Abstract
C10H13N3O·C7H6O3, monoclinic, P21/n (no. 14), a = 7.4038(9) Å, b = 30.448(3) Å, c = 7.6744(8) Å, β = 112.013(3)°, V = 1603.9(3) Å3, Z = 4, R gt (F) = 0.0492, wR ref (F 2) = 0.1384, T = 173 K.
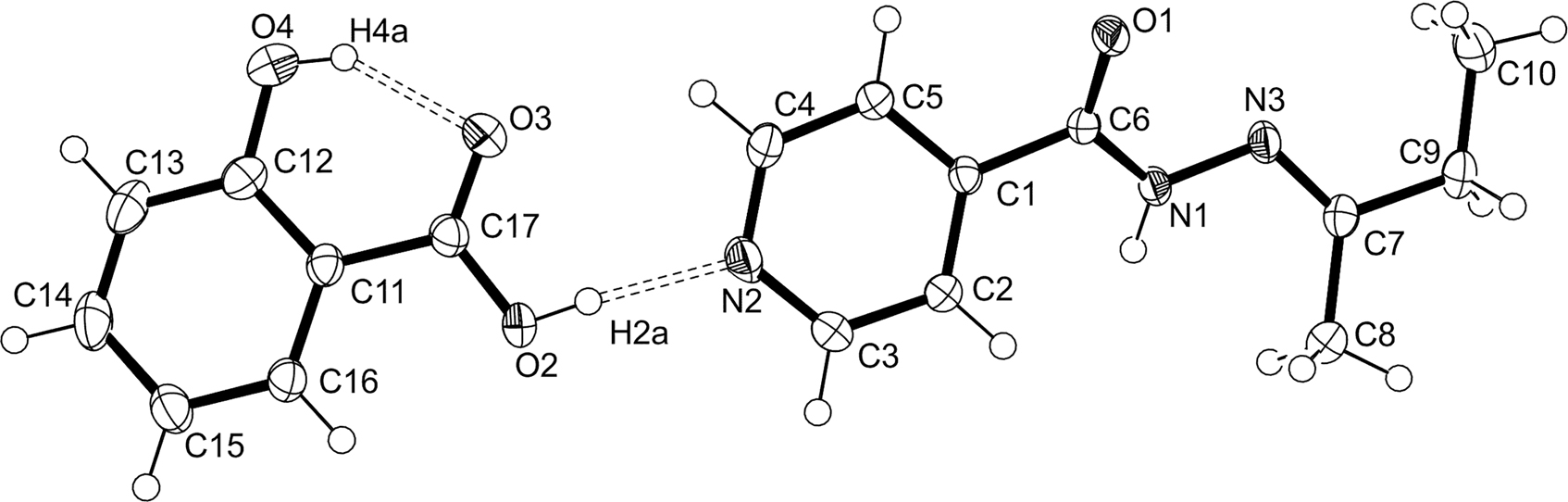
The asymmetric unit of the title crystal structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.43 × 0.34 × 0.31 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.10 mm−1 |
| Diffractometer, scan mode: | Bruker D8 Venture Photon, ω |
| θ max, completeness: | 28.0°, >99% |
| N(hkl) measured, N(hkl)unique, R int: | 77005, 3868, 0.034 |
| Criterion for I obs, N(hkl) gt: | I obs > 2 σ(I obs), 3459 |
| N(param)refined: | 217 |
| Programs: | Bruker [1], SHELX [2], WinGX [3], PLATON [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| C1 | 0.47422 (19) | 0.69710 (4) | 0.79314 (18) | 0.0183 (3) |
| C2 | 0.4466 (2) | 0.71769 (5) | 0.6233 (2) | 0.0232 (3) |
| H2 | 0.4381 | 0.7488 | 0.6132 | 0.028* |
| C3 | 0.4316 (2) | 0.69213 (5) | 0.4685 (2) | 0.0262 (3) |
| H3 | 0.4147 | 0.7064 | 0.3534 | 0.031* |
| C4 | 0.4657 (2) | 0.62846 (5) | 0.6387 (2) | 0.0267 (3) |
| H4 | 0.4704 | 0.5973 | 0.644 | 0.032* |
| C5 | 0.4857 (2) | 0.65154 (5) | 0.8007 (2) | 0.0228 (3) |
| H5 | 0.5069 | 0.6364 | 0.915 | 0.027* |
| C6 | 0.5103 (2) | 0.72181 (4) | 0.97278 (18) | 0.0188 (3) |
| C7 | 0.4555 (2) | 0.82709 (5) | 1.1042 (2) | 0.0218 (3) |
| C8 | 0.4302 (3) | 0.85230 (5) | 0.9284 (2) | 0.0322 (4) |
| H8A | 0.4336 | 0.8839 | 0.9544 | 0.048* |
| H8B | 0.5358 | 0.8448 | 0.886 | 0.048* |
| H8C | 0.3048 | 0.8447 | 0.8302 | 0.048* |
| C9 | 0.4893 (2) | 0.85415 (5) | 1.2773 (2) | 0.0267 (3) |
| H9A | 0.3772 | 0.8743 | 1.2516 | 0.032* |
| H9B | 0.6064 | 0.8725 | 1.3007 | 0.032* |
| C10 | 0.5164 (3) | 0.82882 (6) | 1.4547 (2) | 0.0327 (4) |
| H10A | 0.5372 | 0.8494 | 1.5586 | 0.049* |
| H10B | 0.3998 | 0.8112 | 1.4357 | 0.049* |
| H10C | 0.6297 | 0.8094 | 1.4849 | 0.049* |
| C11 | 0.2919 (2) | 0.52453 (5) | 0.0505 (2) | 0.0222 (3) |
| C12 | 0.2453 (2) | 0.48062 (5) | 0.0713 (2) | 0.0272 (3) |
| C13 | 0.1844 (2) | 0.45300 (5) | −0.0850 (3) | 0.0344 (4) |
| H13 | 0.1503 | 0.4234 | −0.0727 | 0.041* |
| C14 | 0.1737 (3) | 0.46848 (6) | −0.2571 (2) | 0.0362 (4) |
| H14 | 0.1321 | 0.4493 | −0.3625 | 0.043* |
| C15 | 0.2225 (2) | 0.51151 (5) | −0.2795 (2) | 0.0313 (3) |
| H15 | 0.2161 | 0.5218 | −0.3986 | 0.038* |
| C16 | 0.2807 (2) | 0.53909 (5) | −0.1255 (2) | 0.0250 (3) |
| H16 | 0.3138 | 0.5687 | −0.1398 | 0.03* |
| C17 | 0.3518 (2) | 0.55441 (5) | 0.2134 (2) | 0.0248 (3) |
| N1 | 0.42658 (18) | 0.76138 (4) | 0.95584 (16) | 0.0222 (3) |
| H1A | 0.3544 | 0.7721 | 0.8451 | 0.027* |
| N2 | 0.43990 (19) | 0.64817 (4) | 0.47484 (18) | 0.0267 (3) |
| N3 | 0.45845 (18) | 0.78518 (4) | 1.12194 (17) | 0.0226 (3) |
| O1 | 0.61992 (16) | 0.70594 (3) | 1.12281 (14) | 0.0255 (2) |
| O2 | 0.38141 (18) | 0.59551 (3) | 0.17611 (15) | 0.0307 (3) |
| H2A | 0.415 | 0.6104 | 0.2753 | 0.046* |
| O3 | 0.3705 (2) | 0.54221 (4) | 0.37140 (16) | 0.0391 (3) |
| O4 | 0.2580 (2) | 0.46347 (4) | 0.23750 (18) | 0.0404 (3) |
| H4A | 0.2961 | 0.4829 | 0.321 | 0.061* |
Source of material
All reagents used were commercially available and used without further purification. An amount of 0.220 g of isonicotinic acid hydrazide (1.60 mmol), 0.220 g of 2-hydroxy-benzoic acid (1.59 mmol) and 3.0 mL of 2-butanone were added into a screw-top dram vial (with rubber septum) and stirred at 300 rpm at 60 °C for 10 min. The vial was closed, and the solution was allowed to reflux in the vial at 60 °C for 24 h. The solution was then left slightly open to allow slow evaporation at room temperature. Colourless block crystals were observed after three days.
Experimental details
The collection method involved ω-scans of width 0.5°. Data reduction was carried out using SAINT–Plus version 6.02.6 software, and SADABS was used to process empirical absorption corrections [1]. The crystal structure was solved through direct methods using SHELXS-97 [2]. Hydrogen atoms were positioned geometrically and allowed to ride on their respective parent atoms with d(C—H) = 0.95 Å and U iso (H) = 1.2U eq (C). Diagrams and publication material were generated using WinGX [3] and PLATON [4].
Comment
Isoniazid, a synthetic derivative of nicotinic acid [5] is a drug that has been used for many years as a first-line anti–tuberculosis (TB) drug. However, bacterial strains targeted by isoniazid have developed resistance over the years, thus leading to the urgent need to develop novel drugs. There are several ways in which novel anti–TB drugs can be derived. However, one method that stands out is by making modifications to existing drugs so that they can no longer be deactivated by mycobacteria [6]. Therefore, the compound being reported in this article is a modification of isoniazid.
As shown in the figure, the asymmetric unit contains one molecule of N′-(butan-2-ylidene)pyridine-4-carbohydrazide and one molecule of 2-hydroxybenzoic acid (salicylic acid). All bond lengths and angles are as expected [7]. Each carbohydrazide moiety is hydrogen bonded by a O2—H2a⋯N2 hydrogen bond to a salicylic acid molecule to form a co-crystal. As is expected from similar co-crystals reported in the literature [8], this co-crystal is formed through a robust carboxylic acid ⋯ pyridine heterosynthon. The phenol group on the salicylic acid molecule forms an expected intramolecular S(6) hydrogen bond [9], between the H4a donor and the O3 acceptor. Each carbohydrazide moiety is also hydrogen bonded via its N1–H donor to the carbonyl oxygen (O1) acceptor of an adjacent carbohydrazide moiety (not shown in the figure). These hydrogen bonding interactions within the packing form a one dimensional (1D) ribbon along the a-axis. The plane of the salicylic acid is slightly offset from the plane of the pyridine ring by an acute angle of 22°. However, the pyridine rings of the carbohydrazide moiety as well as the benzene rings of salicylic acid lie parallel to each other respectively, and the packing is thus stabilized by π-stacking of these rings along the a-axis.
Funding source: National Research Foundation 10.13039/501100001321
Award Identifier / Grant number: 117850
Award Identifier / Grant number: 118127
Acknowledgement
The authors would like to thank Prof. M. Fernandes (University of the Witwatersrand) for his assistance with crystallographic data collection.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work was supported by the National Research Foundation (NRF) Thuthuka Grant Number 118127 as well as Thuthuka Grant Number 117850.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. SAINT–Plus and SADABS; Bruker AXS Inc.: Madison, WI, USA, 2004.Suche in Google Scholar
2. Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Suche in Google Scholar PubMed
3. Farrugia, L. J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Crystallogr. 1999, 32, 837–838; https://doi.org/10.1107/s0021889899006020.Suche in Google Scholar
4. Spek, A. L. Structure validation in chemical crystallography. Acta Crystallogr. 2009, D65, 148–155; https://doi.org/10.1107/s090744490804362x.Suche in Google Scholar
5. Pubchem. Isoniazid open chemistry database. 2018, 1–75. https://pubchem.ncbi.nml.nih.gov/compound/isoniazid#section=Top.Suche in Google Scholar
6. Hu, Y.-Q., Zhang, S., Zhao, F., Gao, C., Feng, L.-S., Lv, Z.-S. Isoniazid derivatives and their anti-tubercular activity. Eur. J. Med. Chem. 2017, 133, 255–267; https://doi.org/10.1016/j.ejmech.2017.04.002.Suche in Google Scholar PubMed
7. Lemmerer, A., Bernstein, J., Kahlenberg, V. One-pot covalent and supramolecular synthesis of pharmaceutical co-crystals using the API isoniazid: a potential supramolecular reagent. CrystEngComm 2010, 12, 2856–2864; https://doi.org/10.1039/c000473a.Suche in Google Scholar
8. Aakoroy, C. B., Hussain, I., Forbes, S., Desper, J. Exploring the hydrogen-bond preference of N–H moieties in co-crystals assembled via O–H(acid)⃛N(py) intermolecular intereactions. CrystEngComm 2007, 9, 46–54.10.1039/B614984GSuche in Google Scholar
9. Bernstein, J., Davis, R. E., Shimoni, L., Chang, N. L. Patterns in hydrogen bonding: functionality and graph set analysis in crystals. Angew. Chem. Int. Ed. 1995, 34, 1555–1573; https://doi.org/10.1002/anie.199515551.Suche in Google Scholar
© 2021 Itumeleng B. Setshedi and Mark G. Smith, published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of [aqua-(4-iodopyridine-2,6-dicarboxylato-κ3 O,N,O′)-(1,10-phenanothroline-κ2 N,N′)copper(II)] dihydrate, C19H16O7N3CuI
- The crystal structure of tetrakis(1-isopropyl-1H-imidazolium) octamolybdate, C24H44Mo8N8O26
- Crystal structure of catena-poly[bis(µ2-3,5-bis(1-imidazolyl)pyridine-κ2 N:N′)-(µ2-3-nitrophthalato-k3 O,O′:O″)cadmium(II)] dihydrate, C30H25N11O8Cd
- The crystal structure of diaqua-bis(2-(3-(1H-pyrazol-4-yl)-1H-1,2,4-triazol-5-yl)pyridine-κ2 N:N′)-bis(3,5-dicarboxybenzoato-κ1 O)cobalt(II), C38H30CoN12O14
- Crystal structure of the nickel(II) complex aqua-(2,6-di(pyrazin-2-yl)-4,4′-bipyridine-κ3 N,N′,N′′)-(phthalato-κ2 O,O′)nickel(II) tetrahydrate, C26H26N6O9Ni
- The crystal structure of 1-[5-(2-fluorophenyl)-1-(pyridine-3-sulfonyl)-1H-pyrrol-3-yl]-N-methylmethanaminium 3-carboxyprop-2-enoate, C21H20FN3O6S
- The crystal structure of 1,2-bis(4-pyridyl)ethane - 4,4-dihydroxydiphenylmethane (1/1), C25H21N2O2
- Crystal structure of bis(2-((E)-5-chloro-2-hydroxybenzylidene)hydrazineyl)methaniminium trifluoroacetate dihydrate, C34H36Cl4N10O12
- Crystal structure of 1-cyclopropyl-7-ethoxy-6,8-difluoro-4-oxo-1,4-dihydro-quinoline-3-carboxylic acid, C15H13F2NO4
- Crystal structure of methyl 3-(1H-naphtho[1,8-de][1,3,2]diazaborinin-2(3H)-yl)benzoate, C18H15BN2O2
- Crystal structure of (E)-N′-(2-chloro-6-hydroxybenzylidene)-2-hydroxybenzohydrazide, C14H11ClN2O3
- Crystal structure of Al-rich fluorophlogopite, K1.0(Mg2.8Al0.2)(Si2.8Al1.2)O10F2
- The crystal structure of 4,5-diiodo-1,3-dimesityl-1H-1,2,3-triazol-3-ium hexafluoridoantimonate(V), C20H22F6I2N3Sb
- Crystal structure of tris(3-iodopyridin-1-ium) catena-poly[(hexachlorido-κ1 Cl)-(μ2-trichlorido-κ2 Cl:Cl)diantimony(III)], C15H15Cl9I3N3Sb2
- Crystal structure of methyl 2-(1H-naphtho[1,8-de][1.3.2]diazaborinin-2(3H-yl)benzoate C18H15BN2O2
- The crystal structure of 1,8-bis(4-methoxybenzoyl)naphthalene-2,7-diyl dibenzoate, C40H28O8
- Crystal structure of 2-bromo-1,3,6,8-tetramethylBOPHY (BOPHY = bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl)methylene)hydrazine), C14H15B2BrF4N4
- The crystal structure of (E)-3-chloro-2-(2-(2-fluorobenzylidene)hydrazinyl)pyridine, C12H9ClFN3
- Crystal structure of bis(µ2- 4-iodopyridine-2,6-dicarboxylato-κ3O:N:O′)-bis(4-iodopyridine-2,6-dicarboxylato-κ3O:N:O′)-bis(µ2-1-(4-pyridyl)piperazine-κ2N:N′)-hexa-aqua-tetra-copper(II), C46H46Cu4I4N10O22
- Crystal structure of poly[diaqua-(μ2-2,5-dihydroxyterephthalato-κ2O:O′)(μ2-bis(4-pyridylformyl)piperazine-κ2N:N′)cadmium(II)] dihydrate, C24H28CdN4O12
- Crystal structure of poly[aqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)-(μ3-2,3,5,6-tetrafluoroterephthalato-κ3O:O′:O′′)cadmium(II)], C17H14N4O5F4Cd
- Crystal structure of 6-(quinolin-8-yl)benzo[a]phenanthridin-5(6H)-one, C26H16N2O
- The crystal structure of aqua-bis(6-chloropicolinato-κ2N,O)copper(II), C12H8Cl2N2O5Cu
- Crystal structure of catena-poly[diaqua-bis(μ2-4,4′-bipyridyl-κ2N:N′) disilver(I)] 4-oxidopyridine-3-sulfonate trihydrate, C25H29Ag2N5O9S
- The crystal structure of 4-(3-bromophenyl)pyrimidin-2-amine, C10H8BrN3
- Crystal structure of 6-oxo-4-phenyl-1-propyl-1,6-dihydropyridine-3-carbonitrile, C15H14N2O
- Crystal structure of 4-(2,2-difluoroethyl)-2,4-dimethyl-6-(trifluoromethyl)isoquinoline-1,3(2H,4H)-dione, C14H12F5NO2
- Crystal structure of dibromido-(1-methyl-1H-imidazole-κ1N)-(3-(3-methyl-1H-imidazol-3-ium-1-yl)propanoato-κ1O)zinc(II), C11H16Br2N4O2Zn
- The crystal structure of 1,1′-(((2 (dimethylamino)ethyl)azanediyl)bis(methylene)) bis(naphthalen-2-olato-κ4 N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)- titanium(IV) ─ dichloromethane (2/1), C33H29N3O6Ti
- The layered crystal structure of bis(theophyllinium) hexachloridostannate (IV), C14H18N8O8SnCl6
- The crystal structre of 3-(1-ethenyl-1H-imidazol-3-ium-3-yl)propane-1-sulfonate, C8H12N2O3S
- Synthesis and crystal structure of di-tert-butyl 1″-acetyl-2,2″,9′-trioxo-4a′,9a′-dihydro-1′H,3′H,9′H-dispiro[indoline-3,2′-xanthene-4′,3″-indoline]-1,3′-dicarboxylate, C39H38N2O9
- The crystal structure of 4-chloro-2-(quinolin-8-yl)isoindoline-1,3-dione, C17H9ClN2O2
- The crystal structure of 1-fluoro-4-(p-tolylethynyl)benzene, C15H11F
- The crystal structure of bis[4-bromo-2-(1H-pyrazol-3-yl) phenolato-κ2N,O] copper(II), C18H12Br2CuN4O2
- The crystal structure of poly[(μ 3-imidazolato-κ 3 N:N:N′)(tetrahydrofuran- κ 1 O)lithium(I)], C7H11LiN2O
- Crystal structure of N′,N′′′-((1E,1′E)-(propane-2,2-diylbis(1H-pyrrole-5,2diyl))bis(methaneylylidene))di(nicotinohydrazide) pentahydrate, C25H24N8O2·5H2O
- Crystal structure of 3-(2-ethoxy-2-oxoethyl)-1-ethyl-1H-imidazol-3-ium hexafluoridophos-phate(V), C9H15F6N2O2P
- Crystal structure of (1,10-phenanthroline-κ2N,N′)-bis(3-thiophenecarboxylato-κ2O,O′)copper(II), C22H14N2O4S2Cu
- The crystal structure of 2-amino-3-carboxypyridin-1-ium iodide hemihydrate, C6H8IN2O2.5
- Crystal structure of (E)-7-methoxy-2-((6-methoxypyridin-2-yl)methylene)-tetralone, C18H17NO3
- The crystal structure of [μ-hydroxido-bis[(5,5′-dimethyl-2,2′-bipyridine-κ2N,N′)-tricarbonylrhenium(I)] bromide hemihydrate, C30H26N4O9Re2Br
- The crystal structure of 2,5-bis(3,5-dimethylphenyl)thiazolo[5,4-d]thiazole, C20H18N2S2
- The crystal structure of 5-benzoyl-1-[(E)-(4-fluorobenzylidene)amino]-4-phenylpyrimidin-2(1H)-one, C24H16FN3O2
- Crystal structure of monocarbonyl(N-nitroso-N-oxido-phenylamine-κ 2 O,O′)(tricyclohexylphosphine-κP)rhodium(I), C25H39N2O3PRh
- Crystal structure of poly[bis[μ3-1,3,5-tris[(1H-imidazol-1-yl)methyl]benzene-κ3N:N′:N″]nickel(II)] hexafluorosilicate, C36H36N12NiSiF6
- The crystal structure of 13-(pyrazole-1-yl-4-carbonitrile)-matrine, C19H25N5O
- Crystal structure of 3,5-bis((E)-4-methoxy-2-(trifluoromethyl)benzylidene)-1-methylpiperidin-4-one, C24H21F6NO3
- The crystal structure of N,N′-(Disulfanediyldi-2,1-phenylene)di(6′-methylpyridine)-2-carboxamide, C26H22N4O2S2
- Crystal structure of (E)-7-fluoro-2-(4-methoxy-2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C19H14F4O2
- Crystal structure of ethyl 1-(4-fluorophenyl)-4-phenyl-1H-pyrrole-3-carboxylate, C19H16FNO2
- The crystal structure of cis-diaqua-bis (N-butyl-N-(pyridin-2-yl)pyridin-2-amine-κ2N,N′)cobalt(II)] dichloride trihydrate, C28H44Cl2N6O5Co
- Crystal structure of (E)-7-methoxy-2-((6-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C18H17NO3
- Crystal structure of (E)-2-((3-fluoropyridin-4-yl)methylene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- The crystal structure of 6-bromohexanoic acid, C6H11BrO2
- The crystal structure of 4-chloro-thiophenol, C6H5ClS
- The crystal structure of 4-bromobenzyl chloride, C7H6BrCl
- The crystal structure of di-tert-butyl dicarbonate, C10H18O5
- The crystal structure of (2-(4-chlorophenyl)-5-methyl-1,3-dioxan-5-yl)methanol, C12H15ClO3
- The crystal structure of the co-crystal: 2-hydroxybenzoic acid – N′-(butan-2-ylidene)pyridine-4-carbohydrazide, C10H13N3O·C7H6O3
- Crystal structure and anti-inflammatory activity of (E)-7-fluoro-2-((5-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- Crystal structure of (E)-7-fluoro-2-((6-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- Crystal structure of 1,1′-(butane-1,4-diyl)bis(3-propyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C32H56F24N8P4
- The crystal structure of dichlorido-bis(3-methyl-3-imidazolium-1-ylpropionato-κ2)-cadmium(II), C14H20CdCl2N4O4
- Crystal structure of 1-(2-cyanobenzyl)-3-cyano-4-phenyl-4-(2-cyanobenzyl)-1,4-dihydropyridine monohydrate, C56H42N8O
- The crystal structure of 3-(carboxymethyl)-1-ethenyl-1H-imidazol-3-ium chloride, C7H9N2O2Cl
- The crystal structure of adamantylmethoxydiphenylsilane, C23H28OSi
- Redetermination of the crystal structure of (2E,4Z,13E,15Z)-3,5,14,16-tetramethyl-2,6,13,17-tetraazatricyclo[16.4.0.07,12]docosa-1(22),2,4,7,9,11,13,15,18,20-decaene, C22H24N4
- Crystal structure of (E)-7-hydroxy-2-((6-methoxypyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H15NO3
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2 N:N′)cobalt(II)] dinitrate, C18H28N10O8Co
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of [aqua-(4-iodopyridine-2,6-dicarboxylato-κ3 O,N,O′)-(1,10-phenanothroline-κ2 N,N′)copper(II)] dihydrate, C19H16O7N3CuI
- The crystal structure of tetrakis(1-isopropyl-1H-imidazolium) octamolybdate, C24H44Mo8N8O26
- Crystal structure of catena-poly[bis(µ2-3,5-bis(1-imidazolyl)pyridine-κ2 N:N′)-(µ2-3-nitrophthalato-k3 O,O′:O″)cadmium(II)] dihydrate, C30H25N11O8Cd
- The crystal structure of diaqua-bis(2-(3-(1H-pyrazol-4-yl)-1H-1,2,4-triazol-5-yl)pyridine-κ2 N:N′)-bis(3,5-dicarboxybenzoato-κ1 O)cobalt(II), C38H30CoN12O14
- Crystal structure of the nickel(II) complex aqua-(2,6-di(pyrazin-2-yl)-4,4′-bipyridine-κ3 N,N′,N′′)-(phthalato-κ2 O,O′)nickel(II) tetrahydrate, C26H26N6O9Ni
- The crystal structure of 1-[5-(2-fluorophenyl)-1-(pyridine-3-sulfonyl)-1H-pyrrol-3-yl]-N-methylmethanaminium 3-carboxyprop-2-enoate, C21H20FN3O6S
- The crystal structure of 1,2-bis(4-pyridyl)ethane - 4,4-dihydroxydiphenylmethane (1/1), C25H21N2O2
- Crystal structure of bis(2-((E)-5-chloro-2-hydroxybenzylidene)hydrazineyl)methaniminium trifluoroacetate dihydrate, C34H36Cl4N10O12
- Crystal structure of 1-cyclopropyl-7-ethoxy-6,8-difluoro-4-oxo-1,4-dihydro-quinoline-3-carboxylic acid, C15H13F2NO4
- Crystal structure of methyl 3-(1H-naphtho[1,8-de][1,3,2]diazaborinin-2(3H)-yl)benzoate, C18H15BN2O2
- Crystal structure of (E)-N′-(2-chloro-6-hydroxybenzylidene)-2-hydroxybenzohydrazide, C14H11ClN2O3
- Crystal structure of Al-rich fluorophlogopite, K1.0(Mg2.8Al0.2)(Si2.8Al1.2)O10F2
- The crystal structure of 4,5-diiodo-1,3-dimesityl-1H-1,2,3-triazol-3-ium hexafluoridoantimonate(V), C20H22F6I2N3Sb
- Crystal structure of tris(3-iodopyridin-1-ium) catena-poly[(hexachlorido-κ1 Cl)-(μ2-trichlorido-κ2 Cl:Cl)diantimony(III)], C15H15Cl9I3N3Sb2
- Crystal structure of methyl 2-(1H-naphtho[1,8-de][1.3.2]diazaborinin-2(3H-yl)benzoate C18H15BN2O2
- The crystal structure of 1,8-bis(4-methoxybenzoyl)naphthalene-2,7-diyl dibenzoate, C40H28O8
- Crystal structure of 2-bromo-1,3,6,8-tetramethylBOPHY (BOPHY = bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl)methylene)hydrazine), C14H15B2BrF4N4
- The crystal structure of (E)-3-chloro-2-(2-(2-fluorobenzylidene)hydrazinyl)pyridine, C12H9ClFN3
- Crystal structure of bis(µ2- 4-iodopyridine-2,6-dicarboxylato-κ3O:N:O′)-bis(4-iodopyridine-2,6-dicarboxylato-κ3O:N:O′)-bis(µ2-1-(4-pyridyl)piperazine-κ2N:N′)-hexa-aqua-tetra-copper(II), C46H46Cu4I4N10O22
- Crystal structure of poly[diaqua-(μ2-2,5-dihydroxyterephthalato-κ2O:O′)(μ2-bis(4-pyridylformyl)piperazine-κ2N:N′)cadmium(II)] dihydrate, C24H28CdN4O12
- Crystal structure of poly[aqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)-(μ3-2,3,5,6-tetrafluoroterephthalato-κ3O:O′:O′′)cadmium(II)], C17H14N4O5F4Cd
- Crystal structure of 6-(quinolin-8-yl)benzo[a]phenanthridin-5(6H)-one, C26H16N2O
- The crystal structure of aqua-bis(6-chloropicolinato-κ2N,O)copper(II), C12H8Cl2N2O5Cu
- Crystal structure of catena-poly[diaqua-bis(μ2-4,4′-bipyridyl-κ2N:N′) disilver(I)] 4-oxidopyridine-3-sulfonate trihydrate, C25H29Ag2N5O9S
- The crystal structure of 4-(3-bromophenyl)pyrimidin-2-amine, C10H8BrN3
- Crystal structure of 6-oxo-4-phenyl-1-propyl-1,6-dihydropyridine-3-carbonitrile, C15H14N2O
- Crystal structure of 4-(2,2-difluoroethyl)-2,4-dimethyl-6-(trifluoromethyl)isoquinoline-1,3(2H,4H)-dione, C14H12F5NO2
- Crystal structure of dibromido-(1-methyl-1H-imidazole-κ1N)-(3-(3-methyl-1H-imidazol-3-ium-1-yl)propanoato-κ1O)zinc(II), C11H16Br2N4O2Zn
- The crystal structure of 1,1′-(((2 (dimethylamino)ethyl)azanediyl)bis(methylene)) bis(naphthalen-2-olato-κ4 N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)- titanium(IV) ─ dichloromethane (2/1), C33H29N3O6Ti
- The layered crystal structure of bis(theophyllinium) hexachloridostannate (IV), C14H18N8O8SnCl6
- The crystal structre of 3-(1-ethenyl-1H-imidazol-3-ium-3-yl)propane-1-sulfonate, C8H12N2O3S
- Synthesis and crystal structure of di-tert-butyl 1″-acetyl-2,2″,9′-trioxo-4a′,9a′-dihydro-1′H,3′H,9′H-dispiro[indoline-3,2′-xanthene-4′,3″-indoline]-1,3′-dicarboxylate, C39H38N2O9
- The crystal structure of 4-chloro-2-(quinolin-8-yl)isoindoline-1,3-dione, C17H9ClN2O2
- The crystal structure of 1-fluoro-4-(p-tolylethynyl)benzene, C15H11F
- The crystal structure of bis[4-bromo-2-(1H-pyrazol-3-yl) phenolato-κ2N,O] copper(II), C18H12Br2CuN4O2
- The crystal structure of poly[(μ 3-imidazolato-κ 3 N:N:N′)(tetrahydrofuran- κ 1 O)lithium(I)], C7H11LiN2O
- Crystal structure of N′,N′′′-((1E,1′E)-(propane-2,2-diylbis(1H-pyrrole-5,2diyl))bis(methaneylylidene))di(nicotinohydrazide) pentahydrate, C25H24N8O2·5H2O
- Crystal structure of 3-(2-ethoxy-2-oxoethyl)-1-ethyl-1H-imidazol-3-ium hexafluoridophos-phate(V), C9H15F6N2O2P
- Crystal structure of (1,10-phenanthroline-κ2N,N′)-bis(3-thiophenecarboxylato-κ2O,O′)copper(II), C22H14N2O4S2Cu
- The crystal structure of 2-amino-3-carboxypyridin-1-ium iodide hemihydrate, C6H8IN2O2.5
- Crystal structure of (E)-7-methoxy-2-((6-methoxypyridin-2-yl)methylene)-tetralone, C18H17NO3
- The crystal structure of [μ-hydroxido-bis[(5,5′-dimethyl-2,2′-bipyridine-κ2N,N′)-tricarbonylrhenium(I)] bromide hemihydrate, C30H26N4O9Re2Br
- The crystal structure of 2,5-bis(3,5-dimethylphenyl)thiazolo[5,4-d]thiazole, C20H18N2S2
- The crystal structure of 5-benzoyl-1-[(E)-(4-fluorobenzylidene)amino]-4-phenylpyrimidin-2(1H)-one, C24H16FN3O2
- Crystal structure of monocarbonyl(N-nitroso-N-oxido-phenylamine-κ 2 O,O′)(tricyclohexylphosphine-κP)rhodium(I), C25H39N2O3PRh
- Crystal structure of poly[bis[μ3-1,3,5-tris[(1H-imidazol-1-yl)methyl]benzene-κ3N:N′:N″]nickel(II)] hexafluorosilicate, C36H36N12NiSiF6
- The crystal structure of 13-(pyrazole-1-yl-4-carbonitrile)-matrine, C19H25N5O
- Crystal structure of 3,5-bis((E)-4-methoxy-2-(trifluoromethyl)benzylidene)-1-methylpiperidin-4-one, C24H21F6NO3
- The crystal structure of N,N′-(Disulfanediyldi-2,1-phenylene)di(6′-methylpyridine)-2-carboxamide, C26H22N4O2S2
- Crystal structure of (E)-7-fluoro-2-(4-methoxy-2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C19H14F4O2
- Crystal structure of ethyl 1-(4-fluorophenyl)-4-phenyl-1H-pyrrole-3-carboxylate, C19H16FNO2
- The crystal structure of cis-diaqua-bis (N-butyl-N-(pyridin-2-yl)pyridin-2-amine-κ2N,N′)cobalt(II)] dichloride trihydrate, C28H44Cl2N6O5Co
- Crystal structure of (E)-7-methoxy-2-((6-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C18H17NO3
- Crystal structure of (E)-2-((3-fluoropyridin-4-yl)methylene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- The crystal structure of 6-bromohexanoic acid, C6H11BrO2
- The crystal structure of 4-chloro-thiophenol, C6H5ClS
- The crystal structure of 4-bromobenzyl chloride, C7H6BrCl
- The crystal structure of di-tert-butyl dicarbonate, C10H18O5
- The crystal structure of (2-(4-chlorophenyl)-5-methyl-1,3-dioxan-5-yl)methanol, C12H15ClO3
- The crystal structure of the co-crystal: 2-hydroxybenzoic acid – N′-(butan-2-ylidene)pyridine-4-carbohydrazide, C10H13N3O·C7H6O3
- Crystal structure and anti-inflammatory activity of (E)-7-fluoro-2-((5-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- Crystal structure of (E)-7-fluoro-2-((6-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- Crystal structure of 1,1′-(butane-1,4-diyl)bis(3-propyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C32H56F24N8P4
- The crystal structure of dichlorido-bis(3-methyl-3-imidazolium-1-ylpropionato-κ2)-cadmium(II), C14H20CdCl2N4O4
- Crystal structure of 1-(2-cyanobenzyl)-3-cyano-4-phenyl-4-(2-cyanobenzyl)-1,4-dihydropyridine monohydrate, C56H42N8O
- The crystal structure of 3-(carboxymethyl)-1-ethenyl-1H-imidazol-3-ium chloride, C7H9N2O2Cl
- The crystal structure of adamantylmethoxydiphenylsilane, C23H28OSi
- Redetermination of the crystal structure of (2E,4Z,13E,15Z)-3,5,14,16-tetramethyl-2,6,13,17-tetraazatricyclo[16.4.0.07,12]docosa-1(22),2,4,7,9,11,13,15,18,20-decaene, C22H24N4
- Crystal structure of (E)-7-hydroxy-2-((6-methoxypyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H15NO3
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2 N:N′)cobalt(II)] dinitrate, C18H28N10O8Co

