Abstract
C15H20O2S, monoclinic, P21/c (No. 14), a = 12.8520(8) Å, b = 10.5864(8) Å, c = 11.2202(7) Å, β = 104.375(7)°, V = 1478.78(18) Å3, Z = 4, R gt (F) = 0.0655, wR ref (F 2) = 0.1781, T = 293 K.
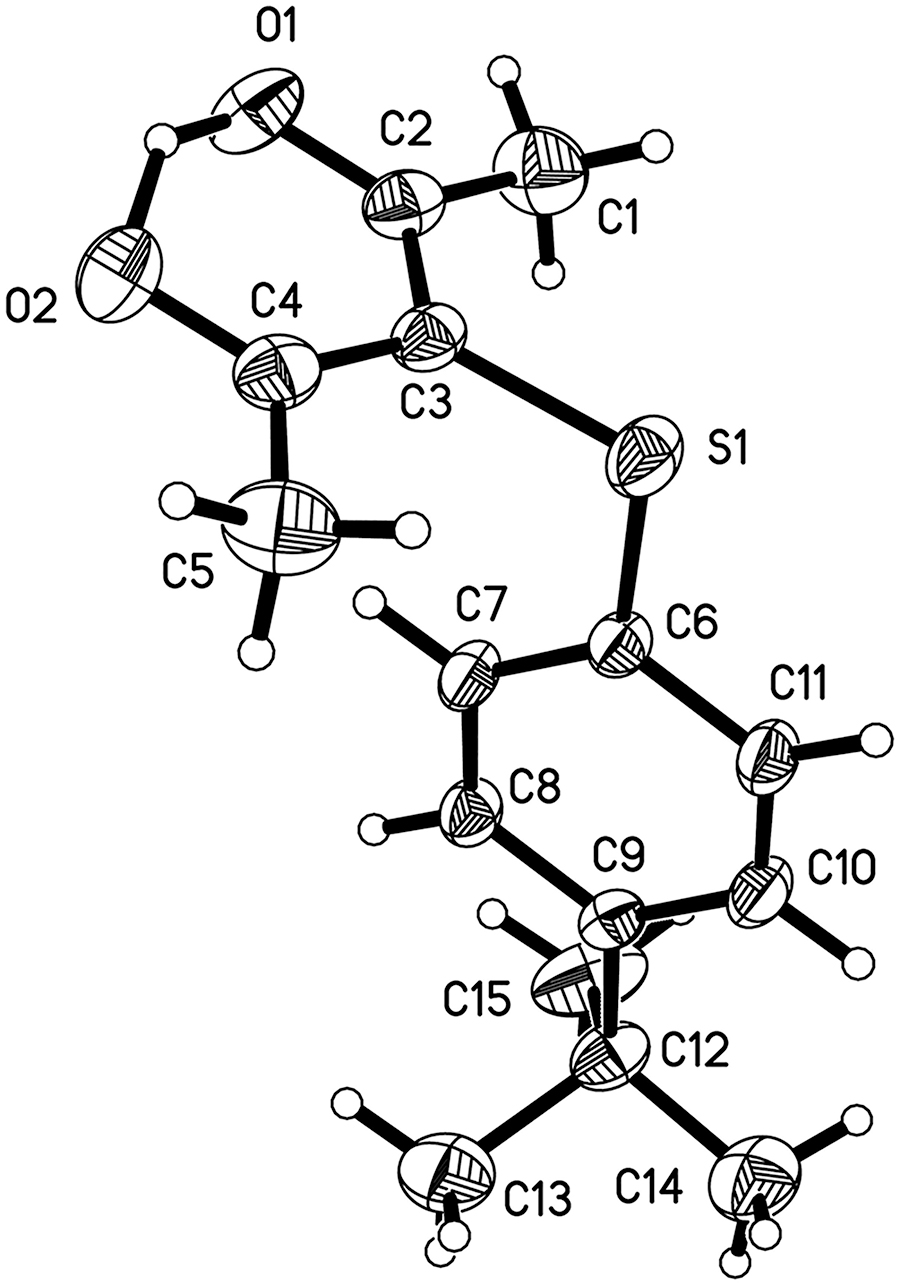
The crystal structure is shown in the figure (for the disordered part only one component list shown). Tables 1 and 2 contain details on crystal structure and measurement conditions and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Block, colourless |
| Size: | 0.27 × 0.25 × 0.24 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.21 mm−1 |
| Diffractometer, scan mode: | SuperNova, ω-scans |
| θ max, completeness: | 28.4°, >99% |
| N(hkl)measured, N(hkl)unique, R int: | 11,385, 3265, 0.033 |
| Criterion for I obs, N(hkl)gt: | I obs > 2 σ(I obs), 2549 |
| N(param)refined: | 181 |
| Programs: | CrysAlisPRO [1], OLEX2 [2], SHELX [3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| S1 | 0.63034 (5) | 0.80321 (7) | 0.49264 (6) | 0.0609 (3) |
| O1 | 0.38156 (15) | 0.8078 (2) | 0.6385 (2) | 0.0758 (6) |
| H1 | 0.4125 | 0.9026 | 0.6875 | 0.114* |
| O2 | 0.50260 (16) | 0.97776 (19) | 0.73228 (18) | 0.0731 (6) |
| C1 | 0.4173 (3) | 0.6610 (3) | 0.4957 (3) | 0.0778 (8) |
| H1A | 0.4651 | 0.5925 | 0.5277 | 0.117* |
| H1B | 0.4224 | 0.6808 | 0.4138 | 0.117* |
| H1C | 0.3449 | 0.6369 | 0.4939 | 0.117* |
| C2 | 0.44780 (18) | 0.7745 (2) | 0.5763 (2) | 0.0541 (6) |
| C3 | 0.54415 (17) | 0.8414 (2) | 0.5865 (2) | 0.0462 (5) |
| C4 | 0.56855 (19) | 0.9438 (2) | 0.6692 (2) | 0.0536 (6) |
| C5 | 0.6696 (2) | 1.0182 (3) | 0.6908 (3) | 0.0818 (9) |
| H5A | 0.6597 | 1.0980 | 0.7272 | 0.123* |
| H5B | 0.6878 | 1.0322 | 0.6139 | 0.123* |
| H5C | 0.7265 | 0.9724 | 0.7453 | 0.123* |
| C6 | 0.71536 (16) | 0.6827 (2) | 0.57366 (19) | 0.0416 (5) |
| C7 | 0.70896 (17) | 0.6326 (2) | 0.68557 (19) | 0.0457 (5) |
| H7 | 0.6580 | 0.6631 | 0.7245 | 0.055* |
| C8 | 0.77863 (17) | 0.5368 (2) | 0.73980 (19) | 0.0452 (5) |
| H8 | 0.7739 | 0.5053 | 0.8156 | 0.054* |
| C9 | 0.85475 (15) | 0.4867 (2) | 0.68514 (19) | 0.0415 (5) |
| C10 | 0.85970 (18) | 0.5392 (2) | 0.5729 (2) | 0.0519 (6) |
| H10 | 0.9104 | 0.5087 | 0.5337 | 0.062* |
| C11 | 0.79198 (18) | 0.6349 (2) | 0.5182 (2) | 0.0508 (6) |
| H11 | 0.7977 | 0.6677 | 0.4432 | 0.061* |
| C12 | 0.92933 (19) | 0.3783 (2) | 0.7442 (2) | 0.0554 (6) |
| C13a | 0.9882 (5) | 0.4224 (6) | 0.8811 (5) | 0.0864 (13) |
| H13Aa | 0.9353 | 0.4431 | 0.9251 | 0.130* |
| H13Ba | 1.0317 | 0.4953 | 0.8773 | 0.130* |
| H13Ca | 1.0329 | 0.3551 | 0.9227 | 0.130* |
| C14a | 1.0205 (5) | 0.3530 (6) | 0.6856 (6) | 0.0844 (13) |
| H14Aa | 1.0590 | 0.4300 | 0.6820 | 0.127* |
| H14Ba | 0.9925 | 0.3211 | 0.6038 | 0.127* |
| H14Ca | 1.0681 | 0.2917 | 0.7335 | 0.127* |
| C15a | 0.8667 (4) | 0.2628 (5) | 0.7556 (7) | 0.0921 (19) |
| H15Aa | 0.9145 | 0.1981 | 0.7971 | 0.138* |
| H15Ba | 0.8299 | 0.2336 | 0.6751 | 0.138* |
| H15Ca | 0.8151 | 0.2820 | 0.8019 | 0.138* |
| C15Ab | 0.8965 (6) | 0.3143 (7) | 0.8474 (9) | 0.0921 (19) |
| H15Db | 0.8241 | 0.2842 | 0.8192 | 0.138* |
| H15Eb | 0.9008 | 0.3731 | 0.9136 | 0.138* |
| H15Fb | 0.9436 | 0.2443 | 0.8758 | 0.138* |
| C13Ab | 1.0427 (5) | 0.4211 (8) | 0.7740 (8) | 0.0864 (13) |
| H13Db | 1.0543 | 0.4817 | 0.8395 | 0.130* |
| H13Eb | 1.0579 | 0.4592 | 0.7026 | 0.130* |
| H13Fb | 1.0893 | 0.3499 | 0.7991 | 0.130* |
| C14Ab | 0.9188 (6) | 0.2733 (7) | 0.6380 (7) | 0.0844 (13) |
| H14Db | 0.9707 | 0.2077 | 0.6660 | 0.127* |
| H14Eb | 0.9316 | 0.3118 | 0.5655 | 0.127* |
| H14Fb | 0.8479 | 0.2378 | 0.6192 | 0.127* |
-
aOccupancy: 0.567(3), bOccupancy: 0.433(3).
Source of material
To a solution of acetylacetone (0.400 g, 4 mmol) and 4-tert-butylbenzenethiol (0.166 g, 1 mmol) in dimethyl sulfoxide (DMSO) (1 mL) was added Na2CO3 (130 mg, 1 mmol). The mixture was stirred at 40 °C under oxygen atmosphere for 17 h. The mixture was then added to water (5 mL). The resulting mixture was extracted with ethylether (10 mL) for three times. The combined organic layers were washed with water and brine, dried over anhydrous Na2SO4 and concentrated under reduced pressure. After removal of the solvent, the residue was then purified by flash column chromatography on silica gel with petroleum ether/ethyl acetate (45:1) to give the desired (238 mg, 90%) as a white solid. Crystals were obtained by crystallization of the title compound from ethyl acetate. Melting point: 104–106 °C. 1H NMR (400 MHz, CDCl3, 298 K) δ 17.26 (s, 1H), 7.31 (d, J = 8.4 Hz, 2H), 7.03 (d, J = 8.4 Hz, 2H), 2.35 (s, 6H), 1.30 (s, 9H). 13C{1H} NMR (101 MHz, CDCl3) δ 198.3, 148.4, 134.2, 126,2 124.6, 102.0, 34.4, 31.4, 24.5.
Experimental details
Hydrogen atoms were placed in their geometrically idealized positions and constrained to ride on their parent atoms.
Discussion
Acetylacetone derivatives are extensively employed in organic synthetic intermediates, analytical reagents, and other essential raw ingredients [4], [5], [6], which have been demonstrated to possess a wide range of biological and pharmaceutical activities such as sulfa-drugs [7], antipyretics [8], diabetes drugs [9], antiviral [10], pesticides [11], spectrofluorimetric method [12]. Besides, acetylacetone and its derivatives were frequently used as chelating ligands [13]. Examples for their coordination behavior were found for main group elements such as iron [14], aluminum [15], and palladium [16]. Up to date, a great number of acetylacetone derivatives have been reported. However, there are relatively few reports about a convenient and efficient protocol for the synthesis of α-sulfenylated carbonyl compounds based acetylacetone derivatives.
In this paper we report the synthesis and crystal structure of a novel sulfenylated carbonyl compound. The asymmetric unit contains one molecule of the title compound, which is constructed by the acetylacetone and the 4-tert-butylbenzenethiol moiety (see the Figure). The acetylacetone together with sulfur atom is almost in a strict plane, whereby the largest deviation for the S1 atom from the acetylacetone plane is 0.078 Å. The dihedral angle between the acetylacetone group and 4-tert-butylbenzenethiol were found to be 85°. The C(6)–S(1)–C(3)–C(2) and C(6)–S(1)–C(3)–C(4) torsion angles are −87.6(2)° and 95.2(2)°. The C(3)–S(1)–C(6) bond angle is 104.97(10)°. The thioether bond distances are 1.755(2) Å for C(3)–S(1) and 1.766(2) Å for C(6)–S(1), respectively, which are typical C–S bond distances. Within the acetylacetone unit, the dimensions and planarity are consistent with their adoption of a localized enol form. The bond lengths of C(3)–C(4), C(3)–C(2) are 1.410(3) and 1.407(3) Å respectively. The only intramolecular O(1)–H(1)⋯O(2) hydrogen bond is observed. The O(1)–H(1) and O(2)–H(1) bond lengths are 1.392 and 1.167 Å. The O(1)⋯O(2) separation is 2.440(3) Å. The structure of the molecule is similar to the stereo-configuration of the compound reported in the references. The bond lengths and angles are all in the expected ranges [17], [18], [19]. And no unusual intermolecular contacts, were observed in this crystal.
Funding source: Programs for Science and Technology Development of Henan Province
Award Identifier / Grant number: 212102210650
Funding source: Key Research Project for Colleges and Universities of Henan Province
Award Identifier / Grant number: 21B530002
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work was supported by the Programs for Science and Technology Development of Henan Province, China (No. 212102210650) and the Key Research Project for Colleges and Universities of Henan Province, China (No. 21B530002).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Oxford Diffraction, CrysAlisPRO (version 1.171.33.42); Oxford Diffraction Ltd.: Oxford, UK, 2009.Suche in Google Scholar
2. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Suche in Google Scholar
3. Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Suche in Google Scholar
4. Al-Adiwish, W. M., Tahir, M. I., Siti-Noor-Adnalizawati, A., Hashi, S. F., Ibrahim, N., Yaacob, W. A. Synthesis, antibacterial activity and cytotoxicity of new fused pyrazolo[1,5-a]pyrimidine and pyrazolo[5,1-c][1,2,4]triazine derivatives from new 5-aminopyrazoles. Eur. J. Med. Chem. 2013, 64, 464–476; https://doi.org/10.1016/j.ejmech.2013.04.029.Suche in Google Scholar
5. Svistunova, I. V., Sharutin, V. V., Tretyakova, G. O., Puzyrkov, Z. N. Synthesis and structure of boron difluoride binuclear β-diketonates. Inorg. Chim. Acta. 2020, 501, 119230–119236; https://doi.org/10.1016/j.ica.2019.119230.Suche in Google Scholar
6. Gupta, V. K., Goyal, R. N., Bachheti, N., Singh, L. P., Agarwal, S. A copper-selective electrode based on bis(acetylacetone)propylenediimine. Talanta 2005, 68, 193–197; https://doi.org/10.1016/j.talanta.2005.06.050.Suche in Google Scholar
7. Darwish, E., Fattah, A., Attaby, F., Al-Shayea, O. Synthesis and antimicrobial evaluation of some novel thiazole, pyridone, pyrazole, chromene, hydrazone derivatives bearing a biologically active sulfonamide moiety. Int. J. Mol. Sci. 2014, 15, 1237–1254; https://doi.org/10.3390/ijms15011237.Suche in Google Scholar
8. Glukhachev, V. S., Il’yasov, S. G., Kazantsev, I. V., Shestakova, E. O., Il’yasov, D. S., Eltsov, I. V., Nefedov, A. A., Gatilov, Y. V. New reaction products of acetylacetone with semicarbazide derivatives. ACS Omega 2021, 6, 8637–8645; https://doi.org/10.1021/acsomega.1c00518.Suche in Google Scholar
9. Sanna, D., Ugone, V., Serra, M., Garribba, E. Speciation of potential anti-diabetic vanadium complexes in real serum samples. J. Inorg. Biochem. 2017, 173, 52–65; https://doi.org/10.1016/j.jinorgbio.2017.04.023.Suche in Google Scholar
10. Krohn, K., Vukics, K. First chemical synthesis of the antiviral agents S2502 and S2507. Synthesis 2007, 2007, 2894–2900; https://doi.org/10.1055/s-2007-983873.Suche in Google Scholar
11. Abunada, N. M., Hassaneen, H. M., Kandile, N. G., Miqdad, O. A. Synthesis and antimicrobial activity of some new pyrazole, fused pyrazolo[3,4-d]-pyrimidine and pyrazolo[4,3-e][1,2,4]-triazolo[1,5-c]pyrimidine derivatives. Med. Sci. 2008, 13, 1501–1517; https://doi.org/10.3390/molecules13071501.Suche in Google Scholar
12. Omar, M. A., Derayea, S. M., Mostafa, I. M. Development and validation of a stability-indicating spectrofluorimetric method for the determination of H1N1 antiviral drug (oseltamivir phosphate) in human plasma through the Hantzsch reaction. RSC Adv. 2015, 5, 27735–27742; https://doi.org/10.1039/c4ra16650g.Suche in Google Scholar
13. Kremer, M., Englert, U. N Donor substituted acetylacetones – versatile ditopic ligands. Z. Kristallogr. - Cryst. Mater. 2018, 233, 437–452; https://doi.org/10.1515/zkri-2017-2131.Suche in Google Scholar
14. Hu, X., Mao, J., Sun, Y., Chen, H., Li, H. Acetylacetone-Fe catalyst modified by imidazol ionic compound and its application in aerobic oxidation of β-isophorone. Catal. Commun. 2009, 10, 1908–1912; https://doi.org/10.1016/j.catcom.2009.06.024.Suche in Google Scholar
15. Pang, X., Chen, X., Du, H., Wang, X., Jing, X. Enolic Schiff-base aluminum complexes and their application in lactide polymerization. J. Organomet. Chem. 2007, 692, 5605–5613; https://doi.org/10.1016/j.jorganchem.2007.09.014.Suche in Google Scholar
16. Khranenko, S. P., Bykova, E. A., Gromilov, S. A., Gallyamov, M. R., Kozlova, S. G., Moroz, N. K., Korenev, S. V. Novel mixed-ligand palladium complexes [Pd2(acac)3NO3] and [Pd(acac)NO3]n involving O,O- and γ-C-bonded acetylacetonate linkers. Polyhedron 2012, 31, 272–277; https://doi.org/10.1016/j.poly.2011.09.026.Suche in Google Scholar
17. Olivier, J. H., Haefele, A., Retailleau, P., Ziessel, R. Borondipyrromethene dyes with pentane-2,4-dione anchors. Org. Lett. 2010, 12, 408–411; https://doi.org/10.1021/ol902386u.Suche in Google Scholar
18. Jing, N., Jian-Hua, W., Zhong-Hai, N. Synthesis, crystal structure and optical property of 1,6-bis(p-tolylthio)pyrene, C30H22S2. Z. Kristallogr. N. Cryst. Struct. 2021, 236, 21–23; https://doi.org/10.1515/ncrs-2020-0466.Suche in Google Scholar
19. Rashid, M. A., Rasool, N., Adeel, M., Reinke, H., Fischer, C., Langer, P. Synthesis of functionalized diaryl sulfides based on regioselective one-pot cyclizations of 1,3-bis(trimethylsilyloxy)-1,3-butadienes. Tetrahedron 2008, 64, 3782–3793; https://doi.org/10.1016/j.tet.2008.02.010.Suche in Google Scholar
© 2021 Le Dong et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of (E)-7-hydroxy-2-((6-methoxypyridin-3-yl)methylene)-3, 4-dihydronaphthalen-1(2H)-one, C17H15NO3
- Crystal structure of (E)-7-methoxy-2-((2-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1 (2H)-one, C18H17NO3
- The crystal structure of N 6,N 6′-di(pyridin-2-yl)-[2,2′-bipyridine]-6,6′-diamine, C20H16N6
- The crystal structure of {N 1,N 2-bis[2,4-dimethyl-6-(4-(tert-butyl)phenyl)(phenyl)methyl]acenaphthylene-1,2-diimino-κ2 N, N′}-dibromido-nickel(II) – dichloromethane(1/2), C64H64Br2Cl4N2Ni
- Synthesis and crystal structure of nonacarbonyltris[(2-thia-1,3,5-triaza-7-phosphatricylco[3.3.1.1]decane-κ1 P)-2,2-dioxide]triruthenium(0) – acetonitrile (7/6), C25.71H32.57N9.86O15P3S3Ru3
- A new polymorph of 1-(4-nitrophenyl)-1H-benzimidazole (C13H9N3O2)
- The crystal structure of 2,2′-((1E,1′E)-(naphthalene-2,3 diylbis(azanylylidene)) bis(methanylylidene))bis(4-methylphenol), C26H22N2O2
- The crystal structure of bis(μ2-iodido)-bis(η6-benzene)-bis(iodido)-diosmium(II), C12H12I4Os2
- Redetermination of the crystal structure of bis{hydridotris(3,5-dimethylpyrazol-1-yl-κN 3)borato}copper(II), C30H44B2CuN12
- Crystal structure of (E)-3-((4-(tert-butyl)phenyl)thio)-4-hydroxypent-3-en-2-one, C15H20O2S
- Crystal structure of 2,2′-(p-tolylazanediyl)bis(1-phenylethan-1-one), C23H21NO2
- Redetermination of the crystal structure of the crystal sponge the poly[tetrakis(μ3-2,4,6-tris(pyridin-4-yl)-1,3,5-triazine)-dodecaiodidohexazinc(II) nitrobenzene solvate], C72H48I12N24Zn6⋅10(C6H5NO2)
- Crystal structure of (4′E)-6′-(diethylamino)-2-[(E)-[(6-methylpyridin-2-yl)methylidene]amino]-4′-{2-[(2E)-1,3,3-trimethyl-2,3-dihydro-1H-indol-2-ylidene]ethylidene}-1′,2,2′,3,3′,4′-hexahydrospiro[isoindole-1,9′-xanthene]-3-one, C44H45N5O2
- Crystal structure of (E)-7-fluoro-2-(3-fluorobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C17H12F2O1
- Crystal structure of tetrabutylammonium sulfanilate – 1-(diaminomethylene)thiourea (1/2)
- Crystal structure of [2,2′-{azanediyl)bis[(propane-3,1-diyl)(azanylylidene)methylylidene]} bis(3,5-dichlorophenolato)-κ2O,O′]-isothiocyanato-κN-iron(III), C21H19Cl4FeN4O2S
- Crystal structure of (4-chlorophenyl)(4-hydroxyphenyl)methanone, C13H9ClO2
- Crystal structure of 6,6′-((pentane-1,3-diylbis(azaneylylidene))bis(methaneylylidene))bis(2,4-dibromolphenolato-κ4 N,N′,O,O′)copper(II),) C19H16Br4CuN2O2
- Chlorido-(2,2′-(ethane-bis(5-methoxyphenolato))-κ4 N,N′,O,O′)manganese(III) monohydrate, C19H18Cl2CuN2O2
- Crystal structure of 2,6-di-tert-butyl-4-(4-methoxybenzylidene)cyclohexa-2,5-dien-1-one, C22H28O2
- Crystal structure of [6,6′-(((2,2-dimethylpropane-1,3-diyl)bis(azanylylidene))bis(methanylylidene))bis(2-chlorophenolato)-κ4N,N′,O,O′]copper(II)
- Crystal structure of 2-chloro-3-((thiophen-2-ylmethyl)amino)naphthalene-1,4-dione, C30H20O4N2Cl2S2
- Crystal structure of bis{hydridotris(3-trifluoromethyl-5-methylpyrazolyl-1-yl)borato-κN 3}manganese(II), C30H26B2F18MnN12
- Crystal structure of 1-(2-methylphenyl)-2-(2-methylbenzo[b]thienyl)-3,3,4,4,5,5-hexafluorocyclopent-ene, C21H14F6S
- Crystal structure of 2-(3-((carbamimidoylthio)methyl)benzyl)isothiouronium hexafluorophosphate monohydrate, C10H17F6N4OPS2
- Crystal structure of 4,5-diiodo-1,3-dimesityl-1H-1,2,3-triazol-3-ium chloride – chloroform (1/1), C21H23Cl4I2N3
- Crystal structure of azido-k1 N-{6,6′-((((methylazanediyl)bis(propane-3,1-diyl))bis(azanylylidene))bis(methanylylidene))bis(2,4-dibromophenolato)k5 N,N′,N″,O,O′}cobalt(III)-methanol (1/1)), C21H23Br4CoN6O3
- The crystal structure of 2-(4-((carbamimidoylthio)methyl)benzyl)isothiouronium hexafluorophosphate monohydrate, C10H17F6N4OPS2
- Crystal structure of 1,1′-(methane-1,1-diyl)bis(3-methyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C9H14F12N4P2
- Crystal structure of (4′E)-6′-(diethylamino)-2-[(E)-[(pyren-1-yl)methylidene]amino]-4′-{2-[(2E)-1,3,3-trimethyl-2,3-dihydro-1H-indol-2-ylidene]ethylidene}-1′,2,2′,3,3′,4′-hexahydrospiro[isoindole-1,9′-xanthene]-3-one, C54H48N4O2
- Crystal structure of poly[bis(μ2-2,6-bis(1-imidazoly)pyridine-κ2 N,N′)-bis(thiocyanato-κ1 N)copper(II)] dithiocyanate, C24H18CuN12S2
- Cones with a three-fold symmetry constructed from three hydrogen bonded theophyllinium cations that coat [FeCl4]− anions in the crystal structure of tris(theophyllinium) bis(tetrachloridoferrate(III)) chloride trihydrate, C21H33Cl9Fe2N12O9
- Crystal structure of 14-O-[(4-(4-hydroxypiperidine-1-yl)-6-methylpyrimidine-2-yl)thioacetyl]-mutilin monohydrate, C32H49N3O6S
- The crystal structure of (E)-3-chloro-2-(2-(4-methylbenzylidene)hydrazinyl)pyridine, C13H12ClN3
- The crystal structure of 4-phenyl-4-[2-(pyridine-4-carbonyl)hydrazinylidene]butanoic acid, C16H15N3O3
- The crystal structure of 6-amino-5-carboxypyridin-1-ium pentaiodide monohydrate C6H9I5N2O3
- Crystal structure of bis(μ3-oxido)-bis(μ2-2-formylbenzoato-k2O:O′)-bis(2-(dimethoxymethyl)-benzoato-κO)-oktakismethyl-tetratin(IV)
- Crystal structure of 2-((E)-(((E)-2-hydroxy-4-methylbenzylidene) hydrazineylidene)methyl)-4-methylphenol, C16H16N2O2
- Crystal structure of (E)-amino(2-((5-methylfuran-2-yl)methylene)hydrazinyl) methaniminium nitrate monohydrate, C14H26N10O10
- The crystal structure of N′-(2-chloro-6-hydroxybenzylidene)thiophene-2-carbohydrazide monohydrate, C12H11ClN2O3S
- Crystal structure of catena-poly[(μ2-1,1′-(biphenyl-4,4′-diyl)bis(1H-imidazol)-κ2N:N′)-bis(4-bromobenzoate-κ1O)zinc(II)], C64H44Br4N8O8Zn2
- The crystal structure of catena-poly[(1-(4-carboxybenzyl)pyridin-1-ium-4-carboxylato-κ1O)-(μ2-oxalato-κ4 O:O′:O″:O‴)dioxidouranium(VI)], C16H11NO10U
- Crystal structure of 3-allyl-4-(2-bromoethyl)-5-(4-methoxyphenyl)-2-phenylfuran, C22H21BrO2
- Halogen bonds in the crystal structure of 4,3′:5′,4″-terpyridine — 1,3-diiodotetrafluorobenzene (1/1), C21H11F4I2N3
- Crystal structure of 2-(1H-indol-3-yl)ethan-1-aminium 2-(4-acetylphenoxy)acetate, C20H22N2O4
- Chalcogen bonds in the crystal structure of 4,7-dibromo-2,1,3-benzoselenadiazole, C6H2Br2N2Se
- The crystal structure of 1,4-bis((1H-benzimidazol-2-yl)methyl)-piperazine-2,5-dione dihydrate, C20H22N6O4
- The crystal structure of C19H20O8
- The crystal structure of KNa3Te8O18·5H2O exhibiting a ∞2[Te4O9]2− layer
- Erratum
- Erratum to: Crystal structure of (Z)-3-(6-bromo-1H-indol-3-yl)-1,3-diphenylprop-2-en-1-one, C23H16BrNO
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of (E)-7-hydroxy-2-((6-methoxypyridin-3-yl)methylene)-3, 4-dihydronaphthalen-1(2H)-one, C17H15NO3
- Crystal structure of (E)-7-methoxy-2-((2-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1 (2H)-one, C18H17NO3
- The crystal structure of N 6,N 6′-di(pyridin-2-yl)-[2,2′-bipyridine]-6,6′-diamine, C20H16N6
- The crystal structure of {N 1,N 2-bis[2,4-dimethyl-6-(4-(tert-butyl)phenyl)(phenyl)methyl]acenaphthylene-1,2-diimino-κ2 N, N′}-dibromido-nickel(II) – dichloromethane(1/2), C64H64Br2Cl4N2Ni
- Synthesis and crystal structure of nonacarbonyltris[(2-thia-1,3,5-triaza-7-phosphatricylco[3.3.1.1]decane-κ1 P)-2,2-dioxide]triruthenium(0) – acetonitrile (7/6), C25.71H32.57N9.86O15P3S3Ru3
- A new polymorph of 1-(4-nitrophenyl)-1H-benzimidazole (C13H9N3O2)
- The crystal structure of 2,2′-((1E,1′E)-(naphthalene-2,3 diylbis(azanylylidene)) bis(methanylylidene))bis(4-methylphenol), C26H22N2O2
- The crystal structure of bis(μ2-iodido)-bis(η6-benzene)-bis(iodido)-diosmium(II), C12H12I4Os2
- Redetermination of the crystal structure of bis{hydridotris(3,5-dimethylpyrazol-1-yl-κN 3)borato}copper(II), C30H44B2CuN12
- Crystal structure of (E)-3-((4-(tert-butyl)phenyl)thio)-4-hydroxypent-3-en-2-one, C15H20O2S
- Crystal structure of 2,2′-(p-tolylazanediyl)bis(1-phenylethan-1-one), C23H21NO2
- Redetermination of the crystal structure of the crystal sponge the poly[tetrakis(μ3-2,4,6-tris(pyridin-4-yl)-1,3,5-triazine)-dodecaiodidohexazinc(II) nitrobenzene solvate], C72H48I12N24Zn6⋅10(C6H5NO2)
- Crystal structure of (4′E)-6′-(diethylamino)-2-[(E)-[(6-methylpyridin-2-yl)methylidene]amino]-4′-{2-[(2E)-1,3,3-trimethyl-2,3-dihydro-1H-indol-2-ylidene]ethylidene}-1′,2,2′,3,3′,4′-hexahydrospiro[isoindole-1,9′-xanthene]-3-one, C44H45N5O2
- Crystal structure of (E)-7-fluoro-2-(3-fluorobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C17H12F2O1
- Crystal structure of tetrabutylammonium sulfanilate – 1-(diaminomethylene)thiourea (1/2)
- Crystal structure of [2,2′-{azanediyl)bis[(propane-3,1-diyl)(azanylylidene)methylylidene]} bis(3,5-dichlorophenolato)-κ2O,O′]-isothiocyanato-κN-iron(III), C21H19Cl4FeN4O2S
- Crystal structure of (4-chlorophenyl)(4-hydroxyphenyl)methanone, C13H9ClO2
- Crystal structure of 6,6′-((pentane-1,3-diylbis(azaneylylidene))bis(methaneylylidene))bis(2,4-dibromolphenolato-κ4 N,N′,O,O′)copper(II),) C19H16Br4CuN2O2
- Chlorido-(2,2′-(ethane-bis(5-methoxyphenolato))-κ4 N,N′,O,O′)manganese(III) monohydrate, C19H18Cl2CuN2O2
- Crystal structure of 2,6-di-tert-butyl-4-(4-methoxybenzylidene)cyclohexa-2,5-dien-1-one, C22H28O2
- Crystal structure of [6,6′-(((2,2-dimethylpropane-1,3-diyl)bis(azanylylidene))bis(methanylylidene))bis(2-chlorophenolato)-κ4N,N′,O,O′]copper(II)
- Crystal structure of 2-chloro-3-((thiophen-2-ylmethyl)amino)naphthalene-1,4-dione, C30H20O4N2Cl2S2
- Crystal structure of bis{hydridotris(3-trifluoromethyl-5-methylpyrazolyl-1-yl)borato-κN 3}manganese(II), C30H26B2F18MnN12
- Crystal structure of 1-(2-methylphenyl)-2-(2-methylbenzo[b]thienyl)-3,3,4,4,5,5-hexafluorocyclopent-ene, C21H14F6S
- Crystal structure of 2-(3-((carbamimidoylthio)methyl)benzyl)isothiouronium hexafluorophosphate monohydrate, C10H17F6N4OPS2
- Crystal structure of 4,5-diiodo-1,3-dimesityl-1H-1,2,3-triazol-3-ium chloride – chloroform (1/1), C21H23Cl4I2N3
- Crystal structure of azido-k1 N-{6,6′-((((methylazanediyl)bis(propane-3,1-diyl))bis(azanylylidene))bis(methanylylidene))bis(2,4-dibromophenolato)k5 N,N′,N″,O,O′}cobalt(III)-methanol (1/1)), C21H23Br4CoN6O3
- The crystal structure of 2-(4-((carbamimidoylthio)methyl)benzyl)isothiouronium hexafluorophosphate monohydrate, C10H17F6N4OPS2
- Crystal structure of 1,1′-(methane-1,1-diyl)bis(3-methyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C9H14F12N4P2
- Crystal structure of (4′E)-6′-(diethylamino)-2-[(E)-[(pyren-1-yl)methylidene]amino]-4′-{2-[(2E)-1,3,3-trimethyl-2,3-dihydro-1H-indol-2-ylidene]ethylidene}-1′,2,2′,3,3′,4′-hexahydrospiro[isoindole-1,9′-xanthene]-3-one, C54H48N4O2
- Crystal structure of poly[bis(μ2-2,6-bis(1-imidazoly)pyridine-κ2 N,N′)-bis(thiocyanato-κ1 N)copper(II)] dithiocyanate, C24H18CuN12S2
- Cones with a three-fold symmetry constructed from three hydrogen bonded theophyllinium cations that coat [FeCl4]− anions in the crystal structure of tris(theophyllinium) bis(tetrachloridoferrate(III)) chloride trihydrate, C21H33Cl9Fe2N12O9
- Crystal structure of 14-O-[(4-(4-hydroxypiperidine-1-yl)-6-methylpyrimidine-2-yl)thioacetyl]-mutilin monohydrate, C32H49N3O6S
- The crystal structure of (E)-3-chloro-2-(2-(4-methylbenzylidene)hydrazinyl)pyridine, C13H12ClN3
- The crystal structure of 4-phenyl-4-[2-(pyridine-4-carbonyl)hydrazinylidene]butanoic acid, C16H15N3O3
- The crystal structure of 6-amino-5-carboxypyridin-1-ium pentaiodide monohydrate C6H9I5N2O3
- Crystal structure of bis(μ3-oxido)-bis(μ2-2-formylbenzoato-k2O:O′)-bis(2-(dimethoxymethyl)-benzoato-κO)-oktakismethyl-tetratin(IV)
- Crystal structure of 2-((E)-(((E)-2-hydroxy-4-methylbenzylidene) hydrazineylidene)methyl)-4-methylphenol, C16H16N2O2
- Crystal structure of (E)-amino(2-((5-methylfuran-2-yl)methylene)hydrazinyl) methaniminium nitrate monohydrate, C14H26N10O10
- The crystal structure of N′-(2-chloro-6-hydroxybenzylidene)thiophene-2-carbohydrazide monohydrate, C12H11ClN2O3S
- Crystal structure of catena-poly[(μ2-1,1′-(biphenyl-4,4′-diyl)bis(1H-imidazol)-κ2N:N′)-bis(4-bromobenzoate-κ1O)zinc(II)], C64H44Br4N8O8Zn2
- The crystal structure of catena-poly[(1-(4-carboxybenzyl)pyridin-1-ium-4-carboxylato-κ1O)-(μ2-oxalato-κ4 O:O′:O″:O‴)dioxidouranium(VI)], C16H11NO10U
- Crystal structure of 3-allyl-4-(2-bromoethyl)-5-(4-methoxyphenyl)-2-phenylfuran, C22H21BrO2
- Halogen bonds in the crystal structure of 4,3′:5′,4″-terpyridine — 1,3-diiodotetrafluorobenzene (1/1), C21H11F4I2N3
- Crystal structure of 2-(1H-indol-3-yl)ethan-1-aminium 2-(4-acetylphenoxy)acetate, C20H22N2O4
- Chalcogen bonds in the crystal structure of 4,7-dibromo-2,1,3-benzoselenadiazole, C6H2Br2N2Se
- The crystal structure of 1,4-bis((1H-benzimidazol-2-yl)methyl)-piperazine-2,5-dione dihydrate, C20H22N6O4
- The crystal structure of C19H20O8
- The crystal structure of KNa3Te8O18·5H2O exhibiting a ∞2[Te4O9]2− layer
- Erratum
- Erratum to: Crystal structure of (Z)-3-(6-bromo-1H-indol-3-yl)-1,3-diphenylprop-2-en-1-one, C23H16BrNO

