Abstract
C60H68O4N12Cl10Mn, triclinic,
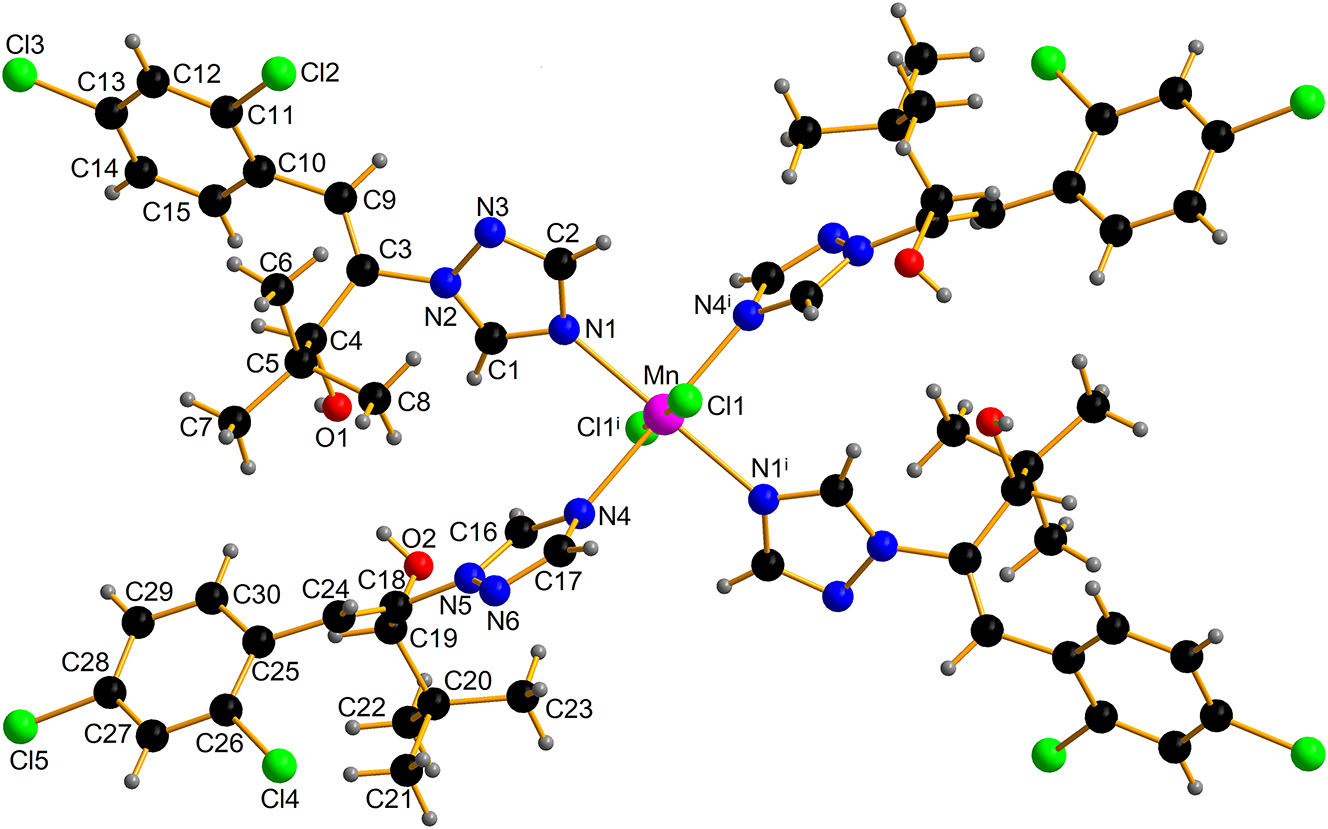
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Pink needle |
| Size: | 0.35 × 0.27 × 0.16 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.62 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| θmax, completeness: | 28.4°, 99 % |
| N(hkl)measured, N(hkl)unique, Rint: | 10,534, 7756, 0.023 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2σ(Iobs), 5739 |
| N(param)refined: | 402 |
| Programs: | Bruker [1], SHELX [2], [3], [4], Diamond [5] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| Mn | 0.500000 | 0.500000 | 0.000000 | 0.03029 (12) |
| Cl1 | 0.74024 (7) | 0.47851 (5) | 0.10640 (4) | 0.04816 (17) |
| Cl2 | 0.39941 (9) | 0.85111 (6) | 0.47832 (5) | 0.0691 (2) |
| Cl3 | −0.17640 (12) | 0.86298 (8) | 0.53008 (7) | 0.0950 (3) |
| Cl4 | −0.12160 (9) | −0.03241 (6) | 0.24942 (6) | 0.0697 (2) |
| Cl5 | −0.66239 (8) | 0.02985 (6) | 0.31674 (5) | 0.0654 (2) |
| N1 | 0.4520 (2) | 0.59086 (16) | 0.11160 (12) | 0.0421 (5) |
| N2 | 0.3430 (2) | 0.63165 (15) | 0.21983 (12) | 0.0370 (4) |
| N3 | 0.4992 (2) | 0.68953 (19) | 0.23896 (14) | 0.0567 (6) |
| N4 | 0.3399 (2) | 0.35657 (15) | 0.04347 (13) | 0.0404 (5) |
| N5 | 0.1333 (2) | 0.26191 (14) | 0.09368 (12) | 0.0353 (4) |
| N6 | 0.2576 (2) | 0.22805 (18) | 0.13018 (16) | 0.0565 (6) |
| O1 | 0.0546 (2) | 0.46907 (13) | 0.22728 (11) | 0.0488 (4) |
| H1A | −0.025303 | 0.482234 | 0.203204 | 0.073* |
| O2 | −0.1437 (2) | 0.31610 (13) | 0.00783 (11) | 0.0483 (4) |
| H2A | −0.171496 | 0.354001 | 0.039883 | 0.072* |
| C1 | 0.3191 (3) | 0.57584 (18) | 0.14375 (14) | 0.0386 (5) |
| H1 | 0.221443 | 0.532060 | 0.116847 | 0.046* |
| C2 | 0.5575 (3) | 0.6611 (2) | 0.17188 (17) | 0.0549 (7) |
| H2 | 0.663701 | 0.687415 | 0.166468 | 0.066* |
| C3 | 0.2310 (3) | 0.63892 (18) | 0.27668 (13) | 0.0356 (5) |
| C4 | 0.1290 (3) | 0.53985 (17) | 0.30288 (15) | 0.0388 (5) |
| H4 | 0.044774 | 0.555014 | 0.329732 | 0.047* |
| C5 | 0.2179 (3) | 0.4843 (2) | 0.37108 (17) | 0.0535 (7) |
| C6 | 0.3072 (5) | 0.5602 (3) | 0.4504 (2) | 0.0875 (12) |
| H6A | 0.346002 | 0.524212 | 0.497474 | 0.131* |
| H6B | 0.236568 | 0.593642 | 0.470492 | 0.131* |
| H6C | 0.394910 | 0.610243 | 0.432868 | 0.131* |
| C7 | 0.0910 (5) | 0.3976 (3) | 0.4027 (2) | 0.0889 (11) |
| H7A | 0.032477 | 0.350621 | 0.353247 | 0.133* |
| H7B | 0.019826 | 0.425568 | 0.428805 | 0.133* |
| H7C | 0.141239 | 0.362286 | 0.445936 | 0.133* |
| C8 | 0.3316 (4) | 0.4390 (3) | 0.3313 (2) | 0.0777 (10) |
| H8A | 0.422304 | 0.493051 | 0.321934 | 0.117* |
| H8B | 0.278673 | 0.401944 | 0.275779 | 0.117* |
| H8C | 0.365415 | 0.393569 | 0.371155 | 0.117* |
| C9 | 0.2349 (3) | 0.73235 (18) | 0.30434 (14) | 0.0399 (5) |
| H9 | 0.310404 | 0.786452 | 0.286010 | 0.048* |
| C10 | 0.1304 (3) | 0.75907 (17) | 0.36181 (14) | 0.0377 (5) |
| C11 | 0.1950 (3) | 0.81740 (18) | 0.44173 (15) | 0.0443 (6) |
| C12 | 0.1021 (4) | 0.8499 (2) | 0.49383 (17) | 0.0536 (7) |
| H12 | 0.147705 | 0.889827 | 0.546484 | 0.064* |
| C13 | −0.0594 (4) | 0.8214 (2) | 0.46547 (18) | 0.0549 (7) |
| C14 | −0.1295 (3) | 0.7634 (2) | 0.38707 (18) | 0.0522 (6) |
| H14 | −0.238824 | 0.745256 | 0.368641 | 0.063* |
| C15 | −0.0328 (3) | 0.7329 (2) | 0.33642 (16) | 0.0465 (6) |
| H15 | −0.079151 | 0.693518 | 0.283565 | 0.056* |
| C16 | 0.1857 (3) | 0.33809 (17) | 0.04256 (15) | 0.0382 (5) |
| H16 | 0.122600 | 0.373076 | 0.010868 | 0.046* |
| C17 | 0.3766 (3) | 0.2874 (2) | 0.09781 (19) | 0.0582 (7) |
| H17 | 0.479182 | 0.282224 | 0.111296 | 0.070* |
| C18 | −0.0221 (2) | 0.22234 (16) | 0.11665 (14) | 0.0334 (5) |
| C19 | −0.1621 (3) | 0.21944 (17) | 0.04521 (14) | 0.0366 (5) |
| H19 | −0.256270 | 0.205691 | 0.073748 | 0.044* |
| C20 | −0.1979 (3) | 0.13581 (19) | −0.03121 (16) | 0.0457 (6) |
| C21 | −0.2114 (4) | 0.0322 (2) | 0.0083 (2) | 0.0796 (10) |
| H21A | −0.289310 | 0.019673 | 0.046555 | 0.119* |
| H21B | −0.110470 | 0.033142 | 0.041584 | 0.119* |
| H21C | −0.242696 | −0.021016 | −0.038674 | 0.119* |
| C22 | −0.3596 (4) | 0.1328 (3) | −0.0856 (2) | 0.0756 (9) |
| H22A | −0.388540 | 0.080191 | −0.132923 | 0.113* |
| H22B | −0.352414 | 0.197658 | −0.109871 | 0.113* |
| H22C | −0.438706 | 0.118846 | −0.048048 | 0.113* |
| C23 | −0.0740 (4) | 0.1573 (3) | −0.09279 (18) | 0.0668 (8) |
| H23A | 0.025435 | 0.153346 | −0.061103 | 0.100* |
| H23B | −0.060715 | 0.224489 | −0.113218 | 0.100* |
| H23C | −0.109114 | 0.107681 | −0.142594 | 0.100* |
| C24 | −0.0321 (3) | 0.18729 (18) | 0.19659 (15) | 0.0401 (5) |
| H24 | 0.061218 | 0.186407 | 0.232829 | 0.048* |
| C25 | −0.1832 (3) | 0.14961 (18) | 0.23122 (14) | 0.0379 (5) |
| C26 | −0.2368 (3) | 0.04964 (19) | 0.25606 (15) | 0.0411 (5) |
| C27 | −0.3814 (3) | 0.01249 (19) | 0.28457 (15) | 0.0458 (6) |
| H27 | −0.414770 | −0.054332 | 0.300645 | 0.055* |
| C28 | −0.4751 (3) | 0.0768 (2) | 0.28862 (15) | 0.0442 (6) |
| C29 | −0.4223 (3) | 0.1778 (2) | 0.26984 (16) | 0.0496 (6) |
| H29 | −0.483036 | 0.221722 | 0.276065 | 0.060* |
| C30 | −0.2775 (3) | 0.21255 (19) | 0.24159 (16) | 0.0465 (6) |
| H30 | −0.241926 | 0.280615 | 0.229091 | 0.056* |
1 Source of material
The (E)-(RS)-1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol (0.653 g, 0.2 mmol) was dissolved in methanol (10 mL). Then, manganese chloride tetrahydrate (0.198 g, 0.1 mmol) was added. Reaction was allowed to stir at room temperature for 2 h. Pink needle crystal of the title compound was obtained by slow evaporation from propanone.
2 Experimental details
Hydrogen atoms were placed in their geometrically idealized positions and constrained to ride on their parent atoms, with C–H = 0.96 Å (methyl), Uiso (H) = 1.5 Ueq(C), C–H = 0.98 Å (methine), Uiso(H) = 1.2 Ueq(C), C–H = 0.93 Å (aromatic and alkenyl), Uiso(H) = 1.2 Ueq(C), and O–H = 0.82 Å (hydroxyl), Uiso(H) = 1.5 Ueq(O).
3 Comment
Triazole fungicides have the advantages of high efficiency, broad spectrum, low toxicity to nontarget organisms, and low resistance. Triazole fungicides can be used to control fungal diseases, such as powdery mildews, rusts, and many leaf spot fungi on a variety of plants [6]. In recent years, they have been widely used in the food and environmental fields [7, 8]. Diniconazole (E)-(RS)-1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1H-1,2,4-triazol-1-yl) pent-1-en-3-ol is a chiral triazole fungicide with protective, therapeutic, and eradicative effects. Its mode of action is to inhibit the demethylation of steroids, which play an important role in the ergosterol biosynthesis of basidiomycetes and ascomycetes, it has strong bactericidal activity to ascomycotina, basidiomycotina, and deuteromycotina [9]. After application, diniconazole can be absorbed by various parts of the plant, and it has been widely used in cereals, fruit trees, vegetables, and so on [10]. The pharmacological and toxicological properties of many drugs are improved when they form metal complexes [11], [12], [13], [14]. In this paper, a new diniconazole Mn(II) complex is described.
The asymmetric unit of the title structure contains a half Mn(II) cation, one chlorine anion and two diniconazole molecules to construct a new mononuclear complex. The Mn(II) cation is six-coordinated by two chlorido ligands and four triazole ligands. The Mn–N length (Mn–N1 = 2.2559(18) Å, Mn–N4 = 2.2576(18) Å) and Mn–Cl length (Mn–Cl1 = 2.5775(6) Å), the N1–Mn–N4, N1–Mn–N4 i , N1–Mn–Cl1, N4–Mn–Cl1, N1–Mn–Cl1 i , and N4–Mn–Cl1 i bond angles of 89.30(7)°, 90.70(7)°, 87.99(5)°, 90.84(5)°, 92.01(5)°, and 89.16(5)°, respectively (symmetry code: (i) −x + 1, −y + 1, −z). Mn(II) cation is the center of symmetry of the title compound, so the bond angle of N1–Mn–N1 i , N4–Mn–N4 i , and Cl1–Mn–Cl1 i is 180°. These are in agreement with closely related Mn(II) complexes reported [15], [16], [17], [18]. The title compound through O1–H1A⋯Cl1 ii (dO1⋯Cl1 = 3.1505(17) Å, 163.2°, symmetry code: (ii) x − 1, y, z) and O2–H2A⋯Cl1 ii (dO2⋯Cl1 = 3.1552(18) Å, 168.8°) hydrogen bond forming a one-dimensional (1D) chain along the a-axis.
Funding source: National Natural Science Foundation of China
Award Identifier / Grant number: (22163012)
Funding source: Key Laboratory Project Foundation of Shaanxi Provincial Education Department in China
Award Identifier / Grant number: (20JS158)
Funding source: Yulin University Graduate Innovation Fund Project
Award Identifier / Grant number: (2023YLYCX01)
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: The work was supported by National Natural Science Foundation of China (22163012), Key Laboratory Project Foundation of Shaanxi Provincial Education Department in China (20JS158), Yulin University Graduate Innovation Fund Project (2023YLYCX01).
References
1. Bruker. APEX2, SAINT and SADABS; Bruker AXS Inc.: Madison, Wisconsin, USA, 2009.Search in Google Scholar
2. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar PubMed PubMed Central
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Search in Google Scholar PubMed
5. Brandenburg, K. DIAMOND; Crystal Impact GbR: Bonn Germany, 2006.Search in Google Scholar
6. Cui, K., Wu, X., Zhang, Y., Cao, J., Wei, D., Xu, J., Dong, F., Liu, X., Zheng, Y. Cumulative risk assessment of dietary exposure to triazole fungicides from 13 daily-consumed foods in China. Environ. Pollut. 2021, 286, 117550; https://doi.org/10.1016/j.envpol.2021.117550.Search in Google Scholar PubMed
7. Zhang, Q., Gao, B., Tian, M., Shi, H., Hua, X., Wang, M. Enantioseparation and determination of triticonazole enantiomers in fruits, vegetables, and soil using efficient extraction and clean-up methods. J. Chromatogr. B 2016, 1009–1010, 130–137; https://doi.org/10.1016/j.jchromb.2015.12.018.Search in Google Scholar PubMed
8. Mai, B., Fan, J., Jiang, Y., He, R., Lai, Y., Zhang, W. Fast enantioselective determination of triadimefon in different matrices by supercritical fluid chromatography. J. Chromatogr. B 2019, 1126–1127, 121740; https://doi.org/10.1016/j.jchromb.2019.121740.Search in Google Scholar PubMed
9. Teng, C., Gu, Y., Wang, Y., Wang, Z., Zhao, H., Qi, P., Guo, C., Xu, H., Di, S., Wang, X. Enantioselective dissipation, residue, and risk assessment of diniconazole enantiomers in four kinds of fruits. J. Agric. Food Chem. 2021, 69, 15512–15520; https://doi.org/10.1021/acs.jafc.1c03852.Search in Google Scholar PubMed
10. Wang, Y., Zhu, W., Wang, D., Teng, M., Yan, J., Miao, J., Zhou, Z. 1H NMR-based metabolomics analysis of adult zebrafish (Danio rerio) after exposure to diniconazole as well as its bioaccumulation behavior. Chemosphere 2017, 168, 1571–1577; https://doi.org/10.1016/j.chemosphere.2016.11.157.Search in Google Scholar PubMed
11. Ren, G.-Y., Li, J., Zhou, J.-H., Yan, B., Ren, Y.-H., Sun, X.-H., Ma, H.-X. Enhanced antifungal activities of four Zn(II) complexes based on uniconazole. Appl. Organomet. Chem. 2018, 32, e4169; https://doi.org/10.1002/aoc.4169.Search in Google Scholar
12. Ren, G.-Y., Li, J., Wei, X.-Z., Zhou, J.-H., Yan, B., Guo, Z.-Q., Ren, Y.-H., Sun, X.-H., Ma, H.-X. The enhanced biological activities of three Zn(II) complexes based on different 1,2,4-triazole fungicides. Appl. Organomet. Chem. 2018, 32, e4474; https://doi.org/10.1002/aoc.4474.Search in Google Scholar
13. Jie, L., Teng, X., Yan, B., Yang, M., Song, J., Ma, H. Two Cu(II) complexes of triadimefon: crystal structure, antifungal activities and structure–activity relationship. New J. Chem. 2015, 39, 6997–7003; https://doi.org/10.1039/c5nj00679a.Search in Google Scholar
14. Jie, L., Teng, X., Yan, B., Guan, Y., Yang, M., Song, J., Ma, H. Synergistic actions between tebuconazole ligand and Cu(II) cation: reasons for the enhanced antifungal activity of four Cu(II) complexes based on the fungicide tebuconazole. New J. Chem. 2015, 39, 9550–9556; https://doi.org/10.1039/c5nj01845e.Search in Google Scholar
15. Lumme, P. O., Lindell, E., Mutikainen, I. Trans-hexakis (pyrazole) manganese (II) bisperchlorate (1) and trans-dichlorotetrakis (pyrazole) manganese(II) (2). Acta Crystallogr. 1988, C44, 967–970; https://doi.org/10.1107/s0108270188001131.Search in Google Scholar
16. Li, J., Yan, B., Li, H. Crystal structure of dichlorido-tetra((E)- (RS)-1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ1N)zinc(II), C60H68O4N12Cl10Zn. Z. Kristallogr. N. Cryst. Struct. 2022, 237, 1121–1123, https://doi.org/10.1515/ncrs-2022-0384.Search in Google Scholar
17. Song, X.-Y., Liu, C.-X., Liu, Q., Nie, X.-L., Wen, S.-H. Dichloridotetrakis(diniconazole)cobalt(II). Acta Crystallogr. 2011, E67, m1214; https://doi.org/10.1107/s1600536811031291.Search in Google Scholar
18. Tang, X., Speldrich, M., Tchougreeff, A. L., Dronskowski, R. Syntheses, crystal structures and magnetic properties of Cr(NCNH2)4Cl2 and Mn(NCNH2)4Cl2. Z. Naturforsch. B 2012, 67, 1205–1211; https://doi.org/10.5560/znb.2012-0234.Search in Google Scholar
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of poly[diaqua-(μ4-3,3′-di(1H-1,2,4-triazol-1-yl)-[1,1′-biphenyl]-4,4′-dicarboxylate-N:N′:O:O′)cadmium(II)], C18H14N6O6Cd
- Crystal structure of (8R,8′S,13S,13′R)-8,8′-bis(hydroxymethyl)-9,9′,10,10′-tetramethoxy-5,5′,6,6′,8,8′,13,13′-octahydro-[13,13′-bi[1,3]dioxolo[4,5-g]isoquinolino[3,2-a]isoquinoline]-7,7′-diium chloride-methanol (1/2), C46H58N2O14Cl2
- The crystal structure of 8-methoxy-2,2-diphenyl-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C29H25BN2O3S
- Crystal structure of aqua-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N″,N‴)copper(II) 5-carboxyisophthalate tetrahydrate, C25H50N4CuO11
- The crystal structure of 1-(naphthalen-2-ylsulfonyl)-2,2-diphenyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ij]quinoline, C31H23BN2O2S
- Crystal structure of iodido-(η6-benzene) (1-(pyridin-2-yl)-N-(p-fluoro-methanamine)-κ2N,Nʹ)ruthenium(II) hexaflourophosphate, (C18H15F7IN2RuP)
- The crystal structure of 1-(3-oxo-1-phenyl-3-(p-tolyl) propylidene)-1,3-dihydro-2H-inden-2-one, C25H20O2
- Crystal structure of tricyclohexyl[4-(4H-1,2,4-triazol-4-yl)-benzoato-κO]tin(IV), C27H39N3O2Sn
- Crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ4N,O,O,O)cadmium(II)]monohydrate, C12H15NO9Cd
- Crystal structure of ethyl 2-((4-(3,5-dimethylisoxazol-4-yl)-2,6-difluorophenyl)amino)benzoate, C20H18F2N2O3
- The crystal structure of 2-(hydroxymethyl)-2-(4H-1,2,4-triazol-4-yl)propane-1,3-diol, C6H11N3O3
- The crystal structure of 1,2-bis(2,4-dinitrophenyl) hydrazine, C12H8N6O8
- Crystal structure of 1-(2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)-1,2-dihydro-4H-benzo[d][1,3]oxazin-4-one, C19H14Cl2N2O3
- The crystal structure of 5-amino-5-oxo-4-(1-oxo-4-(2-oxopyrrolidin-1-yl)isoindolin-2-yl)pentanoic acid, C17H19N3O5
- Crystal structure of N2,N6-bis(2-(((Z)-5-bromo-2-hydroxybenzylidene)amino) phenyl)pyridine-2,6-dicarboxamide, C33H23Br2N5O4
- The crystal structure of (E)-2-methoxy-6-(((5-methyl-1,3,4-thiadiazol-2-yl)imino)methyl)phenol, C11H11N3O2S
- The crystal structure of 3-((tert-butyldiphenylsilyl)methyl)-5,5-diphenyl-6-(p-tolyl) tetrahydro-2H-pyran-2-one, C41H42O2Si
- Crystal structure of 9-fluoro-4-(6-methoxypyridin-3-yl)-5,6-dihydrobenzo[h]quinazolin-2-amine, C18H15FN4O
- The crystal structure of 2-bromo-5-(4-cyanophenoxy)benzyl 1-methyl-1,2,5,6-tetrahydropyridine-3-carboxylate, C21H19BrN2O3
- Crystal structure of 3,3′-(1,4-phenylenebis(methylene))bis(1-isopropyl-1H-imidazol-3-ium) bis(hexafluorophosphate(V)), C10H14F6N2P
- The crystal structure of 2,2-di(thiophen-3-yl)-1-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C24H19BN2O2S3
- Crystal structure of 5-bromo-1-(2-iodobenzoyl)-1H-indole-3-carbaldehyde, C16H9BrINO2
- The crystal structure of monocarbonyl-2-carboxypyridinato-κ2N,O-triphenylphosphine-rhodium(I) acetonitrile solvate, C26H20.50N1.50O3PRh
- Crystal structure of dichlorido-tetrakis(1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ1N)manganese(II), C60H68O4N12Cl10Mn
- Crystal structure of 3-(tert-butyldiphenylsilyl)-1-(2,6-dichlorophenyl)-2,2-diphenylpropan-1-ol, C37H36Cl2OSi
- Crystal structure of langite from Mine du Pradet (France)
- The crystal structure of 5′-(furan-2-yl)-3′-((4-methylphenyl)sulfonamido)-3′,4′,5′,6′-tetrahydro-[1,1′:3′,1″-terphenyl]-4′-carboxylic acid, C30H27NO5S
- Synthesis and crystal structure of bis{2-(((4-acetophenone)imino)methyl)-4-fluorophenolato-κ2N,O}zinc(II), C30H22F2N2O4Zn
- The crystal structure of poly[(tripyridine-κ3N,N′,N″) μ3-(pyridine-3,4-dicarboxylate-κ3N:O:O′) manganese(II)], C22H22N4O8Mn
- The crystal structure of (E)-4-chloro-N′-(1-(4-hydroxyphenyl)propylidene)benzohydrazide, C16H15ClN2O2
- Synthesis and crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino) ethyl)phenyl)imino)methyl)phenolato-κ2N,O}cobalt(II), C40H46CoN4O4
- Crystal structure of tetraaqua-[(1-(carboxymethyl)-1H-pyrazole-3-carboxylato-κ2N,O)cobalt(II)], C6H12CoN2O8
- (6R,7S)-2,3,13-trimethoxy-6,7-dimethyl-5,6,7,8-tetrahydrobenzo[3′,4′]cycloocta [1′,2′:4,5]benzo[1,2-d][1,3]dioxol-1-ol, C22H26O6
- Crystal structure of 2-((2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)amino)benzoic acid, C18H14Cl2N2O3
- Crystal structure of (5aS,6aS,8aR,9R,11aS, 11bS,13R,13aS)-1,1,8a,11a-tetramethyl-9-((S)-1-((S)-5-methyl-6-oxo-3,6-dihydro-2H-pyran-2-yl)ethyl)-3-oxo-1,7,8,8a,9,10,11,11a,11b,12,13,13a-dodecahydro-3H,6H-cyclopenta[5,6]cyclopropa[1,8a]naphtho[2,1-c]oxepin-13-yl acetate, C32H44O6
- Crystal structure of catena-poly[triaqua-(μ2-1-(4-carboxylatophenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylato-O,O′:O″)cobalt(II)], C12H12N2O8Co
- Crystal structure of 3-[(furan-2-ylmethyl)-amino]-2-(2,3,4,5-tetrafluoro-benzoyl)-acrylic acid ethyl ester, C17H13F4NO4
- Crystal structure of methyl 4-(2-ethoxy-2-oxoethoxy)-3-methoxybenzoate, C13H16O6
- Crystal structure of 4-bromo-2-(4-chlorophenyl)-1-methyl-5-(trifluoromethyl)-1H-pyrrole-3-carbonitrile, C13H7BrClF3N2
- The crystal structure of triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ3N,O,O)nickel(II) monohydrate, C12H15NO9Ni
- Crystal structure of dihydroxy(2,4,6-triisopro-pylphenyl)telluronium trifluoromethanesulfonate, C16H25F3O5STe
- The crystal structure of 1-(carboxymethyl)-1H-imidazole 3-oxide
- The crystal structure of 1,3,5-tris(dibromomethyl)benzene, C9H6Br6
- Crystal structure of (Z)-3-(4-methoxyphenyl)-4-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-N-phenylthiazol-2(3H)-imine, C25H21N5OS
- Crystal structure of (Z)-3-(3-(4-hydroxyphenyl)-2-(phenylimino)-2,3-dihydrothiazol-4-yl)-2H-chromen-2-one, C24H16N2O3S
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of poly[diaqua-(μ4-3,3′-di(1H-1,2,4-triazol-1-yl)-[1,1′-biphenyl]-4,4′-dicarboxylate-N:N′:O:O′)cadmium(II)], C18H14N6O6Cd
- Crystal structure of (8R,8′S,13S,13′R)-8,8′-bis(hydroxymethyl)-9,9′,10,10′-tetramethoxy-5,5′,6,6′,8,8′,13,13′-octahydro-[13,13′-bi[1,3]dioxolo[4,5-g]isoquinolino[3,2-a]isoquinoline]-7,7′-diium chloride-methanol (1/2), C46H58N2O14Cl2
- The crystal structure of 8-methoxy-2,2-diphenyl-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C29H25BN2O3S
- Crystal structure of aqua-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N″,N‴)copper(II) 5-carboxyisophthalate tetrahydrate, C25H50N4CuO11
- The crystal structure of 1-(naphthalen-2-ylsulfonyl)-2,2-diphenyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ij]quinoline, C31H23BN2O2S
- Crystal structure of iodido-(η6-benzene) (1-(pyridin-2-yl)-N-(p-fluoro-methanamine)-κ2N,Nʹ)ruthenium(II) hexaflourophosphate, (C18H15F7IN2RuP)
- The crystal structure of 1-(3-oxo-1-phenyl-3-(p-tolyl) propylidene)-1,3-dihydro-2H-inden-2-one, C25H20O2
- Crystal structure of tricyclohexyl[4-(4H-1,2,4-triazol-4-yl)-benzoato-κO]tin(IV), C27H39N3O2Sn
- Crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ4N,O,O,O)cadmium(II)]monohydrate, C12H15NO9Cd
- Crystal structure of ethyl 2-((4-(3,5-dimethylisoxazol-4-yl)-2,6-difluorophenyl)amino)benzoate, C20H18F2N2O3
- The crystal structure of 2-(hydroxymethyl)-2-(4H-1,2,4-triazol-4-yl)propane-1,3-diol, C6H11N3O3
- The crystal structure of 1,2-bis(2,4-dinitrophenyl) hydrazine, C12H8N6O8
- Crystal structure of 1-(2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)-1,2-dihydro-4H-benzo[d][1,3]oxazin-4-one, C19H14Cl2N2O3
- The crystal structure of 5-amino-5-oxo-4-(1-oxo-4-(2-oxopyrrolidin-1-yl)isoindolin-2-yl)pentanoic acid, C17H19N3O5
- Crystal structure of N2,N6-bis(2-(((Z)-5-bromo-2-hydroxybenzylidene)amino) phenyl)pyridine-2,6-dicarboxamide, C33H23Br2N5O4
- The crystal structure of (E)-2-methoxy-6-(((5-methyl-1,3,4-thiadiazol-2-yl)imino)methyl)phenol, C11H11N3O2S
- The crystal structure of 3-((tert-butyldiphenylsilyl)methyl)-5,5-diphenyl-6-(p-tolyl) tetrahydro-2H-pyran-2-one, C41H42O2Si
- Crystal structure of 9-fluoro-4-(6-methoxypyridin-3-yl)-5,6-dihydrobenzo[h]quinazolin-2-amine, C18H15FN4O
- The crystal structure of 2-bromo-5-(4-cyanophenoxy)benzyl 1-methyl-1,2,5,6-tetrahydropyridine-3-carboxylate, C21H19BrN2O3
- Crystal structure of 3,3′-(1,4-phenylenebis(methylene))bis(1-isopropyl-1H-imidazol-3-ium) bis(hexafluorophosphate(V)), C10H14F6N2P
- The crystal structure of 2,2-di(thiophen-3-yl)-1-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C24H19BN2O2S3
- Crystal structure of 5-bromo-1-(2-iodobenzoyl)-1H-indole-3-carbaldehyde, C16H9BrINO2
- The crystal structure of monocarbonyl-2-carboxypyridinato-κ2N,O-triphenylphosphine-rhodium(I) acetonitrile solvate, C26H20.50N1.50O3PRh
- Crystal structure of dichlorido-tetrakis(1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ1N)manganese(II), C60H68O4N12Cl10Mn
- Crystal structure of 3-(tert-butyldiphenylsilyl)-1-(2,6-dichlorophenyl)-2,2-diphenylpropan-1-ol, C37H36Cl2OSi
- Crystal structure of langite from Mine du Pradet (France)
- The crystal structure of 5′-(furan-2-yl)-3′-((4-methylphenyl)sulfonamido)-3′,4′,5′,6′-tetrahydro-[1,1′:3′,1″-terphenyl]-4′-carboxylic acid, C30H27NO5S
- Synthesis and crystal structure of bis{2-(((4-acetophenone)imino)methyl)-4-fluorophenolato-κ2N,O}zinc(II), C30H22F2N2O4Zn
- The crystal structure of poly[(tripyridine-κ3N,N′,N″) μ3-(pyridine-3,4-dicarboxylate-κ3N:O:O′) manganese(II)], C22H22N4O8Mn
- The crystal structure of (E)-4-chloro-N′-(1-(4-hydroxyphenyl)propylidene)benzohydrazide, C16H15ClN2O2
- Synthesis and crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino) ethyl)phenyl)imino)methyl)phenolato-κ2N,O}cobalt(II), C40H46CoN4O4
- Crystal structure of tetraaqua-[(1-(carboxymethyl)-1H-pyrazole-3-carboxylato-κ2N,O)cobalt(II)], C6H12CoN2O8
- (6R,7S)-2,3,13-trimethoxy-6,7-dimethyl-5,6,7,8-tetrahydrobenzo[3′,4′]cycloocta [1′,2′:4,5]benzo[1,2-d][1,3]dioxol-1-ol, C22H26O6
- Crystal structure of 2-((2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)amino)benzoic acid, C18H14Cl2N2O3
- Crystal structure of (5aS,6aS,8aR,9R,11aS, 11bS,13R,13aS)-1,1,8a,11a-tetramethyl-9-((S)-1-((S)-5-methyl-6-oxo-3,6-dihydro-2H-pyran-2-yl)ethyl)-3-oxo-1,7,8,8a,9,10,11,11a,11b,12,13,13a-dodecahydro-3H,6H-cyclopenta[5,6]cyclopropa[1,8a]naphtho[2,1-c]oxepin-13-yl acetate, C32H44O6
- Crystal structure of catena-poly[triaqua-(μ2-1-(4-carboxylatophenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylato-O,O′:O″)cobalt(II)], C12H12N2O8Co
- Crystal structure of 3-[(furan-2-ylmethyl)-amino]-2-(2,3,4,5-tetrafluoro-benzoyl)-acrylic acid ethyl ester, C17H13F4NO4
- Crystal structure of methyl 4-(2-ethoxy-2-oxoethoxy)-3-methoxybenzoate, C13H16O6
- Crystal structure of 4-bromo-2-(4-chlorophenyl)-1-methyl-5-(trifluoromethyl)-1H-pyrrole-3-carbonitrile, C13H7BrClF3N2
- The crystal structure of triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ3N,O,O)nickel(II) monohydrate, C12H15NO9Ni
- Crystal structure of dihydroxy(2,4,6-triisopro-pylphenyl)telluronium trifluoromethanesulfonate, C16H25F3O5STe
- The crystal structure of 1-(carboxymethyl)-1H-imidazole 3-oxide
- The crystal structure of 1,3,5-tris(dibromomethyl)benzene, C9H6Br6
- Crystal structure of (Z)-3-(4-methoxyphenyl)-4-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-N-phenylthiazol-2(3H)-imine, C25H21N5OS
- Crystal structure of (Z)-3-(3-(4-hydroxyphenyl)-2-(phenylimino)-2,3-dihydrothiazol-4-yl)-2H-chromen-2-one, C24H16N2O3S


