Abstract
C12H12N2O8Co, orthorhombic, Pbca (no. 61), a = 11.4542(10) Å, b = 9.2421(8) Å, c = 25.501(2) Å, V = 2699.6(4) Å3, Z = 2, Rgt (F) = 0.0342, wRref (F2) = 0.0888, T = 296 K.
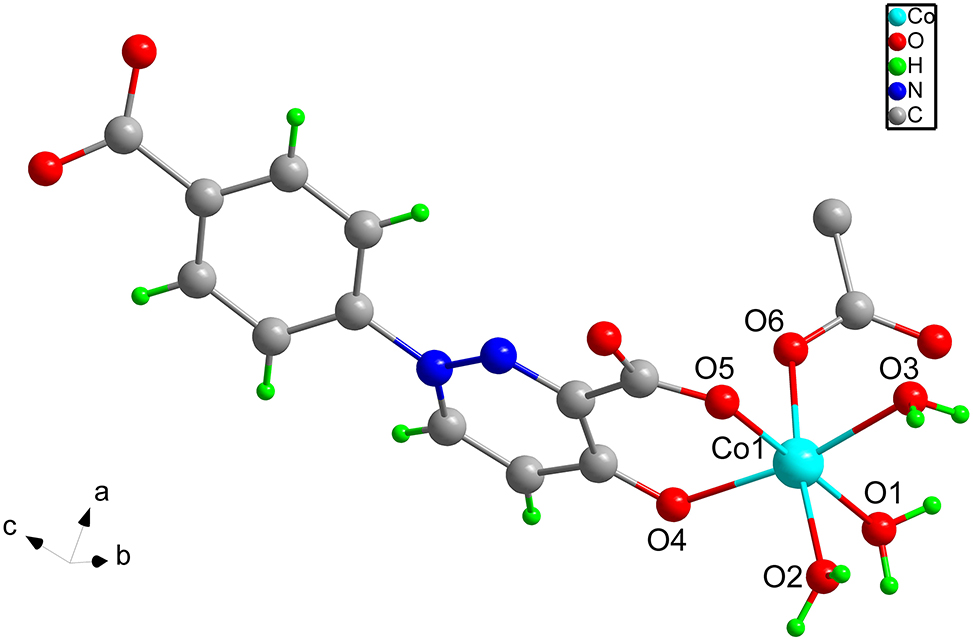
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Pink block |
| Size: | 0.18 × 0.15 × 0.13 mm |
| Wavelength: | MoKα radiation (0.71073 Å) |
| μ: | 1.32 mm−1 |
| Diffractometer, scan mode: | Bruker SMART APEX-II, φ and ω |
| θmax, completeness: | 27.4°, >99 % |
| N(hkl)measured, N(hkl)unique, Rint: | 14,974, 3046, 0.046 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2σ(Iobs), 2504 |
| N(param)refined: | 208 |
| Programs: | Bruker [1], Olex2 [2], SHELX [3, 4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| Co1 | 0.13881 (2) | 0.45219 (3) | −1.14587 (2) | 0.01895 (11) |
| O1 | 0.05773 (15) | 0.65515 (17) | −1.16366 (7) | 0.0290 (4) |
| H1A | 0.001261 | 0.648959 | −1.185176 | 0.044* |
| H1B | 0.105521 | 0.712869 | −1.178226 | 0.044* |
| O2 | 0.04914 (15) | 0.3718 (2) | −1.20910 (7) | 0.0350 (4) |
| H2A | 0.080382 | 0.320590 | −1.232990 | 0.053* |
| H2B | −0.022318 | 0.347170 | −1.209340 | 0.053* |
| O3 | 0.28414 (15) | 0.48307 (19) | −1.19616 (6) | 0.0310 (4) |
| H3A | 0.283613 | 0.419077 | −1.220169 | 0.046* |
| H3B | 0.276973 | 0.562867 | −1.212499 | 0.046* |
| O4 | 0.01823 (13) | 0.39798 (18) | −1.08747 (6) | 0.0255 (4) |
| O5 | 0.21540 (15) | 0.25536 (18) | −1.13058 (6) | 0.0309 (4) |
| O6 | 0.26596 (15) | 0.05578 (17) | −1.08821 (6) | 0.0269 (4) |
| O13 | 0.16708 (16) | −0.2769 (2) | −0.76547 (7) | 0.0394 (5) |
| O14 | 0.34745 (15) | −0.2028 (2) | −0.78060 (7) | 0.0402 (5) |
| N5 | 0.08435 (18) | 0.1433 (2) | −0.96246 (8) | 0.0287 (5) |
| N6 | 0.14938 (17) | 0.1265 (2) | −1.00548 (7) | 0.0269 (4) |
| C25 | 0.2406 (2) | −0.2062 (3) | −0.79095 (9) | 0.0266 (5) |
| C26 | 0.1968 (2) | −0.1133 (3) | −0.83555 (9) | 0.0273 (5) |
| C27 | 0.0827 (2) | −0.0666 (3) | −0.83832 (10) | 0.0294 (5) |
| H27 | 0.030872 | −0.092625 | −0.811890 | 0.035* |
| C28 | 0.0433 (2) | 0.0182 (3) | −0.87955 (9) | 0.0271 (5) |
| H28 | −0.033866 | 0.049225 | −0.880829 | 0.033* |
| C29 | 0.2728 (3) | −0.0710 (4) | −0.87434 (13) | 0.0619 (11) |
| H29 | 0.350811 | −0.098499 | −0.872441 | 0.074* |
| C30 | 0.2350 (3) | 0.0116 (5) | −0.91602 (13) | 0.0707 (13) |
| H30 | 0.286783 | 0.037718 | −0.942453 | 0.085* |
| C31 | 0.1205 (2) | 0.0552 (3) | −0.91837 (9) | 0.0295 (5) |
| C32 | −0.0041 (2) | 0.2400 (3) | −0.96030 (9) | 0.0314 (6) |
| H32 | −0.047643 | 0.248762 | −0.929688 | 0.038* |
| C33 | −0.0299 (2) | 0.3237 (3) | −1.00194 (9) | 0.0304 (5) |
| H33 | −0.092634 | 0.387393 | −0.999992 | 0.036* |
| C34 | 0.03726 (19) | 0.3166 (2) | −1.04898 (8) | 0.0222 (5) |
| C35 | 0.12906 (18) | 0.2065 (2) | −1.04657 (8) | 0.0210 (4) |
| C36 | 0.20972 (19) | 0.1704 (2) | −1.09222 (8) | 0.0206 (4) |
1 Source of material
The mixture of cobalt nitrate hexahydrate 29.1 mg (0.1 mmol), 1-(4-carboxyphenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylic acid 26.1 mg (0.1 mmol), NaOH 4 mg (0.1 mmol) and ethyl alcohol (10 mL) were placed in the autoclave lined with PTFE and heated at 100 °C for 72 h, then cooled up to room temperature over 24 h. Pink needle shaped crystals were collected after cooling to room temperature.
2 Experimental details
Using Olex2 [2], the structure was solved with the ShelXT [3] structure solution program and refined with the ShelXL [4] refinement package.
3 Comment
In recent years, metal coordination polymers have provoked great interest for their promising applications such as laser sensor [5], photosensitive material [6], fluorescent probe [7] and so on. However, the diversity in the framework architectures of such coordination polymers counts on many factors, such as the metal atom species, the coordination geometry of metal centers, the coordination ability of organic ligands and the reaction conditions (pH, temperature, solvent and so on) [8], [9], [10], [11], so that the selection of ligands and metal ions is the key to constructing coordination polymers. The coordination polymers constructed by rigid N-donor and carboxyl groups mixed ligands were a widely effective adopted strategy in this field. For example, the incorporation of functional groups including pyridyl, imidazole and carboxyl group into the internal of coordination polymers could enhance the fluorescence sensing capability through various host–guest interactions, for synthesis of coordination polymers with appropriate luminous performance and are vital. Up to now, several complexes based on 1-(4-carboxyphenyl)-4-oxo-1,4-dihydropyrida zine-3-carboxylic acid have been reported, which exhibited structural diversity and different properties [12, 13].
X-ray single crystal diffraction analysis reveals that the asymmetric unit of the title complex consists of one independent Co(II) ion, one deprotonated 1-(4-carboxyphenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylic acid and three monodentate coordinated water molecules. As show in the Figure, each Co(II) ion is six-coordinated with three oxygen atoms from three monodentate coordinated water molecules and three oxygen atoms from two crystallographically dependent organic ligands. There exists a dihedral angle of 28° between the benzene and pyridazine rings. Among them, the carboxylato group adjacent to pyridazine ring adopt the bridging mode to coordinate the two neighboring Co(II) ions to form a one-dimensional chain [14, 15]. Meanwhile, the carboxylate oxygen of the benzene ring is not involved in coordination. After further modification of the ligand, an interesting 1D structure was formed, which is linked by intermolecular hydrogen bonding to form a 3D supermolecular structure.
Funding source: The work was supported by the Natural Science Foundation of Fujian Province
Award Identifier / Grant number: (2019J05131)
Funding source: Science and Technology Project of Fujian Education Department
Award Identifier / Grant number: (JAT220629)
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: The work was supported by the Natural Science Foundation of Fujian Province (2019J05131) and Science and Technology Project of Fujian Education Department (JAT220629).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. BRUKER. SAINT, APEX2 and SADABS; Bruker AXS Inc.: Madison, Wisconsin, USA, 2009.Search in Google Scholar
2. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
3. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8.10.1107/S2053273314026370Search in Google Scholar PubMed PubMed Central
4. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
5. Li, Y. A., Yang, S., Li, Q. Y., Ma, J. P., Zhang, S. J., Dong, Y. B. UiO-68-ol NMOF-based fluorescent sensor for selective detection of HClO and its application in bioimaging. Inorg. Chem. 2017, 56, 13241–13248; https://doi.org/10.1021/acs.inorgchem.7b02012.Search in Google Scholar PubMed
6. Cheng, W., Sheng, R., Wang, Y., Liu, Y. T., Tong, B. H., Chen, P., Wang, S. Preparation and electroluminescent application of iridium(III) complexes containing sulfur-containing phenylpyridazine ligands. Transition Met. Chem. 2021, 46, 81–89; https://doi.org/10.1007/s11243-020-00424-6.Search in Google Scholar
7. Gao, Y. H., Huang, P. P., Xu, H. T., Huang, P., Liu, B., Lu, J. F., Ge, H. G. Two novel Zn(II) coordination polymers constructed by the same dicarboxylate and different bis-imidazole as co-ligand: syntheses, crystal structures and properties. J. Mol. Struct. 2023, 36, 135106–135114; https://doi.org/10.1016/j.molstruc.2023.135106.Search in Google Scholar
8. Chang, H. N., Liu, L. W., Hao, Z. C., Cui, G. H. A 3D Ag(I) metal-organic framework for sensing luminescence and photocatalytic activities. J. Mol. Struct. 2018, 1155, 496–502; https://doi.org/10.1016/j.molstruc.2017.11.038.Search in Google Scholar
9. Onwudiwe, D. C., Hosten, E. C. Synthesis, structural characterization, and thermal stability studies of heteroleptic cadmium(II) dithiocarbamate with different pyridyl groups. J. Mol. Struct. 2018, 1152, 409–421; https://doi.org/10.1016/j.molstruc.2017.09.076.Search in Google Scholar
10. Wang, J. L., Bai, Y., Pan, H., Zheng, G. S., Dang, D. B. Long-range magnetic ordering in a metal-organic framework based on octanuclear nickel(II) clusters. Dalton Trans. 2017, 46, 12771–12774; https://doi.org/10.1039/c7dt02889j.Search in Google Scholar PubMed
11. Wu, Z. F., Huang, X. Y. A series of Mg–Zn heterometallic coordination polymers: synthesis, characterization, and fluorescence sensing for Fe3+, CS2, and nitroaromatic compounds. Dalton Trans. 2017, 46, 12597–12604; https://doi.org/10.1039/c7dt02800h.Search in Google Scholar PubMed
12. Gao, J. H., Wang, J. X., Huang, P. P., Liu, J., Zheng, N., Shi, J., Xu, H. T., Yue, S. Y., Lu, J. F. A new pyrazine carboxyl derivative and its two d10 metal coordination polymers: syntheses, characterization, DFT and property. J. Mol. Struct. 2023, 1290, 135935–135946; https://doi.org/10.1016/j.molstruc.2023.135935.Search in Google Scholar
13. Gao, J. H., Huang, P. P., Liu, J., Zheng, N., Wang, D., Yue, S. Y., Liu, E. N., Liu, Q., Liu, B., Lu, J. F. Construction of three novel Co/Zn/Cd(II) coordination polymers based on the same ligands: different spatial structures, electrocatalysis and photoluminescence properties. Arab. J. Chem. 2023, 16, 105314–105330; https://doi.org/10.1016/j.arabjc.2023.105314.Search in Google Scholar
14. Zhao, J., Liu, M.-L., Guo, S.-B., Sun, M., Zheng, J.-L., Ma, S.-T., Lu, J.-F., Ge, H.-G. Two Cu(II) complexes constructed by pyridazine carboxyl derivatives: synthesis, crystal structure and property. Inorg. Nano-Met. Chem. 2021, 52, 753–759; https://doi.org/10.1080/24701556.2021.1952247.Search in Google Scholar
15. Gao, J.-H., Wang, J.-X., Huang, P.-P., Liu, J., Zheng, N., Shi, J., Xu, H.-T., Yue, S.-Y., Lu, J.-F. A new pyrazine carboxyl derivative and its two d10 metal coordination polymers: syntheses, characterization, DFT and property. J. Mol. Struct. 2023, 1290, 135935; https://doi.org/10.1016/j.molstruc.2023.135935.Search in Google Scholar
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of poly[diaqua-(μ4-3,3′-di(1H-1,2,4-triazol-1-yl)-[1,1′-biphenyl]-4,4′-dicarboxylate-N:N′:O:O′)cadmium(II)], C18H14N6O6Cd
- Crystal structure of (8R,8′S,13S,13′R)-8,8′-bis(hydroxymethyl)-9,9′,10,10′-tetramethoxy-5,5′,6,6′,8,8′,13,13′-octahydro-[13,13′-bi[1,3]dioxolo[4,5-g]isoquinolino[3,2-a]isoquinoline]-7,7′-diium chloride-methanol (1/2), C46H58N2O14Cl2
- The crystal structure of 8-methoxy-2,2-diphenyl-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C29H25BN2O3S
- Crystal structure of aqua-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N″,N‴)copper(II) 5-carboxyisophthalate tetrahydrate, C25H50N4CuO11
- The crystal structure of 1-(naphthalen-2-ylsulfonyl)-2,2-diphenyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ij]quinoline, C31H23BN2O2S
- Crystal structure of iodido-(η6-benzene) (1-(pyridin-2-yl)-N-(p-fluoro-methanamine)-κ2N,Nʹ)ruthenium(II) hexaflourophosphate, (C18H15F7IN2RuP)
- The crystal structure of 1-(3-oxo-1-phenyl-3-(p-tolyl) propylidene)-1,3-dihydro-2H-inden-2-one, C25H20O2
- Crystal structure of tricyclohexyl[4-(4H-1,2,4-triazol-4-yl)-benzoato-κO]tin(IV), C27H39N3O2Sn
- Crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ4N,O,O,O)cadmium(II)]monohydrate, C12H15NO9Cd
- Crystal structure of ethyl 2-((4-(3,5-dimethylisoxazol-4-yl)-2,6-difluorophenyl)amino)benzoate, C20H18F2N2O3
- The crystal structure of 2-(hydroxymethyl)-2-(4H-1,2,4-triazol-4-yl)propane-1,3-diol, C6H11N3O3
- The crystal structure of 1,2-bis(2,4-dinitrophenyl) hydrazine, C12H8N6O8
- Crystal structure of 1-(2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)-1,2-dihydro-4H-benzo[d][1,3]oxazin-4-one, C19H14Cl2N2O3
- The crystal structure of 5-amino-5-oxo-4-(1-oxo-4-(2-oxopyrrolidin-1-yl)isoindolin-2-yl)pentanoic acid, C17H19N3O5
- Crystal structure of N2,N6-bis(2-(((Z)-5-bromo-2-hydroxybenzylidene)amino) phenyl)pyridine-2,6-dicarboxamide, C33H23Br2N5O4
- The crystal structure of (E)-2-methoxy-6-(((5-methyl-1,3,4-thiadiazol-2-yl)imino)methyl)phenol, C11H11N3O2S
- The crystal structure of 3-((tert-butyldiphenylsilyl)methyl)-5,5-diphenyl-6-(p-tolyl) tetrahydro-2H-pyran-2-one, C41H42O2Si
- Crystal structure of 9-fluoro-4-(6-methoxypyridin-3-yl)-5,6-dihydrobenzo[h]quinazolin-2-amine, C18H15FN4O
- The crystal structure of 2-bromo-5-(4-cyanophenoxy)benzyl 1-methyl-1,2,5,6-tetrahydropyridine-3-carboxylate, C21H19BrN2O3
- Crystal structure of 3,3′-(1,4-phenylenebis(methylene))bis(1-isopropyl-1H-imidazol-3-ium) bis(hexafluorophosphate(V)), C10H14F6N2P
- The crystal structure of 2,2-di(thiophen-3-yl)-1-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C24H19BN2O2S3
- Crystal structure of 5-bromo-1-(2-iodobenzoyl)-1H-indole-3-carbaldehyde, C16H9BrINO2
- The crystal structure of monocarbonyl-2-carboxypyridinato-κ2N,O-triphenylphosphine-rhodium(I) acetonitrile solvate, C26H20.50N1.50O3PRh
- Crystal structure of dichlorido-tetrakis(1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ1N)manganese(II), C60H68O4N12Cl10Mn
- Crystal structure of 3-(tert-butyldiphenylsilyl)-1-(2,6-dichlorophenyl)-2,2-diphenylpropan-1-ol, C37H36Cl2OSi
- Crystal structure of langite from Mine du Pradet (France)
- The crystal structure of 5′-(furan-2-yl)-3′-((4-methylphenyl)sulfonamido)-3′,4′,5′,6′-tetrahydro-[1,1′:3′,1″-terphenyl]-4′-carboxylic acid, C30H27NO5S
- Synthesis and crystal structure of bis{2-(((4-acetophenone)imino)methyl)-4-fluorophenolato-κ2N,O}zinc(II), C30H22F2N2O4Zn
- The crystal structure of poly[(tripyridine-κ3N,N′,N″) μ3-(pyridine-3,4-dicarboxylate-κ3N:O:O′) manganese(II)], C22H22N4O8Mn
- The crystal structure of (E)-4-chloro-N′-(1-(4-hydroxyphenyl)propylidene)benzohydrazide, C16H15ClN2O2
- Synthesis and crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino) ethyl)phenyl)imino)methyl)phenolato-κ2N,O}cobalt(II), C40H46CoN4O4
- Crystal structure of tetraaqua-[(1-(carboxymethyl)-1H-pyrazole-3-carboxylato-κ2N,O)cobalt(II)], C6H12CoN2O8
- (6R,7S)-2,3,13-trimethoxy-6,7-dimethyl-5,6,7,8-tetrahydrobenzo[3′,4′]cycloocta [1′,2′:4,5]benzo[1,2-d][1,3]dioxol-1-ol, C22H26O6
- Crystal structure of 2-((2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)amino)benzoic acid, C18H14Cl2N2O3
- Crystal structure of (5aS,6aS,8aR,9R,11aS, 11bS,13R,13aS)-1,1,8a,11a-tetramethyl-9-((S)-1-((S)-5-methyl-6-oxo-3,6-dihydro-2H-pyran-2-yl)ethyl)-3-oxo-1,7,8,8a,9,10,11,11a,11b,12,13,13a-dodecahydro-3H,6H-cyclopenta[5,6]cyclopropa[1,8a]naphtho[2,1-c]oxepin-13-yl acetate, C32H44O6
- Crystal structure of catena-poly[triaqua-(μ2-1-(4-carboxylatophenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylato-O,O′:O″)cobalt(II)], C12H12N2O8Co
- Crystal structure of 3-[(furan-2-ylmethyl)-amino]-2-(2,3,4,5-tetrafluoro-benzoyl)-acrylic acid ethyl ester, C17H13F4NO4
- Crystal structure of methyl 4-(2-ethoxy-2-oxoethoxy)-3-methoxybenzoate, C13H16O6
- Crystal structure of 4-bromo-2-(4-chlorophenyl)-1-methyl-5-(trifluoromethyl)-1H-pyrrole-3-carbonitrile, C13H7BrClF3N2
- The crystal structure of triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ3N,O,O)nickel(II) monohydrate, C12H15NO9Ni
- Crystal structure of dihydroxy(2,4,6-triisopro-pylphenyl)telluronium trifluoromethanesulfonate, C16H25F3O5STe
- The crystal structure of 1-(carboxymethyl)-1H-imidazole 3-oxide
- The crystal structure of 1,3,5-tris(dibromomethyl)benzene, C9H6Br6
- Crystal structure of (Z)-3-(4-methoxyphenyl)-4-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-N-phenylthiazol-2(3H)-imine, C25H21N5OS
- Crystal structure of (Z)-3-(3-(4-hydroxyphenyl)-2-(phenylimino)-2,3-dihydrothiazol-4-yl)-2H-chromen-2-one, C24H16N2O3S
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of poly[diaqua-(μ4-3,3′-di(1H-1,2,4-triazol-1-yl)-[1,1′-biphenyl]-4,4′-dicarboxylate-N:N′:O:O′)cadmium(II)], C18H14N6O6Cd
- Crystal structure of (8R,8′S,13S,13′R)-8,8′-bis(hydroxymethyl)-9,9′,10,10′-tetramethoxy-5,5′,6,6′,8,8′,13,13′-octahydro-[13,13′-bi[1,3]dioxolo[4,5-g]isoquinolino[3,2-a]isoquinoline]-7,7′-diium chloride-methanol (1/2), C46H58N2O14Cl2
- The crystal structure of 8-methoxy-2,2-diphenyl-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C29H25BN2O3S
- Crystal structure of aqua-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N″,N‴)copper(II) 5-carboxyisophthalate tetrahydrate, C25H50N4CuO11
- The crystal structure of 1-(naphthalen-2-ylsulfonyl)-2,2-diphenyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ij]quinoline, C31H23BN2O2S
- Crystal structure of iodido-(η6-benzene) (1-(pyridin-2-yl)-N-(p-fluoro-methanamine)-κ2N,Nʹ)ruthenium(II) hexaflourophosphate, (C18H15F7IN2RuP)
- The crystal structure of 1-(3-oxo-1-phenyl-3-(p-tolyl) propylidene)-1,3-dihydro-2H-inden-2-one, C25H20O2
- Crystal structure of tricyclohexyl[4-(4H-1,2,4-triazol-4-yl)-benzoato-κO]tin(IV), C27H39N3O2Sn
- Crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ4N,O,O,O)cadmium(II)]monohydrate, C12H15NO9Cd
- Crystal structure of ethyl 2-((4-(3,5-dimethylisoxazol-4-yl)-2,6-difluorophenyl)amino)benzoate, C20H18F2N2O3
- The crystal structure of 2-(hydroxymethyl)-2-(4H-1,2,4-triazol-4-yl)propane-1,3-diol, C6H11N3O3
- The crystal structure of 1,2-bis(2,4-dinitrophenyl) hydrazine, C12H8N6O8
- Crystal structure of 1-(2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)-1,2-dihydro-4H-benzo[d][1,3]oxazin-4-one, C19H14Cl2N2O3
- The crystal structure of 5-amino-5-oxo-4-(1-oxo-4-(2-oxopyrrolidin-1-yl)isoindolin-2-yl)pentanoic acid, C17H19N3O5
- Crystal structure of N2,N6-bis(2-(((Z)-5-bromo-2-hydroxybenzylidene)amino) phenyl)pyridine-2,6-dicarboxamide, C33H23Br2N5O4
- The crystal structure of (E)-2-methoxy-6-(((5-methyl-1,3,4-thiadiazol-2-yl)imino)methyl)phenol, C11H11N3O2S
- The crystal structure of 3-((tert-butyldiphenylsilyl)methyl)-5,5-diphenyl-6-(p-tolyl) tetrahydro-2H-pyran-2-one, C41H42O2Si
- Crystal structure of 9-fluoro-4-(6-methoxypyridin-3-yl)-5,6-dihydrobenzo[h]quinazolin-2-amine, C18H15FN4O
- The crystal structure of 2-bromo-5-(4-cyanophenoxy)benzyl 1-methyl-1,2,5,6-tetrahydropyridine-3-carboxylate, C21H19BrN2O3
- Crystal structure of 3,3′-(1,4-phenylenebis(methylene))bis(1-isopropyl-1H-imidazol-3-ium) bis(hexafluorophosphate(V)), C10H14F6N2P
- The crystal structure of 2,2-di(thiophen-3-yl)-1-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C24H19BN2O2S3
- Crystal structure of 5-bromo-1-(2-iodobenzoyl)-1H-indole-3-carbaldehyde, C16H9BrINO2
- The crystal structure of monocarbonyl-2-carboxypyridinato-κ2N,O-triphenylphosphine-rhodium(I) acetonitrile solvate, C26H20.50N1.50O3PRh
- Crystal structure of dichlorido-tetrakis(1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ1N)manganese(II), C60H68O4N12Cl10Mn
- Crystal structure of 3-(tert-butyldiphenylsilyl)-1-(2,6-dichlorophenyl)-2,2-diphenylpropan-1-ol, C37H36Cl2OSi
- Crystal structure of langite from Mine du Pradet (France)
- The crystal structure of 5′-(furan-2-yl)-3′-((4-methylphenyl)sulfonamido)-3′,4′,5′,6′-tetrahydro-[1,1′:3′,1″-terphenyl]-4′-carboxylic acid, C30H27NO5S
- Synthesis and crystal structure of bis{2-(((4-acetophenone)imino)methyl)-4-fluorophenolato-κ2N,O}zinc(II), C30H22F2N2O4Zn
- The crystal structure of poly[(tripyridine-κ3N,N′,N″) μ3-(pyridine-3,4-dicarboxylate-κ3N:O:O′) manganese(II)], C22H22N4O8Mn
- The crystal structure of (E)-4-chloro-N′-(1-(4-hydroxyphenyl)propylidene)benzohydrazide, C16H15ClN2O2
- Synthesis and crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino) ethyl)phenyl)imino)methyl)phenolato-κ2N,O}cobalt(II), C40H46CoN4O4
- Crystal structure of tetraaqua-[(1-(carboxymethyl)-1H-pyrazole-3-carboxylato-κ2N,O)cobalt(II)], C6H12CoN2O8
- (6R,7S)-2,3,13-trimethoxy-6,7-dimethyl-5,6,7,8-tetrahydrobenzo[3′,4′]cycloocta [1′,2′:4,5]benzo[1,2-d][1,3]dioxol-1-ol, C22H26O6
- Crystal structure of 2-((2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)amino)benzoic acid, C18H14Cl2N2O3
- Crystal structure of (5aS,6aS,8aR,9R,11aS, 11bS,13R,13aS)-1,1,8a,11a-tetramethyl-9-((S)-1-((S)-5-methyl-6-oxo-3,6-dihydro-2H-pyran-2-yl)ethyl)-3-oxo-1,7,8,8a,9,10,11,11a,11b,12,13,13a-dodecahydro-3H,6H-cyclopenta[5,6]cyclopropa[1,8a]naphtho[2,1-c]oxepin-13-yl acetate, C32H44O6
- Crystal structure of catena-poly[triaqua-(μ2-1-(4-carboxylatophenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylato-O,O′:O″)cobalt(II)], C12H12N2O8Co
- Crystal structure of 3-[(furan-2-ylmethyl)-amino]-2-(2,3,4,5-tetrafluoro-benzoyl)-acrylic acid ethyl ester, C17H13F4NO4
- Crystal structure of methyl 4-(2-ethoxy-2-oxoethoxy)-3-methoxybenzoate, C13H16O6
- Crystal structure of 4-bromo-2-(4-chlorophenyl)-1-methyl-5-(trifluoromethyl)-1H-pyrrole-3-carbonitrile, C13H7BrClF3N2
- The crystal structure of triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ3N,O,O)nickel(II) monohydrate, C12H15NO9Ni
- Crystal structure of dihydroxy(2,4,6-triisopro-pylphenyl)telluronium trifluoromethanesulfonate, C16H25F3O5STe
- The crystal structure of 1-(carboxymethyl)-1H-imidazole 3-oxide
- The crystal structure of 1,3,5-tris(dibromomethyl)benzene, C9H6Br6
- Crystal structure of (Z)-3-(4-methoxyphenyl)-4-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-N-phenylthiazol-2(3H)-imine, C25H21N5OS
- Crystal structure of (Z)-3-(3-(4-hydroxyphenyl)-2-(phenylimino)-2,3-dihydrothiazol-4-yl)-2H-chromen-2-one, C24H16N2O3S

