, C31H23BN2O2S
- Crystal structure of iodido-(η6-benzene) (1-(pyridin-2-yl)-N-(p-fluoro-methanamine)-κ2N,Nʹ)ruthenium(II) hexaflourophosphate, (C18H15F7IN2RuP)
- The crystal structure of 1-(3-oxo-1-phenyl-3-(p-tolyl) propylidene)-1,3-dihydro-2H-inden-2-one, C25H20O2
- Crystal structure of tricyclohexyl[4-(4H-1,2,4-triazol-4-yl)-benzoato-κO]tin(IV), C27H39N3O2Sn
- Crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ4N,O,O,O)cadmium(II)]monohydrate, C12H15NO9Cd
- Crystal structure of ethyl 2-((4-(3,5-dimethylisoxazol-4-yl)-2,6-difluorophenyl)amino)benzoate, C20H18F2N2O3
- The crystal structure of 2-(hydroxymethyl)-2-(4H-1,2,4-triazol-4-yl)propane-1,3-diol, C6H11N3O3
- The crystal structure of 1,2-bis(2,4-dinitrophenyl) hydrazine, C12H8N6O8
- Crystal structure of 1-(2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)-1,2-dihydro-4H-benzo[d][1,3]oxazin-4-one, C19H14Cl2N2O3
- The crystal structure of 5-amino-5-oxo-4-(1-oxo-4-(2-oxopyrrolidin-1-yl)isoindolin-2-yl)pentanoic acid, C17H19N3O5
- Crystal structure of N2,N6-bis(2-(((Z)-5-bromo-2-hydroxybenzylidene)amino) phenyl)pyridine-2,6-dicarboxamide, C33H23Br2N5O4
- The crystal structure of (E)-2-methoxy-6-(((5-methyl-1,3,4-thiadiazol-2-yl)imino)methyl)phenol, C11H11N3O2S
- The crystal structure of 3-((tert-butyldiphenylsilyl)methyl)-5,5-diphenyl-6-(p-tolyl) tetrahydro-2H-pyran-2-one, C41H42O2Si
- Crystal structure of 9-fluoro-4-(6-methoxypyridin-3-yl)-5,6-dihydrobenzo[h]quinazolin-2-amine, C18H15FN4O
- The crystal structure of 2-bromo-5-(4-cyanophenoxy)benzyl 1-methyl-1,2,5,6-tetrahydropyridine-3-carboxylate, C21H19BrN2O3
- Crystal structure of 3,3′-(1,4-phenylenebis(methylene))bis(1-isopropyl-1H-imidazol-3-ium) bis(hexafluorophosphate(V)), C10H14F6N2P
- The crystal structure of 2,2-di(thiophen-3-yl)-1-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C24H19BN2O2S3
- Crystal structure of 5-bromo-1-(2-iodobenzoyl)-1H-indole-3-carbaldehyde, C16H9BrINO2
- The crystal structure of monocarbonyl-2-carboxypyridinato-κ2N,O-triphenylphosphine-rhodium(I) acetonitrile solvate, C26H20.50N1.50O3PRh
- Crystal structure of dichlorido-tetrakis(1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ1N)manganese(II), C60H68O4N12Cl10Mn
- Crystal structure of 3-(tert-butyldiphenylsilyl)-1-(2,6-dichlorophenyl)-2,2-diphenylpropan-1-ol, C37H36Cl2OSi
- Crystal structure of langite from Mine du Pradet (France)
- The crystal structure of 5′-(furan-2-yl)-3′-((4-methylphenyl)sulfonamido)-3′,4′,5′,6′-tetrahydro-[1,1′:3′,1″-terphenyl]-4′-carboxylic acid, C30H27NO5S
- Synthesis and crystal structure of bis{2-(((4-acetophenone)imino)methyl)-4-fluorophenolato-κ2N,O}zinc(II), C30H22F2N2O4Zn
- The crystal structure of poly[(tripyridine-κ3N,N′,N″) μ3-(pyridine-3,4-dicarboxylate-κ3N:O:O′) manganese(II)], C22H22N4O8Mn
- The crystal structure of (E)-4-chloro-N′-(1-(4-hydroxyphenyl)propylidene)benzohydrazide, C16H15ClN2O2
- Synthesis and crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino) ethyl)phenyl)imino)methyl)phenolato-κ2N,O}cobalt(II), C40H46CoN4O4
- Crystal structure of tetraaqua-[(1-(carboxymethyl)-1H-pyrazole-3-carboxylato-κ2N,O)cobalt(II)], C6H12CoN2O8
- (6R,7S)-2,3,13-trimethoxy-6,7-dimethyl-5,6,7,8-tetrahydrobenzo[3′,4′]cycloocta [1′,2′:4,5]benzo[1,2-d][1,3]dioxol-1-ol, C22H26O6
- Crystal structure of 2-((2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)amino)benzoic acid, C18H14Cl2N2O3
- Crystal structure of (5aS,6aS,8aR,9R,11aS, 11bS,13R,13aS)-1,1,8a,11a-tetramethyl-9-((S)-1-((S)-5-methyl-6-oxo-3,6-dihydro-2H-pyran-2-yl)ethyl)-3-oxo-1,7,8,8a,9,10,11,11a,11b,12,13,13a-dodecahydro-3H,6H-cyclopenta[5,6]cyclopropa[1,8a]naphtho[2,1-c]oxepin-13-yl acetate, C32H44O6
- Crystal structure of catena-poly[triaqua-(μ2-1-(4-carboxylatophenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylato-O,O′:O″)cobalt(II)], C12H12N2O8Co
- Crystal structure of 3-[(furan-2-ylmethyl)-amino]-2-(2,3,4,5-tetrafluoro-benzoyl)-acrylic acid ethyl ester, C17H13F4NO4
- Crystal structure of methyl 4-(2-ethoxy-2-oxoethoxy)-3-methoxybenzoate, C13H16O6
- Crystal structure of 4-bromo-2-(4-chlorophenyl)-1-methyl-5-(trifluoromethyl)-1H-pyrrole-3-carbonitrile, C13H7BrClF3N2
- The crystal structure of triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ3N,O,O)nickel(II) monohydrate, C12H15NO9Ni
- Crystal structure of dihydroxy(2,4,6-triisopro-pylphenyl)telluronium trifluoromethanesulfonate, C16H25F3O5STe
- The crystal structure of 1-(carboxymethyl)-1H-imidazole 3-oxide
- The crystal structure of 1,3,5-tris(dibromomethyl)benzene, C9H6Br6
- Crystal structure of (Z)-3-(4-methoxyphenyl)-4-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-N-phenylthiazol-2(3H)-imine, C25H21N5OS
- Crystal structure of (Z)-3-(3-(4-hydroxyphenyl)-2-(phenylimino)-2,3-dihydrothiazol-4-yl)-2H-chromen-2-one, C24H16N2O3S

