Abstract
C6H5ClS, monoclinic, P21/n (no. 14), a = 5.7461(2) Å, b = 9.7601(4) Å, c = 5.7466(3) Å, β = 95.869(2)°, V = 320.59(2) Å3, Z = 2, R gt (F) = 0.0216, wRref(F2) = 0.0545, T = 200 K.
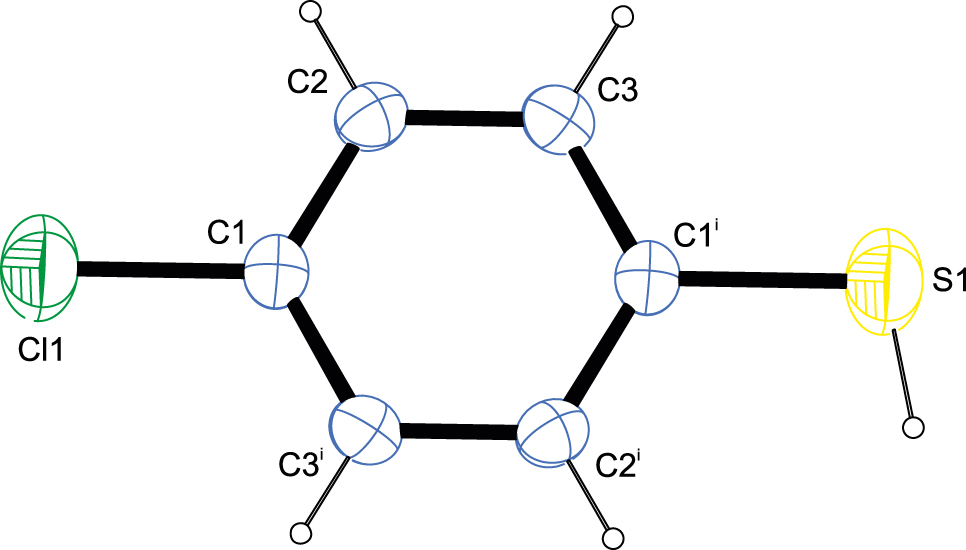
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.55 × 0.33 × 0.16 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.80 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| θmax, completeness: | 28.3°, 99% |
| N(hkl)measured, N(hkl)unique, Rint: | 2752, 795, 0.012 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 740 |
| N(param)refined: | 54 |
| Programs: | Bruker [1, 2], SHELX [3], WinGX/ORTEP [4], Mercury [5], PLATON [6] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1 | 0.37593 (19) | 0.59088 (10) | 0.62410 (19) | 0.0267 (2) |
| C2 | 0.28114 (19) | 0.54565 (11) | 0.40579 (19) | 0.0292 (3) |
| H2 | 0.134751 | 0.576427 | 0.343306 | 0.035* |
| C3 | 0.40571 (19) | 0.45433 (11) | 0.28115 (19) | 0.0291 (3) |
| H3 | 0.343171 | 0.423547 | 0.134799 | 0.035* |
| Cl1a | 0.2180 (4) | 0.7058 (2) | 0.7821 (3) | 0.0273 (5) |
| S1a | 0.2173 (7) | 0.7058 (4) | 0.7826 (6) | 0.0556 (13) |
| H1Ab | 0.354 (12) | 0.724 (6) | 0.942 (12) | 0.037 (15)* |
| H1Bb | 0.044 (11) | 0.716 (6) | 0.661 (11) | 0.030 (14)* |
-
aOccupancy: 0.5, bOccupancy: 0.25.
Source of material
The compound was obtained commercially (Fluka). Crystals suitable for the diffraction study were obtained upon repeated slow sublimation and resublimation of the compound in a closed vessel kept at room temperature.
Experimental details
Carbon-bound H atoms were placed in calculated positions (C–H 0.95 Å) and were included in the refinement in the riding model approximation, with U(H) set to 1.2Ueq(C).
The sulfur-bound H atom was located on a difference Fourier map and refined freely. It shows orientational disorder over two positions.
The molecule shows pseudo-centrosymmetry due to the almost identical number of electrons for sulfur and chlorine.
Comment
While phenol and its core-substituted derivatives as well as the pertaining deprotonated species have received considerable attention as ligands in main group and transition metal chemistry and found to exhibit a variety of coordination modes (e.g., monodentate towards tin [7], bidentate towards tantalum [8], bridging tridentate towards zinc [9, 10] and bridging tetradentate towards sodium [11]) considerable less information is at hand for comparable thiophenols. It is especially surprising to find that structural information about the simplest thiophenol derivatives is scant and limited to, e.g., thiophenol as such [12] as well as the 4-methyl-substituted [13] and perchlorinated derivatives [14]. In continuation of our interest in halogenated derivatives of phenols and thiophenols [15, 16] we determined the molecular and crystal structure of the title compound.
The title compound is the para-chlorinated derivative of thiophenol. Due to the similarity in the number of electrons for the two heteroatoms, the molecule shows pseudo-centrosymmetry with the chlorine and sulfur atom sites being superimposed. The C–S bond length of 1.758(4) Å as well as the C–Cl bond length of 1.753(2) Å are in very good agreement with the values reported for the corresponding bonds in the Cambridge Structural Database [17] for two other thiophenol derivatives featuring this bonding pattern [18, 19]. The C–C–C angles cover a narrow range of 119.69(10)–120.61(10)° only. The hydrogen atom of the thiol group shows orientational disorder over two positions.
In the crystal, hydrogen bonds supported by the thiol group can be observed. The superposition of the two heteratoms on grounds of the pseudo-centrosymmetric nature of the title compound as well as the orientational disorder of the sulfur-bound hydrogen atom allows for the interpretation of these hydrogen bonds of being either of the S–H···Cl type or the cooperative S–H···S type. While both interpretations see the molecules connected to infinite chains along [1 0 1], a graph-set analysis [20, 21] would require a C11(2) descriptor at the unitary level in case of the cooperative interpretation and a C11(7) descriptor at the unitary level in case of the chlorine atom acting as acceptor.
While one could discuss the presence of dispersive interactions between the halogen atom and the aromatic system, π-stacking is not a prominent feature in the crystal structure of the title compound.
Funding source: National Research Foundation
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This study was funded by the National Research Foundation.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. BRUKER. APEX2 and SAINT; Bruker AXS Inc.: Madison, Wisconsin, USA, 2012.Suche in Google Scholar
2. Bruker. SADABS; Bruker AXS Inc.; Madison, Wisconsin, USA, 2008.Suche in Google Scholar
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2008, C71, 3–8; https://doi.org/10.1107/s0108767307043930.Suche in Google Scholar PubMed
4. Farrugia, L. J. WinGX and ORTEP for Windows: an update. J. Appl. Crystallogr. 2012, 45, 849–854; https://doi.org/10.1107/s0021889812029111.Suche in Google Scholar
5. Macrae, C. F., Sovago, I., Cottrell, S. J., Galek, P. T. A., McCabe, P., Pidcock, E., Platings, M., Platings, G. P., Stevens, J. S., Towler, M., Wood, P. A. Mercury 4.0: from visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 63, 226–235; https://doi.org/10.1107/s0021889807067908.Suche in Google Scholar
6. Spek, A. L. Structure validation in chemical crystallography. Acta Crystallogr. 2009, D65, 148–155; https://doi.org/10.1107/s090744490804362x.Suche in Google Scholar
7. Chernov, O. V., Smirnov, A. Y., Portnyagin, I. A., Khrustalev, V. N., Nechaev, M. S. Heteroleptic tin (II) dialkoxides stabilized by intramolecular coordination Sn(OCH2CH2NMe2)(OR) (R = Me, Et, iPr, tBu, Ph). Synthesis, structure and catalytic activity in polyurethane synthesis. J. Organomet. Chem. 2009, 694, 3184–3189; https://doi.org/10.1016/j.jorganchem.2009.05.014.Suche in Google Scholar
8. Bortoluzzi, M., Guazzelli, N., Marchetti, F., Pampaloni, G., Zacchini, S. Convenient synthesis of fluoride-alkoxides of Nb(V) and Ta(V): a spectroscopic, crystallographic and computational study. Dalton Trans. 2012, 41, 12898–12906; https://doi.org/10.1039/c2dt31453c.Suche in Google Scholar PubMed
9. Boersma, J., Spek, A. L., Noltes, J. G. Coordination complexes of bis(2,2-dimethyl-3,5-hexanedionato) zinc with organozinc-oxygen and -nitrogen compounds. crystal structure of the complex formed with phenylzinc phenoxide. J. Organomet. Chem. 1974, 81, 7–15; https://doi.org/10.1016/s0022-328x(00)87880-8.Suche in Google Scholar
10. Enthaler, S., Eckhardt, B., Inoue, S., Irran, E., Driess, M. Facile and efficient reduction of ketones in the presence of zinc catalysts modified by phenol ligands. Chem. Asian J. 2010, 5, 2027–2035; https://doi.org/10.1002/asia.201000317.Suche in Google Scholar PubMed
11. Kunert, M., Dinjus, E., Nauck, M., Sieler, J. Structure and reactivity of sodium phenoxide - following the course of the Kolbe–Schmitt reaction. Chem. Ber. 1997, 130, 1461–1465; https://doi.org/10.1002/cber.19971301017.Suche in Google Scholar
12. Thomas, S. P., Sathishkumar, R., Guru Row, T. N. Organic alloys of room temperature liquids thiophenol and selenophenol. Chem. Commun. 2015, 51, 14255–14258; https://doi.org/10.1039/c5cc03322e.Suche in Google Scholar PubMed
13. Forbes, C. R., Sinha, S. K., Ganguly, H. K., Bai, S., Yap, G. P. A., Patel, S., Zondlo, N. J. Insights into thiol–aromatic interactions: a stereoelectronic basis for S–H/π interactions. J. Am. Chem. Soc. 2017, 139, 1842–1855; https://doi.org/10.1021/jacs.6b08415.Suche in Google Scholar PubMed PubMed Central
14. Wojcik, G., Charbonneau, G. P., Delugeard, Y., Toupet, L. The disordered crystal structure of pentachlorothiophenol. Acta Crystallogr. 1980, B36, 506–507; https://doi.org/10.1107/s0567740880003706.Suche in Google Scholar
15. Betz, R., Klüfers, P., Mayer, P. 2,3,4,5,6-Pentabromophenol. Acta Crystallogr. 2008, E64, o1921; https://doi.org/10.1107/s1600536808028602.Suche in Google Scholar PubMed PubMed Central
16. Hosten, E., Betz, R. Redetermination of the crystal structure of 5,7-dibromoquinolin-8-ol, at K – analysis of intermolecular forces, C9H5Br2NO. Z. Kristallogr. NCS. 2014, 229, 285–286; https://doi.org/10.1515/ncrs-2014-0140.Suche in Google Scholar
17. Groom, C. R., Allen, F. H. The Cambridge Structural Database in retrospect and prospect. Angew. Chem. Int. Ed. 2014, 53, 663–671; https://doi.org/10.1515/ncrs-2014-0140.Suche in Google Scholar
18. Ballmann, J., Fuchs, M. G. G., Dechert, S., John, M., Meyer, F. Synthesis and coordination properties of chelating dithiophenolate ligands. Inorg. Chem. 2009, 48, 90–99; https://doi.org/10.1021/ic801285u.Suche in Google Scholar PubMed
19. Trifonov, A. L., Panferova, L. I., Levin, V. V., Volodin, A. D., Korlyukov, A. A., Dilman, A. D. Interaction of difluoromethylene phosphobetaine with heteroatom-centered electrophiles. J. Fluor. Chem. 2019, 220, 78–82; https://doi.org/10.1016/j.jfluchem.2019.02.008.Suche in Google Scholar
20. Bernstein, J., Davis, R. E., Shimoni, L., Chang, N.-L. Patterns in hydrogen bonding: functionality and graph set analysis in crystals. Angew Chem. Int. Ed. Engl. 1995, 34, 1555–1573; https://doi.org/10.1002/anie.199515551.Suche in Google Scholar
21. Etter, M. C., MacDonald, J. C., Bernstein, J. Graph-set analysis of hydrogen-bond patterns in organic crystals. Acta Crystallogr. 1990, B46, 256–262; https://doi.org/10.1107/s0108768189012929.Suche in Google Scholar PubMed
© 2021 Pholani Manana et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of [aqua-(4-iodopyridine-2,6-dicarboxylato-κ3 O,N,O′)-(1,10-phenanothroline-κ2 N,N′)copper(II)] dihydrate, C19H16O7N3CuI
- The crystal structure of tetrakis(1-isopropyl-1H-imidazolium) octamolybdate, C24H44Mo8N8O26
- Crystal structure of catena-poly[bis(µ2-3,5-bis(1-imidazolyl)pyridine-κ2 N:N′)-(µ2-3-nitrophthalato-k3 O,O′:O″)cadmium(II)] dihydrate, C30H25N11O8Cd
- The crystal structure of diaqua-bis(2-(3-(1H-pyrazol-4-yl)-1H-1,2,4-triazol-5-yl)pyridine-κ2 N:N′)-bis(3,5-dicarboxybenzoato-κ1 O)cobalt(II), C38H30CoN12O14
- Crystal structure of the nickel(II) complex aqua-(2,6-di(pyrazin-2-yl)-4,4′-bipyridine-κ3 N,N′,N′′)-(phthalato-κ2 O,O′)nickel(II) tetrahydrate, C26H26N6O9Ni
- The crystal structure of 1-[5-(2-fluorophenyl)-1-(pyridine-3-sulfonyl)-1H-pyrrol-3-yl]-N-methylmethanaminium 3-carboxyprop-2-enoate, C21H20FN3O6S
- The crystal structure of 1,2-bis(4-pyridyl)ethane - 4,4-dihydroxydiphenylmethane (1/1), C25H21N2O2
- Crystal structure of bis(2-((E)-5-chloro-2-hydroxybenzylidene)hydrazineyl)methaniminium trifluoroacetate dihydrate, C34H36Cl4N10O12
- Crystal structure of 1-cyclopropyl-7-ethoxy-6,8-difluoro-4-oxo-1,4-dihydro-quinoline-3-carboxylic acid, C15H13F2NO4
- Crystal structure of methyl 3-(1H-naphtho[1,8-de][1,3,2]diazaborinin-2(3H)-yl)benzoate, C18H15BN2O2
- Crystal structure of (E)-N′-(2-chloro-6-hydroxybenzylidene)-2-hydroxybenzohydrazide, C14H11ClN2O3
- Crystal structure of Al-rich fluorophlogopite, K1.0(Mg2.8Al0.2)(Si2.8Al1.2)O10F2
- The crystal structure of 4,5-diiodo-1,3-dimesityl-1H-1,2,3-triazol-3-ium hexafluoridoantimonate(V), C20H22F6I2N3Sb
- Crystal structure of tris(3-iodopyridin-1-ium) catena-poly[(hexachlorido-κ1 Cl)-(μ2-trichlorido-κ2 Cl:Cl)diantimony(III)], C15H15Cl9I3N3Sb2
- Crystal structure of methyl 2-(1H-naphtho[1,8-de][1.3.2]diazaborinin-2(3H-yl)benzoate C18H15BN2O2
- The crystal structure of 1,8-bis(4-methoxybenzoyl)naphthalene-2,7-diyl dibenzoate, C40H28O8
- Crystal structure of 2-bromo-1,3,6,8-tetramethylBOPHY (BOPHY = bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl)methylene)hydrazine), C14H15B2BrF4N4
- The crystal structure of (E)-3-chloro-2-(2-(2-fluorobenzylidene)hydrazinyl)pyridine, C12H9ClFN3
- Crystal structure of bis(µ2- 4-iodopyridine-2,6-dicarboxylato-κ3O:N:O′)-bis(4-iodopyridine-2,6-dicarboxylato-κ3O:N:O′)-bis(µ2-1-(4-pyridyl)piperazine-κ2N:N′)-hexa-aqua-tetra-copper(II), C46H46Cu4I4N10O22
- Crystal structure of poly[diaqua-(μ2-2,5-dihydroxyterephthalato-κ2O:O′)(μ2-bis(4-pyridylformyl)piperazine-κ2N:N′)cadmium(II)] dihydrate, C24H28CdN4O12
- Crystal structure of poly[aqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)-(μ3-2,3,5,6-tetrafluoroterephthalato-κ3O:O′:O′′)cadmium(II)], C17H14N4O5F4Cd
- Crystal structure of 6-(quinolin-8-yl)benzo[a]phenanthridin-5(6H)-one, C26H16N2O
- The crystal structure of aqua-bis(6-chloropicolinato-κ2N,O)copper(II), C12H8Cl2N2O5Cu
- Crystal structure of catena-poly[diaqua-bis(μ2-4,4′-bipyridyl-κ2N:N′) disilver(I)] 4-oxidopyridine-3-sulfonate trihydrate, C25H29Ag2N5O9S
- The crystal structure of 4-(3-bromophenyl)pyrimidin-2-amine, C10H8BrN3
- Crystal structure of 6-oxo-4-phenyl-1-propyl-1,6-dihydropyridine-3-carbonitrile, C15H14N2O
- Crystal structure of 4-(2,2-difluoroethyl)-2,4-dimethyl-6-(trifluoromethyl)isoquinoline-1,3(2H,4H)-dione, C14H12F5NO2
- Crystal structure of dibromido-(1-methyl-1H-imidazole-κ1N)-(3-(3-methyl-1H-imidazol-3-ium-1-yl)propanoato-κ1O)zinc(II), C11H16Br2N4O2Zn
- The crystal structure of 1,1′-(((2 (dimethylamino)ethyl)azanediyl)bis(methylene)) bis(naphthalen-2-olato-κ4 N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)- titanium(IV) ─ dichloromethane (2/1), C33H29N3O6Ti
- The layered crystal structure of bis(theophyllinium) hexachloridostannate (IV), C14H18N8O8SnCl6
- The crystal structre of 3-(1-ethenyl-1H-imidazol-3-ium-3-yl)propane-1-sulfonate, C8H12N2O3S
- Synthesis and crystal structure of di-tert-butyl 1″-acetyl-2,2″,9′-trioxo-4a′,9a′-dihydro-1′H,3′H,9′H-dispiro[indoline-3,2′-xanthene-4′,3″-indoline]-1,3′-dicarboxylate, C39H38N2O9
- The crystal structure of 4-chloro-2-(quinolin-8-yl)isoindoline-1,3-dione, C17H9ClN2O2
- The crystal structure of 1-fluoro-4-(p-tolylethynyl)benzene, C15H11F
- The crystal structure of bis[4-bromo-2-(1H-pyrazol-3-yl) phenolato-κ2N,O] copper(II), C18H12Br2CuN4O2
- The crystal structure of poly[(μ 3-imidazolato-κ 3 N:N:N′)(tetrahydrofuran- κ 1 O)lithium(I)], C7H11LiN2O
- Crystal structure of N′,N′′′-((1E,1′E)-(propane-2,2-diylbis(1H-pyrrole-5,2diyl))bis(methaneylylidene))di(nicotinohydrazide) pentahydrate, C25H24N8O2·5H2O
- Crystal structure of 3-(2-ethoxy-2-oxoethyl)-1-ethyl-1H-imidazol-3-ium hexafluoridophos-phate(V), C9H15F6N2O2P
- Crystal structure of (1,10-phenanthroline-κ2N,N′)-bis(3-thiophenecarboxylato-κ2O,O′)copper(II), C22H14N2O4S2Cu
- The crystal structure of 2-amino-3-carboxypyridin-1-ium iodide hemihydrate, C6H8IN2O2.5
- Crystal structure of (E)-7-methoxy-2-((6-methoxypyridin-2-yl)methylene)-tetralone, C18H17NO3
- The crystal structure of [μ-hydroxido-bis[(5,5′-dimethyl-2,2′-bipyridine-κ2N,N′)-tricarbonylrhenium(I)] bromide hemihydrate, C30H26N4O9Re2Br
- The crystal structure of 2,5-bis(3,5-dimethylphenyl)thiazolo[5,4-d]thiazole, C20H18N2S2
- The crystal structure of 5-benzoyl-1-[(E)-(4-fluorobenzylidene)amino]-4-phenylpyrimidin-2(1H)-one, C24H16FN3O2
- Crystal structure of monocarbonyl(N-nitroso-N-oxido-phenylamine-κ 2 O,O′)(tricyclohexylphosphine-κP)rhodium(I), C25H39N2O3PRh
- Crystal structure of poly[bis[μ3-1,3,5-tris[(1H-imidazol-1-yl)methyl]benzene-κ3N:N′:N″]nickel(II)] hexafluorosilicate, C36H36N12NiSiF6
- The crystal structure of 13-(pyrazole-1-yl-4-carbonitrile)-matrine, C19H25N5O
- Crystal structure of 3,5-bis((E)-4-methoxy-2-(trifluoromethyl)benzylidene)-1-methylpiperidin-4-one, C24H21F6NO3
- The crystal structure of N,N′-(Disulfanediyldi-2,1-phenylene)di(6′-methylpyridine)-2-carboxamide, C26H22N4O2S2
- Crystal structure of (E)-7-fluoro-2-(4-methoxy-2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C19H14F4O2
- Crystal structure of ethyl 1-(4-fluorophenyl)-4-phenyl-1H-pyrrole-3-carboxylate, C19H16FNO2
- The crystal structure of cis-diaqua-bis (N-butyl-N-(pyridin-2-yl)pyridin-2-amine-κ2N,N′)cobalt(II)] dichloride trihydrate, C28H44Cl2N6O5Co
- Crystal structure of (E)-7-methoxy-2-((6-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C18H17NO3
- Crystal structure of (E)-2-((3-fluoropyridin-4-yl)methylene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- The crystal structure of 6-bromohexanoic acid, C6H11BrO2
- The crystal structure of 4-chloro-thiophenol, C6H5ClS
- The crystal structure of 4-bromobenzyl chloride, C7H6BrCl
- The crystal structure of di-tert-butyl dicarbonate, C10H18O5
- The crystal structure of (2-(4-chlorophenyl)-5-methyl-1,3-dioxan-5-yl)methanol, C12H15ClO3
- The crystal structure of the co-crystal: 2-hydroxybenzoic acid – N′-(butan-2-ylidene)pyridine-4-carbohydrazide, C10H13N3O·C7H6O3
- Crystal structure and anti-inflammatory activity of (E)-7-fluoro-2-((5-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- Crystal structure of (E)-7-fluoro-2-((6-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- Crystal structure of 1,1′-(butane-1,4-diyl)bis(3-propyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C32H56F24N8P4
- The crystal structure of dichlorido-bis(3-methyl-3-imidazolium-1-ylpropionato-κ2)-cadmium(II), C14H20CdCl2N4O4
- Crystal structure of 1-(2-cyanobenzyl)-3-cyano-4-phenyl-4-(2-cyanobenzyl)-1,4-dihydropyridine monohydrate, C56H42N8O
- The crystal structure of 3-(carboxymethyl)-1-ethenyl-1H-imidazol-3-ium chloride, C7H9N2O2Cl
- The crystal structure of adamantylmethoxydiphenylsilane, C23H28OSi
- Redetermination of the crystal structure of (2E,4Z,13E,15Z)-3,5,14,16-tetramethyl-2,6,13,17-tetraazatricyclo[16.4.0.07,12]docosa-1(22),2,4,7,9,11,13,15,18,20-decaene, C22H24N4
- Crystal structure of (E)-7-hydroxy-2-((6-methoxypyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H15NO3
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2 N:N′)cobalt(II)] dinitrate, C18H28N10O8Co
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of [aqua-(4-iodopyridine-2,6-dicarboxylato-κ3 O,N,O′)-(1,10-phenanothroline-κ2 N,N′)copper(II)] dihydrate, C19H16O7N3CuI
- The crystal structure of tetrakis(1-isopropyl-1H-imidazolium) octamolybdate, C24H44Mo8N8O26
- Crystal structure of catena-poly[bis(µ2-3,5-bis(1-imidazolyl)pyridine-κ2 N:N′)-(µ2-3-nitrophthalato-k3 O,O′:O″)cadmium(II)] dihydrate, C30H25N11O8Cd
- The crystal structure of diaqua-bis(2-(3-(1H-pyrazol-4-yl)-1H-1,2,4-triazol-5-yl)pyridine-κ2 N:N′)-bis(3,5-dicarboxybenzoato-κ1 O)cobalt(II), C38H30CoN12O14
- Crystal structure of the nickel(II) complex aqua-(2,6-di(pyrazin-2-yl)-4,4′-bipyridine-κ3 N,N′,N′′)-(phthalato-κ2 O,O′)nickel(II) tetrahydrate, C26H26N6O9Ni
- The crystal structure of 1-[5-(2-fluorophenyl)-1-(pyridine-3-sulfonyl)-1H-pyrrol-3-yl]-N-methylmethanaminium 3-carboxyprop-2-enoate, C21H20FN3O6S
- The crystal structure of 1,2-bis(4-pyridyl)ethane - 4,4-dihydroxydiphenylmethane (1/1), C25H21N2O2
- Crystal structure of bis(2-((E)-5-chloro-2-hydroxybenzylidene)hydrazineyl)methaniminium trifluoroacetate dihydrate, C34H36Cl4N10O12
- Crystal structure of 1-cyclopropyl-7-ethoxy-6,8-difluoro-4-oxo-1,4-dihydro-quinoline-3-carboxylic acid, C15H13F2NO4
- Crystal structure of methyl 3-(1H-naphtho[1,8-de][1,3,2]diazaborinin-2(3H)-yl)benzoate, C18H15BN2O2
- Crystal structure of (E)-N′-(2-chloro-6-hydroxybenzylidene)-2-hydroxybenzohydrazide, C14H11ClN2O3
- Crystal structure of Al-rich fluorophlogopite, K1.0(Mg2.8Al0.2)(Si2.8Al1.2)O10F2
- The crystal structure of 4,5-diiodo-1,3-dimesityl-1H-1,2,3-triazol-3-ium hexafluoridoantimonate(V), C20H22F6I2N3Sb
- Crystal structure of tris(3-iodopyridin-1-ium) catena-poly[(hexachlorido-κ1 Cl)-(μ2-trichlorido-κ2 Cl:Cl)diantimony(III)], C15H15Cl9I3N3Sb2
- Crystal structure of methyl 2-(1H-naphtho[1,8-de][1.3.2]diazaborinin-2(3H-yl)benzoate C18H15BN2O2
- The crystal structure of 1,8-bis(4-methoxybenzoyl)naphthalene-2,7-diyl dibenzoate, C40H28O8
- Crystal structure of 2-bromo-1,3,6,8-tetramethylBOPHY (BOPHY = bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl)methylene)hydrazine), C14H15B2BrF4N4
- The crystal structure of (E)-3-chloro-2-(2-(2-fluorobenzylidene)hydrazinyl)pyridine, C12H9ClFN3
- Crystal structure of bis(µ2- 4-iodopyridine-2,6-dicarboxylato-κ3O:N:O′)-bis(4-iodopyridine-2,6-dicarboxylato-κ3O:N:O′)-bis(µ2-1-(4-pyridyl)piperazine-κ2N:N′)-hexa-aqua-tetra-copper(II), C46H46Cu4I4N10O22
- Crystal structure of poly[diaqua-(μ2-2,5-dihydroxyterephthalato-κ2O:O′)(μ2-bis(4-pyridylformyl)piperazine-κ2N:N′)cadmium(II)] dihydrate, C24H28CdN4O12
- Crystal structure of poly[aqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)-(μ3-2,3,5,6-tetrafluoroterephthalato-κ3O:O′:O′′)cadmium(II)], C17H14N4O5F4Cd
- Crystal structure of 6-(quinolin-8-yl)benzo[a]phenanthridin-5(6H)-one, C26H16N2O
- The crystal structure of aqua-bis(6-chloropicolinato-κ2N,O)copper(II), C12H8Cl2N2O5Cu
- Crystal structure of catena-poly[diaqua-bis(μ2-4,4′-bipyridyl-κ2N:N′) disilver(I)] 4-oxidopyridine-3-sulfonate trihydrate, C25H29Ag2N5O9S
- The crystal structure of 4-(3-bromophenyl)pyrimidin-2-amine, C10H8BrN3
- Crystal structure of 6-oxo-4-phenyl-1-propyl-1,6-dihydropyridine-3-carbonitrile, C15H14N2O
- Crystal structure of 4-(2,2-difluoroethyl)-2,4-dimethyl-6-(trifluoromethyl)isoquinoline-1,3(2H,4H)-dione, C14H12F5NO2
- Crystal structure of dibromido-(1-methyl-1H-imidazole-κ1N)-(3-(3-methyl-1H-imidazol-3-ium-1-yl)propanoato-κ1O)zinc(II), C11H16Br2N4O2Zn
- The crystal structure of 1,1′-(((2 (dimethylamino)ethyl)azanediyl)bis(methylene)) bis(naphthalen-2-olato-κ4 N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)- titanium(IV) ─ dichloromethane (2/1), C33H29N3O6Ti
- The layered crystal structure of bis(theophyllinium) hexachloridostannate (IV), C14H18N8O8SnCl6
- The crystal structre of 3-(1-ethenyl-1H-imidazol-3-ium-3-yl)propane-1-sulfonate, C8H12N2O3S
- Synthesis and crystal structure of di-tert-butyl 1″-acetyl-2,2″,9′-trioxo-4a′,9a′-dihydro-1′H,3′H,9′H-dispiro[indoline-3,2′-xanthene-4′,3″-indoline]-1,3′-dicarboxylate, C39H38N2O9
- The crystal structure of 4-chloro-2-(quinolin-8-yl)isoindoline-1,3-dione, C17H9ClN2O2
- The crystal structure of 1-fluoro-4-(p-tolylethynyl)benzene, C15H11F
- The crystal structure of bis[4-bromo-2-(1H-pyrazol-3-yl) phenolato-κ2N,O] copper(II), C18H12Br2CuN4O2
- The crystal structure of poly[(μ 3-imidazolato-κ 3 N:N:N′)(tetrahydrofuran- κ 1 O)lithium(I)], C7H11LiN2O
- Crystal structure of N′,N′′′-((1E,1′E)-(propane-2,2-diylbis(1H-pyrrole-5,2diyl))bis(methaneylylidene))di(nicotinohydrazide) pentahydrate, C25H24N8O2·5H2O
- Crystal structure of 3-(2-ethoxy-2-oxoethyl)-1-ethyl-1H-imidazol-3-ium hexafluoridophos-phate(V), C9H15F6N2O2P
- Crystal structure of (1,10-phenanthroline-κ2N,N′)-bis(3-thiophenecarboxylato-κ2O,O′)copper(II), C22H14N2O4S2Cu
- The crystal structure of 2-amino-3-carboxypyridin-1-ium iodide hemihydrate, C6H8IN2O2.5
- Crystal structure of (E)-7-methoxy-2-((6-methoxypyridin-2-yl)methylene)-tetralone, C18H17NO3
- The crystal structure of [μ-hydroxido-bis[(5,5′-dimethyl-2,2′-bipyridine-κ2N,N′)-tricarbonylrhenium(I)] bromide hemihydrate, C30H26N4O9Re2Br
- The crystal structure of 2,5-bis(3,5-dimethylphenyl)thiazolo[5,4-d]thiazole, C20H18N2S2
- The crystal structure of 5-benzoyl-1-[(E)-(4-fluorobenzylidene)amino]-4-phenylpyrimidin-2(1H)-one, C24H16FN3O2
- Crystal structure of monocarbonyl(N-nitroso-N-oxido-phenylamine-κ 2 O,O′)(tricyclohexylphosphine-κP)rhodium(I), C25H39N2O3PRh
- Crystal structure of poly[bis[μ3-1,3,5-tris[(1H-imidazol-1-yl)methyl]benzene-κ3N:N′:N″]nickel(II)] hexafluorosilicate, C36H36N12NiSiF6
- The crystal structure of 13-(pyrazole-1-yl-4-carbonitrile)-matrine, C19H25N5O
- Crystal structure of 3,5-bis((E)-4-methoxy-2-(trifluoromethyl)benzylidene)-1-methylpiperidin-4-one, C24H21F6NO3
- The crystal structure of N,N′-(Disulfanediyldi-2,1-phenylene)di(6′-methylpyridine)-2-carboxamide, C26H22N4O2S2
- Crystal structure of (E)-7-fluoro-2-(4-methoxy-2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C19H14F4O2
- Crystal structure of ethyl 1-(4-fluorophenyl)-4-phenyl-1H-pyrrole-3-carboxylate, C19H16FNO2
- The crystal structure of cis-diaqua-bis (N-butyl-N-(pyridin-2-yl)pyridin-2-amine-κ2N,N′)cobalt(II)] dichloride trihydrate, C28H44Cl2N6O5Co
- Crystal structure of (E)-7-methoxy-2-((6-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C18H17NO3
- Crystal structure of (E)-2-((3-fluoropyridin-4-yl)methylene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- The crystal structure of 6-bromohexanoic acid, C6H11BrO2
- The crystal structure of 4-chloro-thiophenol, C6H5ClS
- The crystal structure of 4-bromobenzyl chloride, C7H6BrCl
- The crystal structure of di-tert-butyl dicarbonate, C10H18O5
- The crystal structure of (2-(4-chlorophenyl)-5-methyl-1,3-dioxan-5-yl)methanol, C12H15ClO3
- The crystal structure of the co-crystal: 2-hydroxybenzoic acid – N′-(butan-2-ylidene)pyridine-4-carbohydrazide, C10H13N3O·C7H6O3
- Crystal structure and anti-inflammatory activity of (E)-7-fluoro-2-((5-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- Crystal structure of (E)-7-fluoro-2-((6-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- Crystal structure of 1,1′-(butane-1,4-diyl)bis(3-propyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C32H56F24N8P4
- The crystal structure of dichlorido-bis(3-methyl-3-imidazolium-1-ylpropionato-κ2)-cadmium(II), C14H20CdCl2N4O4
- Crystal structure of 1-(2-cyanobenzyl)-3-cyano-4-phenyl-4-(2-cyanobenzyl)-1,4-dihydropyridine monohydrate, C56H42N8O
- The crystal structure of 3-(carboxymethyl)-1-ethenyl-1H-imidazol-3-ium chloride, C7H9N2O2Cl
- The crystal structure of adamantylmethoxydiphenylsilane, C23H28OSi
- Redetermination of the crystal structure of (2E,4Z,13E,15Z)-3,5,14,16-tetramethyl-2,6,13,17-tetraazatricyclo[16.4.0.07,12]docosa-1(22),2,4,7,9,11,13,15,18,20-decaene, C22H24N4
- Crystal structure of (E)-7-hydroxy-2-((6-methoxypyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H15NO3
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2 N:N′)cobalt(II)] dinitrate, C18H28N10O8Co


