Abstract
C18H17NO3, triclinic,
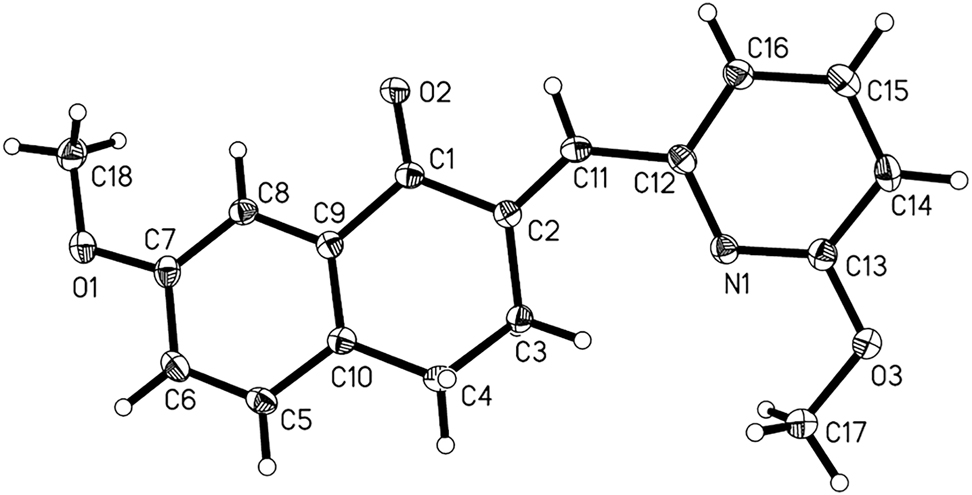
The molecular structure is shown in Figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.15 × 0.12 × 0.11 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.09 mm−1 |
| Diffractometer, scan mode: | SuperNova, |
| θmax, completeness: | 25.5°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 4714, 2660, 0.042 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2σ(Iobs), 2124 |
| N(param)refined: | 201 |
| Programs: | CrysAlisPRO [1], Shelx [2, 3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1 | 0.8932 (5) | 0.65626 (14) | 0.16741 (13) | 0.0215 (4) |
| C2 | 0.7312 (5) | 0.72771 (14) | 0.21974 (13) | 0.0203 (4) |
| C3 | 0.7835 (5) | 0.69310 (13) | 0.32835 (13) | 0.0224 (4) |
| H3A | 0.639175 | 0.736137 | 0.357335 | 0.027* |
| H3B | 1.007410 | 0.706690 | 0.339160 | 0.027* |
| C4 | 0.7151 (5) | 0.57074 (14) | 0.37833 (13) | 0.0226 (4) |
| H4A | 0.771521 | 0.548179 | 0.447041 | 0.027* |
| H4B | 0.483280 | 0.559332 | 0.376104 | 0.027* |
| C5 | 0.9975 (5) | 0.39189 (15) | 0.38072 (14) | 0.0248 (5) |
| H5 | 0.935214 | 0.361534 | 0.447066 | 0.030* |
| C6 | 1.1765 (5) | 0.32829 (15) | 0.33572 (14) | 0.0273 (5) |
| H6 | 1.232012 | 0.255789 | 0.371612 | 0.033* |
| C7 | 1.2744 (5) | 0.37250 (15) | 0.23654 (14) | 0.0249 (5) |
| C8 | 1.1857 (5) | 0.47998 (14) | 0.18311 (14) | 0.0226 (4) |
| H8 | 1.248110 | 0.509892 | 0.116740 | 0.027* |
| C9 | 1.0022 (5) | 0.54312 (14) | 0.22931 (13) | 0.0204 (4) |
| C10 | 0.9074 (5) | 0.50092 (14) | 0.32894 (13) | 0.0210 (4) |
| C11 | 0.5586 (5) | 0.81598 (14) | 0.16365 (13) | 0.0225 (4) |
| H11 | 0.551297 | 0.821417 | 0.097805 | 0.027* |
| C12 | 0.3809 (5) | 0.90467 (14) | 0.18738 (13) | 0.0223 (4) |
| C13 | 0.2055 (5) | 0.98777 (14) | 0.29846 (14) | 0.0244 (5) |
| C14 | 0.0570 (5) | 1.07664 (15) | 0.22600 (15) | 0.0288 (5) |
| H14 | −0.049705 | 1.133695 | 0.241718 | 0.035* |
| C15 | 0.0759 (5) | 1.07572 (15) | 0.13119 (14) | 0.0295 (5) |
| H15 | −0.017966 | 1.132873 | 0.080569 | 0.035* |
| C16 | 0.2371 (5) | 0.98832 (15) | 0.11154 (14) | 0.0249 (5) |
| H16 | 0.248408 | 0.986000 | 0.047716 | 0.030* |
| C17 | 0.3289(6) | 0.89823 (15) | 0.46620 (14) | 0.0325 (5) |
| H17A | 0.238639 | 0.831305 | 0.465483 | 0.049* |
| H17B | 0.286711 | 0.905347 | 0.529839 | 0.049* |
| H17C | 0.560790 | 0.896779 | 0.452808 | 0.049* |
| C18 | 1.5485 (6) | 0.34415 (17) | 0.09731 (15) | 0.0344 (5) |
| H18A | 1.355831 | 0.363651 | 0.059011 | 0.052* |
| H18B | 1.673117 | 0.288442 | 0.080016 | 0.052* |
| H18C | 1.679046 | 0.407776 | 0.084055 | 0.052* |
| N1 | 0.3651 (4) | 0.90504 (11) | 0.28168 (11) | 0.0225 (4) |
| O1 | 1.4560 (4) | 0.30353 (10) | 0.19940 (10) | 0.0328 (4) |
| O2 | 0.9342 (4) | 0.68720 (10) | 0.07762 (9) | 0.0310 (4) |
| O3 | 0.1806 (4) | 0.98961 (10) | 0.39229 (10) | 0.0302 (4) |
Source of material
The title compound {systematic name: (E)-7-methoxy-2-((6-methoxypyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one} was prepared according to a literature protocol [4]. 7-Methoxy-1-tetralone (0.53 g, 3.0 mmol) and 6-methoxy-2-pyridinecarbaldehyde (0.41 g, 3.0 mmol) were dissolved in methanol (10 mL). Aqueous NaOH (0.60 g, 15.0 mmol) solution (3 mL) was added to the above solution. The reaction mixture was stirred until completion of the reaction (monitored by TLC). Then, it was cooled in an ice bath for 20 min. The solids were filtered off and the residues were purified on a silica gel by column chromatography using petroleum ether/ethyl acetate (2:1, v/v) as the eluent to produce light yellow powders. Crystals suitable for X-ray diffraction were obtained by slow evaporation from methanol at room temperature.
Experimental details
The H atoms were placed in idealized positions and treated as riding on their parent atoms, with d (C–H) = 0.93 Å (aromatic) and 0.97 Å (methylene), Uiso(H) = 1.2Ueq(C), and d(C–H) = 0.96 Å (methyl), Uiso(H) = 1.5Ueq(C). Displacement ellipsoids are drawn at the 35% probability level.
Comment
1-Tetralones, also known as 3,4-dihydronaphthalen-1(2H)-ones, have attracted the attention of chemists and pharmacists because of their potential applications for novel modulators of allergic and inflammatory phenomena and inhibitors of retinoic acid (RA)-metabollizing enzymes [5, 6]. In order to obtain novel pharmaceutical agents, 3,4-dihydronaphthalen-1(2H)-one and its derivatives were designed and synthesized to innovative 1-tetralone derivatives with judicious functional groups and different substituents. (E)-2-(3,4-dimethoxybenzylidene)-7-methoxy-tetralone exhibits obvious inhibition activity against Bcl-2 (B cell lymphoma 2) protein [7]. 2-(2-Bromo-3,4,5-trimethoxybenzylidene)-6-methoxy-tetralone possesses potent inhibitory activity against monoamine oxidase and acetylcholinesterase, which can be a promising agent for the treatment of Alzheimer’s disease [8]. Some 2-arylidene-1-tetralones with methoxy groups showed the antioxidant activity against radical scavenging [9]. These results demonstrate that active 1-tetralone derivatives all contain the fraction of α,β-unsaturated keto and methoxy group. The pharmacophore of α,β-unsaturated keto can establish the primary binding interaction with bio-thiols from susceptible neoplasms with lower toxicity [10]. The methoxy group can improve molecular lipophilicity and increase the ability of the membrane permeability. Based on these consideration, 2-arylidene-1-tetralones with methoxy groups were designed and synthesized in our laboratory, which show evident anti-neuroinflammatory property with relatively low toxicity [11]. Additionally, 1-tetralone derivatives that contain pyridinyl groups demonstrated antiproliferative activity against a variety of cancer cell lines [12]. As a part of the search for new anti-inflammatory and antitumor agents, a new 1-tetralone derivative with methoxy group and pyridine ring was synthesized by the aldol condensation reaction.
There is one independent molecule in the asymmetric unit (cf. the Figure). The bond length of C2–C11 is 1.349(2) Å, which represents a typical C=C double bond, and the ethylene moiety in the enone linkage adopts an E configuration [13]. Other bond lengths and bond angles are all in the normal ranges [14–17]. In the title molecule, the cyclohexanone ring displays an envelope conformation with the flap atom C4 deviating by 0.493(3) Å from the least-squares plane of the ring. The dihedral angle between the benzene and pyridine rings is 24.28(7)°. In the crystal, molecules are connected through weak C–H–O hydrogen bonds. It is noteworthy that the peripheric heteroatoms with free electron pairs (such as O and N) can be considered as the potential hydrogen bonding acceptors and such weak interactions will play a crucial role in the biological activity [18].
Funding source: Project of Shandong Province Higher Educational Science and Technology Program 10.13039/501100015642
Award Identifier / Grant number: J18KA092
Acknowledgements
This work was supported by Project of the Shandong Province Higher Educational Science and Technology Program (J18KA092).
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: Project of the Shandong Province Higher Educational Science and Technology Program (J18KA092).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Rigaku, O. D. CrysAlisPRO; Rigaku Oxford Diffraction Ltd: Yarnton, Oxfordshire, England, 2017.Suche in Google Scholar
2. Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Suche in Google Scholar PubMed
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar
4. Pramila, K., Aastha, S., Aarajana, S., Ritina, S., Pil-Hoon, P., Eung-Seok, L. Introduction of amino moiety enhances the inhibitory potency of 1-tetralone chalcone derivatives against LPS-stimulated reactive oxygen species production in RAW 264.7 macrophages. Bioorg. Chem. 2019, 87, 494–505.10.1016/j.bioorg.2019.03.055Suche in Google Scholar PubMed
5. Barlow, J. W., Zhang, T., Woods, O., Byrne, A. J., Walsh, J. J. Novel mast cell-stabilising amine derivatives of 3,4-dihydronaphthalen-1(2H)-one and 6,7,8,9-tetrahydro-5H-benzo[7]annulen-5-one. Med. Chem. 2011, 7, 213–223; https://doi.org/10.2174/157340611795564222.Suche in Google Scholar PubMed
6. Kirby, A. J., Lain, R. L., Maharlouie, F., Mason, P., Nicholls, P. J., John Smith, H., Simons, C. Inhibition of retinoic acid metabolising enzymes by 2-(4-aminophenylmethyl)-6-hydroxy-3,4-dihydronaphthalen-1(2H)-one and related compounds. J. Enzym. Inhib. Med. Chem. 2003, 18, 27–33; https://doi.org/10.1080/1475636021000049221.Suche in Google Scholar PubMed
7. Wang, F., Zhang, R., Cui, Y., Sheng, L., Sun, Y., Tian, W., Liu, X., Liang, S. Design, synthesis and biological evaluation of 3,4-dihydronaphthalen-1(2H)-one derivatives as Bcl-2 inhibitors. Res. Chem. Intermed. 2017, 43, 5933–5942; https://doi.org/10.1007/s11164-017-2972-x.Suche in Google Scholar
8. Leng, J., Qin, H. L., Zhu, K., Jantan, I., Hussain, M. A., Sher, M., Amjad, M. W., Naeem-Ul-Hassan, M., Ahmad, W., Bukhari, S. N. Evaluation of multifunctional synthetic tetralone derivatives for treatment of Alzheimer’s disease. Chem. Biol. Drug Des. 2016, 88, 889–898; https://doi.org/10.1111/cbdd.12822.Suche in Google Scholar PubMed
9. Ranjbar, S., Akbari, A., Edraki, N., Khoshneviszadeh, M., Hemmatian, H., Firuzi, O., Khoshneviszadeh, M. 6-Methoxy-3,4-dihydronaphthalenone chalcone-like derivatives as potent tyrosinase inhibitors and radical scavengers. Lett. Drug Des. Discov. 2018, 15, 1170–1179; https://doi.org/10.2174/1570180815666180219155027.Suche in Google Scholar
10. Sun, Y., Gao, Z., Wang, C., Hou, G. Synthesis, crystal structures and anti-inflammatory activity of fluorine-substituted 1,4,5,6-tetrahydrobenzo[h]quinazolin-2-amine derivatives. Acta Crystallogr. 2018, C74, 659–665.10.1107/S2053229619010118Suche in Google Scholar PubMed
11. Sun, Y., Zhou, Y. Q., Liu, Y. K., Zhang, H. Q., Hou, G. G., Meng, Q. G. Potential anti-neuroinflammatory NF-κB inhibitors based on 3,4-dihydronaphthalen-1(2H)-one derivatives. J. Enzym. Inhib. Med. Chem. 2020, 35, 1631–1640; https://doi.org/10.1080/14756366.2020.1804899.Suche in Google Scholar PubMed PubMed Central
12. Gibson, M. Z., Nguyen, M. A., Zingales, S. K. Design, synthesis, and evaluation of (2-(pyridinyl)methylene)-1-tetralone chalcones for anticancer and antimicrobial activity. Med. Chem. 2018, 14, 333–343; https://doi.org/10.2174/1573406413666171020121244.Suche in Google Scholar PubMed
13. Baddeley, T. C., Gomes, L. R., Low, J. N., Turner, A. B., Wardell, J. L., Watson, G. J. R. Structural studies of (E)-2-(benzylidene)-1-tetralone derivatives: crystal structures and Hirshfeld surface analysis. Z. Kristallogr. NCS 2017, 232, 697–718; https://doi.org/10.1515/zkri-2017-2048.Suche in Google Scholar
14. El-Sayed, N. N. E., Almaneai, N. M., Ghabbour, H. A., Alafeefy, A. M. Crystal structure of (E)-2-(4-hydroxy-3-methoxybenzylidene)-6-methoxy- 3,4-dihydronaphthalen-1(2H)-one, C19H18O4. Z. Kristallogr. NCS 2017, 232, 203–205; https://doi.org/10.1515/ncrs-2016-0195.Suche in Google Scholar
15. Luan, M.-Z., Wang, H.-Y., Zhang, M., Song, J., Xu, Y.-R., Zhao, F.-L., Meng, Q.-G. Crystal structure of (E)-2-(4-fluoro-2-(trifluoromethyl) benzylidene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C19H14F4O2. Z. Kristallogr. NCS 2021, 236, 245–247; https://doi.org/10.1515/ncrs-2020-0484.Suche in Google Scholar
16. Luan, M.-Z., Wang, H.-Y., Zhang, M., Song, J., Hou, G.-G., Zhao, F.-L., Meng, Q.-G. Crystal structure of (E)-2-(3,5-bis(trifluoromethyl)benzylidene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C20H14F6O2. Z. Kristallogr. NCS 2021, 236, 61–63; https://doi.org/10.1515/ncrs-2020-0446.Suche in Google Scholar
17. Zhang, X.-F., Wang, H.-Y., Zhao, S.-N., Zhang, S.-N., Zhao, F.-L., Meng, Q.-G. Crystal structure of (E)-2-(4-fluoro-3-(trifluoromethyl)benzylidene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C19H14F4O2. Z. Kristallogr. NCS 2021, 236, 47–49; https://doi.org/10.1515/ncrs-2020-0448.Suche in Google Scholar
18. Amakali, K. T., Legoabe, L. J., Petzer, A., Petzer, J. P. Synthesis and in vitro evaluation of 2-heteroarylidene-1-tetralone derivatives as monoamine oxidase inhibitors. Drug Res. 2018, 68, 687–695; https://doi.org/10.1055/a-0620-8309.Suche in Google Scholar PubMed
© 2021 Lei Wang et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of [aqua-(4-iodopyridine-2,6-dicarboxylato-κ3 O,N,O′)-(1,10-phenanothroline-κ2 N,N′)copper(II)] dihydrate, C19H16O7N3CuI
- The crystal structure of tetrakis(1-isopropyl-1H-imidazolium) octamolybdate, C24H44Mo8N8O26
- Crystal structure of catena-poly[bis(µ2-3,5-bis(1-imidazolyl)pyridine-κ2 N:N′)-(µ2-3-nitrophthalato-k3 O,O′:O″)cadmium(II)] dihydrate, C30H25N11O8Cd
- The crystal structure of diaqua-bis(2-(3-(1H-pyrazol-4-yl)-1H-1,2,4-triazol-5-yl)pyridine-κ2 N:N′)-bis(3,5-dicarboxybenzoato-κ1 O)cobalt(II), C38H30CoN12O14
- Crystal structure of the nickel(II) complex aqua-(2,6-di(pyrazin-2-yl)-4,4′-bipyridine-κ3 N,N′,N′′)-(phthalato-κ2 O,O′)nickel(II) tetrahydrate, C26H26N6O9Ni
- The crystal structure of 1-[5-(2-fluorophenyl)-1-(pyridine-3-sulfonyl)-1H-pyrrol-3-yl]-N-methylmethanaminium 3-carboxyprop-2-enoate, C21H20FN3O6S
- The crystal structure of 1,2-bis(4-pyridyl)ethane - 4,4-dihydroxydiphenylmethane (1/1), C25H21N2O2
- Crystal structure of bis(2-((E)-5-chloro-2-hydroxybenzylidene)hydrazineyl)methaniminium trifluoroacetate dihydrate, C34H36Cl4N10O12
- Crystal structure of 1-cyclopropyl-7-ethoxy-6,8-difluoro-4-oxo-1,4-dihydro-quinoline-3-carboxylic acid, C15H13F2NO4
- Crystal structure of methyl 3-(1H-naphtho[1,8-de][1,3,2]diazaborinin-2(3H)-yl)benzoate, C18H15BN2O2
- Crystal structure of (E)-N′-(2-chloro-6-hydroxybenzylidene)-2-hydroxybenzohydrazide, C14H11ClN2O3
- Crystal structure of Al-rich fluorophlogopite, K1.0(Mg2.8Al0.2)(Si2.8Al1.2)O10F2
- The crystal structure of 4,5-diiodo-1,3-dimesityl-1H-1,2,3-triazol-3-ium hexafluoridoantimonate(V), C20H22F6I2N3Sb
- Crystal structure of tris(3-iodopyridin-1-ium) catena-poly[(hexachlorido-κ1 Cl)-(μ2-trichlorido-κ2 Cl:Cl)diantimony(III)], C15H15Cl9I3N3Sb2
- Crystal structure of methyl 2-(1H-naphtho[1,8-de][1.3.2]diazaborinin-2(3H-yl)benzoate C18H15BN2O2
- The crystal structure of 1,8-bis(4-methoxybenzoyl)naphthalene-2,7-diyl dibenzoate, C40H28O8
- Crystal structure of 2-bromo-1,3,6,8-tetramethylBOPHY (BOPHY = bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl)methylene)hydrazine), C14H15B2BrF4N4
- The crystal structure of (E)-3-chloro-2-(2-(2-fluorobenzylidene)hydrazinyl)pyridine, C12H9ClFN3
- Crystal structure of bis(µ2- 4-iodopyridine-2,6-dicarboxylato-κ3O:N:O′)-bis(4-iodopyridine-2,6-dicarboxylato-κ3O:N:O′)-bis(µ2-1-(4-pyridyl)piperazine-κ2N:N′)-hexa-aqua-tetra-copper(II), C46H46Cu4I4N10O22
- Crystal structure of poly[diaqua-(μ2-2,5-dihydroxyterephthalato-κ2O:O′)(μ2-bis(4-pyridylformyl)piperazine-κ2N:N′)cadmium(II)] dihydrate, C24H28CdN4O12
- Crystal structure of poly[aqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)-(μ3-2,3,5,6-tetrafluoroterephthalato-κ3O:O′:O′′)cadmium(II)], C17H14N4O5F4Cd
- Crystal structure of 6-(quinolin-8-yl)benzo[a]phenanthridin-5(6H)-one, C26H16N2O
- The crystal structure of aqua-bis(6-chloropicolinato-κ2N,O)copper(II), C12H8Cl2N2O5Cu
- Crystal structure of catena-poly[diaqua-bis(μ2-4,4′-bipyridyl-κ2N:N′) disilver(I)] 4-oxidopyridine-3-sulfonate trihydrate, C25H29Ag2N5O9S
- The crystal structure of 4-(3-bromophenyl)pyrimidin-2-amine, C10H8BrN3
- Crystal structure of 6-oxo-4-phenyl-1-propyl-1,6-dihydropyridine-3-carbonitrile, C15H14N2O
- Crystal structure of 4-(2,2-difluoroethyl)-2,4-dimethyl-6-(trifluoromethyl)isoquinoline-1,3(2H,4H)-dione, C14H12F5NO2
- Crystal structure of dibromido-(1-methyl-1H-imidazole-κ1N)-(3-(3-methyl-1H-imidazol-3-ium-1-yl)propanoato-κ1O)zinc(II), C11H16Br2N4O2Zn
- The crystal structure of 1,1′-(((2 (dimethylamino)ethyl)azanediyl)bis(methylene)) bis(naphthalen-2-olato-κ4 N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)- titanium(IV) ─ dichloromethane (2/1), C33H29N3O6Ti
- The layered crystal structure of bis(theophyllinium) hexachloridostannate (IV), C14H18N8O8SnCl6
- The crystal structre of 3-(1-ethenyl-1H-imidazol-3-ium-3-yl)propane-1-sulfonate, C8H12N2O3S
- Synthesis and crystal structure of di-tert-butyl 1″-acetyl-2,2″,9′-trioxo-4a′,9a′-dihydro-1′H,3′H,9′H-dispiro[indoline-3,2′-xanthene-4′,3″-indoline]-1,3′-dicarboxylate, C39H38N2O9
- The crystal structure of 4-chloro-2-(quinolin-8-yl)isoindoline-1,3-dione, C17H9ClN2O2
- The crystal structure of 1-fluoro-4-(p-tolylethynyl)benzene, C15H11F
- The crystal structure of bis[4-bromo-2-(1H-pyrazol-3-yl) phenolato-κ2N,O] copper(II), C18H12Br2CuN4O2
- The crystal structure of poly[(μ 3-imidazolato-κ 3 N:N:N′)(tetrahydrofuran- κ 1 O)lithium(I)], C7H11LiN2O
- Crystal structure of N′,N′′′-((1E,1′E)-(propane-2,2-diylbis(1H-pyrrole-5,2diyl))bis(methaneylylidene))di(nicotinohydrazide) pentahydrate, C25H24N8O2·5H2O
- Crystal structure of 3-(2-ethoxy-2-oxoethyl)-1-ethyl-1H-imidazol-3-ium hexafluoridophos-phate(V), C9H15F6N2O2P
- Crystal structure of (1,10-phenanthroline-κ2N,N′)-bis(3-thiophenecarboxylato-κ2O,O′)copper(II), C22H14N2O4S2Cu
- The crystal structure of 2-amino-3-carboxypyridin-1-ium iodide hemihydrate, C6H8IN2O2.5
- Crystal structure of (E)-7-methoxy-2-((6-methoxypyridin-2-yl)methylene)-tetralone, C18H17NO3
- The crystal structure of [μ-hydroxido-bis[(5,5′-dimethyl-2,2′-bipyridine-κ2N,N′)-tricarbonylrhenium(I)] bromide hemihydrate, C30H26N4O9Re2Br
- The crystal structure of 2,5-bis(3,5-dimethylphenyl)thiazolo[5,4-d]thiazole, C20H18N2S2
- The crystal structure of 5-benzoyl-1-[(E)-(4-fluorobenzylidene)amino]-4-phenylpyrimidin-2(1H)-one, C24H16FN3O2
- Crystal structure of monocarbonyl(N-nitroso-N-oxido-phenylamine-κ 2 O,O′)(tricyclohexylphosphine-κP)rhodium(I), C25H39N2O3PRh
- Crystal structure of poly[bis[μ3-1,3,5-tris[(1H-imidazol-1-yl)methyl]benzene-κ3N:N′:N″]nickel(II)] hexafluorosilicate, C36H36N12NiSiF6
- The crystal structure of 13-(pyrazole-1-yl-4-carbonitrile)-matrine, C19H25N5O
- Crystal structure of 3,5-bis((E)-4-methoxy-2-(trifluoromethyl)benzylidene)-1-methylpiperidin-4-one, C24H21F6NO3
- The crystal structure of N,N′-(Disulfanediyldi-2,1-phenylene)di(6′-methylpyridine)-2-carboxamide, C26H22N4O2S2
- Crystal structure of (E)-7-fluoro-2-(4-methoxy-2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C19H14F4O2
- Crystal structure of ethyl 1-(4-fluorophenyl)-4-phenyl-1H-pyrrole-3-carboxylate, C19H16FNO2
- The crystal structure of cis-diaqua-bis (N-butyl-N-(pyridin-2-yl)pyridin-2-amine-κ2N,N′)cobalt(II)] dichloride trihydrate, C28H44Cl2N6O5Co
- Crystal structure of (E)-7-methoxy-2-((6-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C18H17NO3
- Crystal structure of (E)-2-((3-fluoropyridin-4-yl)methylene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- The crystal structure of 6-bromohexanoic acid, C6H11BrO2
- The crystal structure of 4-chloro-thiophenol, C6H5ClS
- The crystal structure of 4-bromobenzyl chloride, C7H6BrCl
- The crystal structure of di-tert-butyl dicarbonate, C10H18O5
- The crystal structure of (2-(4-chlorophenyl)-5-methyl-1,3-dioxan-5-yl)methanol, C12H15ClO3
- The crystal structure of the co-crystal: 2-hydroxybenzoic acid – N′-(butan-2-ylidene)pyridine-4-carbohydrazide, C10H13N3O·C7H6O3
- Crystal structure and anti-inflammatory activity of (E)-7-fluoro-2-((5-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- Crystal structure of (E)-7-fluoro-2-((6-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- Crystal structure of 1,1′-(butane-1,4-diyl)bis(3-propyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C32H56F24N8P4
- The crystal structure of dichlorido-bis(3-methyl-3-imidazolium-1-ylpropionato-κ2)-cadmium(II), C14H20CdCl2N4O4
- Crystal structure of 1-(2-cyanobenzyl)-3-cyano-4-phenyl-4-(2-cyanobenzyl)-1,4-dihydropyridine monohydrate, C56H42N8O
- The crystal structure of 3-(carboxymethyl)-1-ethenyl-1H-imidazol-3-ium chloride, C7H9N2O2Cl
- The crystal structure of adamantylmethoxydiphenylsilane, C23H28OSi
- Redetermination of the crystal structure of (2E,4Z,13E,15Z)-3,5,14,16-tetramethyl-2,6,13,17-tetraazatricyclo[16.4.0.07,12]docosa-1(22),2,4,7,9,11,13,15,18,20-decaene, C22H24N4
- Crystal structure of (E)-7-hydroxy-2-((6-methoxypyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H15NO3
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2 N:N′)cobalt(II)] dinitrate, C18H28N10O8Co
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of [aqua-(4-iodopyridine-2,6-dicarboxylato-κ3 O,N,O′)-(1,10-phenanothroline-κ2 N,N′)copper(II)] dihydrate, C19H16O7N3CuI
- The crystal structure of tetrakis(1-isopropyl-1H-imidazolium) octamolybdate, C24H44Mo8N8O26
- Crystal structure of catena-poly[bis(µ2-3,5-bis(1-imidazolyl)pyridine-κ2 N:N′)-(µ2-3-nitrophthalato-k3 O,O′:O″)cadmium(II)] dihydrate, C30H25N11O8Cd
- The crystal structure of diaqua-bis(2-(3-(1H-pyrazol-4-yl)-1H-1,2,4-triazol-5-yl)pyridine-κ2 N:N′)-bis(3,5-dicarboxybenzoato-κ1 O)cobalt(II), C38H30CoN12O14
- Crystal structure of the nickel(II) complex aqua-(2,6-di(pyrazin-2-yl)-4,4′-bipyridine-κ3 N,N′,N′′)-(phthalato-κ2 O,O′)nickel(II) tetrahydrate, C26H26N6O9Ni
- The crystal structure of 1-[5-(2-fluorophenyl)-1-(pyridine-3-sulfonyl)-1H-pyrrol-3-yl]-N-methylmethanaminium 3-carboxyprop-2-enoate, C21H20FN3O6S
- The crystal structure of 1,2-bis(4-pyridyl)ethane - 4,4-dihydroxydiphenylmethane (1/1), C25H21N2O2
- Crystal structure of bis(2-((E)-5-chloro-2-hydroxybenzylidene)hydrazineyl)methaniminium trifluoroacetate dihydrate, C34H36Cl4N10O12
- Crystal structure of 1-cyclopropyl-7-ethoxy-6,8-difluoro-4-oxo-1,4-dihydro-quinoline-3-carboxylic acid, C15H13F2NO4
- Crystal structure of methyl 3-(1H-naphtho[1,8-de][1,3,2]diazaborinin-2(3H)-yl)benzoate, C18H15BN2O2
- Crystal structure of (E)-N′-(2-chloro-6-hydroxybenzylidene)-2-hydroxybenzohydrazide, C14H11ClN2O3
- Crystal structure of Al-rich fluorophlogopite, K1.0(Mg2.8Al0.2)(Si2.8Al1.2)O10F2
- The crystal structure of 4,5-diiodo-1,3-dimesityl-1H-1,2,3-triazol-3-ium hexafluoridoantimonate(V), C20H22F6I2N3Sb
- Crystal structure of tris(3-iodopyridin-1-ium) catena-poly[(hexachlorido-κ1 Cl)-(μ2-trichlorido-κ2 Cl:Cl)diantimony(III)], C15H15Cl9I3N3Sb2
- Crystal structure of methyl 2-(1H-naphtho[1,8-de][1.3.2]diazaborinin-2(3H-yl)benzoate C18H15BN2O2
- The crystal structure of 1,8-bis(4-methoxybenzoyl)naphthalene-2,7-diyl dibenzoate, C40H28O8
- Crystal structure of 2-bromo-1,3,6,8-tetramethylBOPHY (BOPHY = bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl)methylene)hydrazine), C14H15B2BrF4N4
- The crystal structure of (E)-3-chloro-2-(2-(2-fluorobenzylidene)hydrazinyl)pyridine, C12H9ClFN3
- Crystal structure of bis(µ2- 4-iodopyridine-2,6-dicarboxylato-κ3O:N:O′)-bis(4-iodopyridine-2,6-dicarboxylato-κ3O:N:O′)-bis(µ2-1-(4-pyridyl)piperazine-κ2N:N′)-hexa-aqua-tetra-copper(II), C46H46Cu4I4N10O22
- Crystal structure of poly[diaqua-(μ2-2,5-dihydroxyterephthalato-κ2O:O′)(μ2-bis(4-pyridylformyl)piperazine-κ2N:N′)cadmium(II)] dihydrate, C24H28CdN4O12
- Crystal structure of poly[aqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)-(μ3-2,3,5,6-tetrafluoroterephthalato-κ3O:O′:O′′)cadmium(II)], C17H14N4O5F4Cd
- Crystal structure of 6-(quinolin-8-yl)benzo[a]phenanthridin-5(6H)-one, C26H16N2O
- The crystal structure of aqua-bis(6-chloropicolinato-κ2N,O)copper(II), C12H8Cl2N2O5Cu
- Crystal structure of catena-poly[diaqua-bis(μ2-4,4′-bipyridyl-κ2N:N′) disilver(I)] 4-oxidopyridine-3-sulfonate trihydrate, C25H29Ag2N5O9S
- The crystal structure of 4-(3-bromophenyl)pyrimidin-2-amine, C10H8BrN3
- Crystal structure of 6-oxo-4-phenyl-1-propyl-1,6-dihydropyridine-3-carbonitrile, C15H14N2O
- Crystal structure of 4-(2,2-difluoroethyl)-2,4-dimethyl-6-(trifluoromethyl)isoquinoline-1,3(2H,4H)-dione, C14H12F5NO2
- Crystal structure of dibromido-(1-methyl-1H-imidazole-κ1N)-(3-(3-methyl-1H-imidazol-3-ium-1-yl)propanoato-κ1O)zinc(II), C11H16Br2N4O2Zn
- The crystal structure of 1,1′-(((2 (dimethylamino)ethyl)azanediyl)bis(methylene)) bis(naphthalen-2-olato-κ4 N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)- titanium(IV) ─ dichloromethane (2/1), C33H29N3O6Ti
- The layered crystal structure of bis(theophyllinium) hexachloridostannate (IV), C14H18N8O8SnCl6
- The crystal structre of 3-(1-ethenyl-1H-imidazol-3-ium-3-yl)propane-1-sulfonate, C8H12N2O3S
- Synthesis and crystal structure of di-tert-butyl 1″-acetyl-2,2″,9′-trioxo-4a′,9a′-dihydro-1′H,3′H,9′H-dispiro[indoline-3,2′-xanthene-4′,3″-indoline]-1,3′-dicarboxylate, C39H38N2O9
- The crystal structure of 4-chloro-2-(quinolin-8-yl)isoindoline-1,3-dione, C17H9ClN2O2
- The crystal structure of 1-fluoro-4-(p-tolylethynyl)benzene, C15H11F
- The crystal structure of bis[4-bromo-2-(1H-pyrazol-3-yl) phenolato-κ2N,O] copper(II), C18H12Br2CuN4O2
- The crystal structure of poly[(μ 3-imidazolato-κ 3 N:N:N′)(tetrahydrofuran- κ 1 O)lithium(I)], C7H11LiN2O
- Crystal structure of N′,N′′′-((1E,1′E)-(propane-2,2-diylbis(1H-pyrrole-5,2diyl))bis(methaneylylidene))di(nicotinohydrazide) pentahydrate, C25H24N8O2·5H2O
- Crystal structure of 3-(2-ethoxy-2-oxoethyl)-1-ethyl-1H-imidazol-3-ium hexafluoridophos-phate(V), C9H15F6N2O2P
- Crystal structure of (1,10-phenanthroline-κ2N,N′)-bis(3-thiophenecarboxylato-κ2O,O′)copper(II), C22H14N2O4S2Cu
- The crystal structure of 2-amino-3-carboxypyridin-1-ium iodide hemihydrate, C6H8IN2O2.5
- Crystal structure of (E)-7-methoxy-2-((6-methoxypyridin-2-yl)methylene)-tetralone, C18H17NO3
- The crystal structure of [μ-hydroxido-bis[(5,5′-dimethyl-2,2′-bipyridine-κ2N,N′)-tricarbonylrhenium(I)] bromide hemihydrate, C30H26N4O9Re2Br
- The crystal structure of 2,5-bis(3,5-dimethylphenyl)thiazolo[5,4-d]thiazole, C20H18N2S2
- The crystal structure of 5-benzoyl-1-[(E)-(4-fluorobenzylidene)amino]-4-phenylpyrimidin-2(1H)-one, C24H16FN3O2
- Crystal structure of monocarbonyl(N-nitroso-N-oxido-phenylamine-κ 2 O,O′)(tricyclohexylphosphine-κP)rhodium(I), C25H39N2O3PRh
- Crystal structure of poly[bis[μ3-1,3,5-tris[(1H-imidazol-1-yl)methyl]benzene-κ3N:N′:N″]nickel(II)] hexafluorosilicate, C36H36N12NiSiF6
- The crystal structure of 13-(pyrazole-1-yl-4-carbonitrile)-matrine, C19H25N5O
- Crystal structure of 3,5-bis((E)-4-methoxy-2-(trifluoromethyl)benzylidene)-1-methylpiperidin-4-one, C24H21F6NO3
- The crystal structure of N,N′-(Disulfanediyldi-2,1-phenylene)di(6′-methylpyridine)-2-carboxamide, C26H22N4O2S2
- Crystal structure of (E)-7-fluoro-2-(4-methoxy-2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C19H14F4O2
- Crystal structure of ethyl 1-(4-fluorophenyl)-4-phenyl-1H-pyrrole-3-carboxylate, C19H16FNO2
- The crystal structure of cis-diaqua-bis (N-butyl-N-(pyridin-2-yl)pyridin-2-amine-κ2N,N′)cobalt(II)] dichloride trihydrate, C28H44Cl2N6O5Co
- Crystal structure of (E)-7-methoxy-2-((6-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C18H17NO3
- Crystal structure of (E)-2-((3-fluoropyridin-4-yl)methylene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- The crystal structure of 6-bromohexanoic acid, C6H11BrO2
- The crystal structure of 4-chloro-thiophenol, C6H5ClS
- The crystal structure of 4-bromobenzyl chloride, C7H6BrCl
- The crystal structure of di-tert-butyl dicarbonate, C10H18O5
- The crystal structure of (2-(4-chlorophenyl)-5-methyl-1,3-dioxan-5-yl)methanol, C12H15ClO3
- The crystal structure of the co-crystal: 2-hydroxybenzoic acid – N′-(butan-2-ylidene)pyridine-4-carbohydrazide, C10H13N3O·C7H6O3
- Crystal structure and anti-inflammatory activity of (E)-7-fluoro-2-((5-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- Crystal structure of (E)-7-fluoro-2-((6-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- Crystal structure of 1,1′-(butane-1,4-diyl)bis(3-propyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C32H56F24N8P4
- The crystal structure of dichlorido-bis(3-methyl-3-imidazolium-1-ylpropionato-κ2)-cadmium(II), C14H20CdCl2N4O4
- Crystal structure of 1-(2-cyanobenzyl)-3-cyano-4-phenyl-4-(2-cyanobenzyl)-1,4-dihydropyridine monohydrate, C56H42N8O
- The crystal structure of 3-(carboxymethyl)-1-ethenyl-1H-imidazol-3-ium chloride, C7H9N2O2Cl
- The crystal structure of adamantylmethoxydiphenylsilane, C23H28OSi
- Redetermination of the crystal structure of (2E,4Z,13E,15Z)-3,5,14,16-tetramethyl-2,6,13,17-tetraazatricyclo[16.4.0.07,12]docosa-1(22),2,4,7,9,11,13,15,18,20-decaene, C22H24N4
- Crystal structure of (E)-7-hydroxy-2-((6-methoxypyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H15NO3
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2 N:N′)cobalt(II)] dinitrate, C18H28N10O8Co

