Synthesis and crystal structure of di-tert-butyl 1″-acetyl-2,2″,9′-trioxo-4a′,9a′-dihydro-1′H,3′H,9′H-dispiro[indoline-3,2′-xanthene-4′,3″-indoline]-1,3′-dicarboxylate, C39H38N2O9
Abstract
C39H38N2O9, monoclinic, P21/c (no. 14), a = 9.9846(6) Å, b = 31.4308(13) Å, c = 11.5739(6) Å, β = 110.712(7)°, V = 3397.4(3) Å3, Z = 4, R gt (F) = 0.0596, wRref(F2) = 0.1221, T = 293 K.
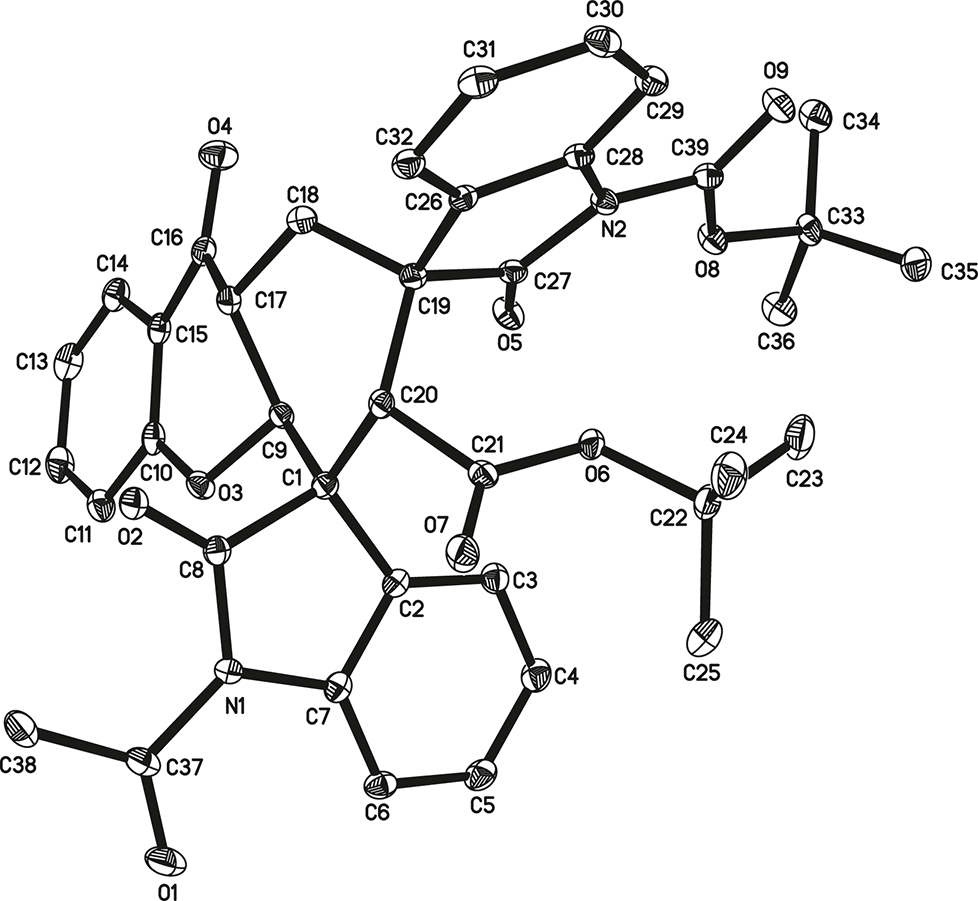
The molecular structure is shown in the figure (hydrogen atoms were omitted for clarity). Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.16 × 0.13 × 0.11 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.10 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| θmax, completeness: | 29.6°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 20424, 8056, 0.051 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 5688 |
| N(param)refined: | 458 |
| Programs: | Bruker [1], SHELX [2] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| O1 | 0.49268 (18) | 0.18408 (5) | 0.79183 (15) | 0.0368 (4) |
| O2 | 0.64878 (14) | 0.28258 (4) | 0.63529 (13) | 0.0215 (3) |
| O3 | 0.74880 (14) | 0.31560 (4) | 0.90630 (12) | 0.0201 (3) |
| O4 | 0.92507 (16) | 0.42983 (5) | 0.87169 (14) | 0.0306 (4) |
| O5 | 0.49526 (15) | 0.42826 (4) | 0.82492 (12) | 0.0223 (3) |
| O6 | 0.23033 (13) | 0.38045 (4) | 0.60009 (12) | 0.0183 (3) |
| O7 | 0.25144 (15) | 0.31789 (4) | 0.51315 (14) | 0.0263 (3) |
| O8 | 0.31723 (15) | 0.49176 (4) | 0.79910 (12) | 0.0212 (3) |
| O9 | 0.24867 (15) | 0.52715 (4) | 0.61685 (13) | 0.0249 (3) |
| N1 | 0.52248 (17) | 0.25325 (5) | 0.75146 (15) | 0.0178 (4) |
| N2 | 0.37647 (17) | 0.46539 (5) | 0.64275 (14) | 0.0163 (3) |
| C1 | 0.5324 (2) | 0.32910 (6) | 0.74002 (18) | 0.0166 (4) |
| C2 | 0.43796 (19) | 0.31519 (6) | 0.80994 (17) | 0.0162 (4) |
| C3 | 0.3683 (2) | 0.33940 (6) | 0.87029 (18) | 0.0210 (4) |
| H3 | 0.369890 | 0.368940 | 0.866324 | 0.025* |
| C4 | 0.2954 (2) | 0.31906 (7) | 0.93747 (19) | 0.0249 (5) |
| H4 | 0.246592 | 0.334953 | 0.977736 | 0.030* |
| C5 | 0.2960 (2) | 0.27518 (7) | 0.94391 (19) | 0.0250 (5) |
| H5 | 0.247062 | 0.261898 | 0.988952 | 0.030* |
| C6 | 0.3675 (2) | 0.25034 (6) | 0.88514 (18) | 0.0214 (4) |
| H6 | 0.368012 | 0.220821 | 0.890802 | 0.026* |
| C7 | 0.4379 (2) | 0.27106 (6) | 0.81786 (17) | 0.0180 (4) |
| C8 | 0.5777 (2) | 0.28625 (6) | 0.69990 (17) | 0.0171 (4) |
| C9 | 0.6681 (2) | 0.35001 (6) | 0.83104 (18) | 0.0169 (4) |
| H9 | 0.639916 | 0.369778 | 0.883865 | 0.020* |
| C10 | 0.8779 (2) | 0.32722 (6) | 0.99111 (18) | 0.0202 (4) |
| C11 | 0.9436 (2) | 0.29799 (7) | 1.08407 (19) | 0.0257 (5) |
| H11 | 0.901493 | 0.271667 | 1.085194 | 0.031* |
| C12 | 1.0722 (2) | 0.30861 (7) | 1.1748 (2) | 0.0295 (5) |
| H12 | 1.117060 | 0.289172 | 1.237076 | 0.035* |
| C13 | 1.1353 (2) | 0.34790 (7) | 1.1741 (2) | 0.0295 (5) |
| H13 | 1.220749 | 0.354919 | 1.236757 | 0.035* |
| C14 | 1.0714 (2) | 0.37641 (7) | 1.08101 (19) | 0.0250 (5) |
| H14 | 1.114289 | 0.402666 | 1.080817 | 0.030* |
| C15 | 0.9421 (2) | 0.36633 (6) | 0.98604 (18) | 0.0199 (4) |
| C16 | 0.8807 (2) | 0.39410 (6) | 0.87733 (19) | 0.0203 (4) |
| C17 | 0.7599 (2) | 0.37376 (6) | 0.77198 (18) | 0.0177 (4) |
| H17 | 0.802843 | 0.352534 | 0.733661 | 0.021* |
| C18 | 0.6779 (2) | 0.40547 (6) | 0.67114 (19) | 0.0203 (4) |
| H18A | 0.704284 | 0.400462 | 0.599278 | 0.024* |
| H18B | 0.709312 | 0.433962 | 0.700578 | 0.024* |
| C19 | 0.5124 (2) | 0.40397 (6) | 0.62977 (17) | 0.0166 (4) |
| C20 | 0.4640 (2) | 0.35701 (6) | 0.62150 (17) | 0.0159 (4) |
| H20 | 0.498611 | 0.344297 | 0.559933 | 0.019* |
| C21 | 0.3025 (2) | 0.34942 (6) | 0.57068 (17) | 0.0168 (4) |
| C22 | 0.0706 (2) | 0.37942 (6) | 0.55657 (19) | 0.0222 (4) |
| C23 | 0.0371 (2) | 0.42098 (7) | 0.6058 (2) | 0.0357 (6) |
| H23A | 0.084355 | 0.421828 | 0.693864 | 0.054* |
| H23B | −0.064497 | 0.423339 | 0.585978 | 0.054* |
| H23C | 0.069954 | 0.444194 | 0.568963 | 0.054* |
| C24 | 0.0102 (2) | 0.37871 (8) | 0.4169 (2) | 0.0327 (5) |
| H24A | 0.059461 | 0.399262 | 0.385260 | 0.049* |
| H24B | −0.089981 | 0.385530 | 0.388797 | 0.049* |
| H24C | 0.022641 | 0.350876 | 0.388075 | 0.049* |
| C25 | 0.0219 (2) | 0.34165 (7) | 0.6131 (2) | 0.0311 (5) |
| H25A | 0.055126 | 0.315822 | 0.587999 | 0.047* |
| H25B | −0.080747 | 0.341321 | 0.585665 | 0.047* |
| H25C | 0.060573 | 0.343890 | 0.701541 | 0.047* |
| C26 | 0.44382 (19) | 0.42554 (6) | 0.50591 (17) | 0.0162 (4) |
| C27 | 0.4613 (2) | 0.43241 (6) | 0.71501 (18) | 0.0174 (4) |
| C28 | 0.3649 (2) | 0.46072 (6) | 0.51691 (17) | 0.0159 (4) |
| C29 | 0.2889 (2) | 0.48476 (6) | 0.41492 (18) | 0.0194 (4) |
| H29 | 0.234244 | 0.507913 | 0.421966 | 0.023* |
| C30 | 0.2964 (2) | 0.47328 (6) | 0.30135 (18) | 0.0211 (4) |
| H30 | 0.245992 | 0.489034 | 0.231467 | 0.025* |
| C31 | 0.3774 (2) | 0.43895 (6) | 0.29027 (18) | 0.0209 (4) |
| H31 | 0.382392 | 0.432151 | 0.213687 | 0.025* |
| C32 | 0.4516 (2) | 0.41451 (6) | 0.39325 (18) | 0.0192 (4) |
| H32 | 0.505347 | 0.391158 | 0.386180 | 0.023* |
| C33 | 0.2469 (2) | 0.52174 (6) | 0.85992 (18) | 0.0212 (4) |
| C34 | 0.3150 (2) | 0.56543 (6) | 0.8695 (2) | 0.0263 (5) |
| H34A | 0.416180 | 0.563227 | 0.913497 | 0.040* |
| H34B | 0.273669 | 0.584306 | 0.912976 | 0.040* |
| H34C | 0.298006 | 0.576379 | 0.788123 | 0.040* |
| C35 | 0.0868 (2) | 0.52217 (7) | 0.7905 (2) | 0.0274 (5) |
| H35A | 0.067435 | 0.532713 | 0.708333 | 0.041* |
| H35B | 0.041595 | 0.540269 | 0.832701 | 0.041* |
| H35C | 0.049801 | 0.493796 | 0.786470 | 0.041* |
| C36 | 0.2811 (3) | 0.50167 (7) | 0.9859 (2) | 0.0293 (5) |
| H36A | 0.246674 | 0.472893 | 0.976435 | 0.044* |
| H36B | 0.235438 | 0.517591 | 1.032424 | 0.044* |
| H36C | 0.382823 | 0.501794 | 1.028791 | 0.044* |
| C37 | 0.5490 (2) | 0.20934 (6) | 0.74495 (19) | 0.0236 (5) |
| C38 | 0.6490 (2) | 0.19630 (7) | 0.6812 (2) | 0.0313 (5) |
| H38A | 0.657248 | 0.165863 | 0.682714 | 0.047* |
| H38B | 0.741453 | 0.208681 | 0.722864 | 0.047* |
| H38C | 0.612451 | 0.205984 | 0.597151 | 0.047* |
| C39 | 0.3073 (2) | 0.49828 (6) | 0.68328 (18) | 0.0186 (4) |
Source of material
The mixture of tert-butyl 2-oxo-3-((4-oxo-4H -chromen-3-yl)methyl)indoline-1-carboxylate (0.2 mmol), tert-butyl (E)-2-(1-acetyl-2-oxoindolin-3-ylidene)acetate (0.3 mmol), 5 Å molecular sieves 125 mg, catalyst (3,5-bis(trifluoromethyl)phenyl)-3-((S)-(6-methoxyquinolin-4-yl)((1S,2S,4S,5R)-5-vinylquinuclidin-2-yl)methyl)thiourea (10 mol %) and 6.0 mL of freshly distilled Et2O was maintained at room temperature for 84 h. Then concentration by evaporation under reduced pressure gave a crude product, which was purified by column chromatography on a silica gel column using hexane/EtOAc (8/1, v/v) to give the corresponding pure products [3].
Experimental details
All hydrogen atoms were placed in geometrically idealized positions. The Uiso values of the hydrogen atoms of methyl groups were set to 1.5Ueq(C) and the Uiso values of all other hydrogen atons were set to 1.2Ueq(C).
Comment
The spirooxindole system is the core structure of some natural alkaloids. Moreover, they have momentous medicinal properties including anticancer [4], antioxidant [5], antimicrobial [6], antifungal [7], anti HIV [8] and antitubercular activities [9]. Due to the aforesaid properties a variety of methods using diverse types of catalysts have been reported in the literature for the procurement of these types of compounds [10–13]. On the other hand, 4H-chromen-4-ones, a well-known class of oxygenated heterocyclic compounds, play an important role in nature due to their recognized biological, pharmacological and biocidal activities [14–16]. Due to the significance of hybrid systems in drug discovery [17], there is an urgent need to assemble multiple pharmacophores into a single molecule. According to physiological activity structure combination strategy, spirooxindole skeleton and 4H-chromen-4-ones ring were joined together and the title compound was synthesized.
X-ray crystal structural analysis indicates that the molecular structure of the title structure consists of a 1,2,3,4,4a,9a-hexahydro-9H-xanthen-9-one ring, a 1-acetylindoline-2-one ring, a tert-butyl 2-oxoindoline-1-carboxylate ring and a tert-butyloxycarbonyl moiety (cf. the figure). The indoline-2-one rings are essentially planar, with a mean deviation from plane of 0.0168(3) Å for 1-acetylindoline-2-one ring and 0.0120(2) Å for tert-butyl 2-oxoindoline-1-carboxylate ring. Xanthen and indoline-2-one rings form spiro structural feature through atom C1 and C19. Because C1 and C19 are sp3 carbon atoms, the indoline-2-one rings are non-coplanar with the xanthen ring. Bond lengths and angles in the title molecule are all in the expected ranges [18, 19].
Funding source: Scientific Research Program Funded by Shaanxi Provincial Education Department
Award Identifier / Grant number: 18JK0837
Funding source: Natural Science Basic Research Plan Funded by Shaanxi Province of China
Award Identifier / Grant number: 2018JM2045
Funding source: Scientific Research Project Funded by Xianyang Normal University
Award Identifier / Grant number: XSYK18006
Funding source: University Students Research and Innovation Training Program of Ministry of Education
Award Identifier / Grant number: S202010722009
Funding source: Qing–Lan Talents Project Funded by Xianyang Normal University
Award Identifier / Grant number: XSYQL201904
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This research was supported by Scientific Research Program Funded by Shaanxi Provincial Education Department (No. 18JK0837), Natural Science Basic Research Plan Funded by Shaanxi Province of China (No. 2018JM2045), Scientific Research Project Funded by Xianyang Normal University (No. XSYK18006), University Students Research and Innovation Training Program of Ministry of Education (S202010722009) and Qing–Lan Talents Project Funded by Xianyang Normal University (No. XSYQL201904).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. APEX2 and SAINT; Bruker AXS Inc.: Madison, Wisconsin, USA, 2012.Suche in Google Scholar
2. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar
3. Liu, X. L., Zhou, G., Gong, Y., Yao, Z., Zuo, X., Zhang, W. H., Zhou, Y. Stereocontrolled synthesis of bispirooxindole-based hexahydroxanthones with five contiguous stereocenters. Org. Lett. 2019, 21, 1428–1431; https://doi.org/10.1021/acs.orglett.9b00139.Suche in Google Scholar PubMed
4. Ding, K., Lu, Y., Nikolovska-Coleska, Z., Qiu, S., Ding, Y., Gao, W., Stuckey, J., Krajewski, K., Roller, P. P., Tomita, Y., Parrish, D. A., Deschamps, J. R., Wang, S. Structure-based design of potent non-peptide MDM2 inhibitors. J. Am. Chem. Soc. 2005, 127, 10130–10131; https://doi.org/10.1021/ja051147z.Suche in Google Scholar PubMed
5. Mathusalini, S., Arasakumar, T., Lakshmi, K., Lin, C. H., Mohan, P. S., Ramnath, M. G., Thirugnanasampandan, R. Synthesis and biological evaluation of new spiro oxindoles with embedded pharmacophores. New J. Chem. 2016, 40, 5164–5169; https://doi.org/10.1039/c6nj00534a.Suche in Google Scholar
6. Ramadoss, H., Saravanan, D., Sudhan, S. P. N., Mansoor, S. S. Synthesis and antimicrobial evaluation of diversely substituted spirooxindole derivatives. Der Pharm. Lett. 2016, 8, 25–29.Suche in Google Scholar
7. Abdel-Rahman, A. H., Keshk, E. M., Hanna, M. A., El-Bady, S. M. Synthesis and evaluation of some new spiro indoline-based heterocycles as potentially active antimicrobial agents. Bioorg. Med. Chem. 2004, 12, 2483–2488; https://doi.org/10.1016/j.bmc.2003.10.063.Suche in Google Scholar PubMed
8. Kumari, G., Modi, M., Gupta, S. K., Singh, R. K. Rhodium(II) acetate-catalyzed stereoselective synthesis, SAR and anti-HIV activity of novel oxindoles bearing cyclopropane ring. Eur. J. Med. Chem. 2011, 46, 1181–1188; https://doi.org/10.1016/j.ejmech.2011.01.037.Suche in Google Scholar PubMed
9. Vintonyak, V. V., Warburg, K., Kruse, H., Grimme, S., Hübel, K., Rauh, D., Waldmann, H. Identification of thiazolidinones spiro-fused to indolin-2-ones as potent and selective inhibitors of the mycobacterium tuberculosis protein tyrosine phosphatase B. Angew. Chem. Int. Ed. Engl. 2010, 49, 5902–5905; https://doi.org/10.1002/anie.201002138.Suche in Google Scholar PubMed
10. Rao, B. M., Niranjan Reddy, G., Vijaikumar Reddy, T., Prabhavathi Devi, B. L. A., Prasad, R. B. N., Yadav, J. S., Reddy, B. V. S. Carbon—SO3H: a novel and recyclable solid acid catalyst for the synthesis of spiro[4H-pyran-3,3′-oxindoles]. Tetrahedron Lett. 2013, 54, 2466–2471.10.1016/j.tetlet.2013.02.089Suche in Google Scholar
11. Rad-Moghadam, K., Youseftabar-Miri, L. Ambient synthesis of spiro[4H-pyran-oxindole] derivatives under [BMIm]BF4 catalysis. Tetrahedron 2011, 67, 5693–5699; https://doi.org/10.1016/j.tet.2011.05.077.Suche in Google Scholar
12. Wagh, Y. B., Tayade, Y. A., Padvi, S. A., Patil, B. S., Patil, N. B., Dalal, D. S. A cesium fluoride promoted efficient and rapid multicomponent synthesis of functionalized 2-amino-3-cyano-4H-pyran and spirooxindole derivatives. Chin. Chem. Lett. 2015, 26, 1273–1277; https://doi.org/10.1016/j.cclet.2015.06.014.Suche in Google Scholar
13. Li, Y., Chen, H., Shi, C., Shi, D., Ji, S. Efficient one-pot synthesis of spirooxindole derivatives catalyzed by L-proline in aqueous medium. J. Comb. Chem. 2010, 12, 231–237; https://doi.org/10.1021/cc9001185.Suche in Google Scholar PubMed
14. Keri, R. S., Budagumpi, S., Pai, R. K., Balakrishna, R. G. Chromones as a privileged scaffold in drug discovery: a review. Eur. J. Med. Chem. 2014, 78, 340–374; https://doi.org/10.1016/j.ejmech.2014.03.047.Suche in Google Scholar PubMed
15. Gaspar, A., Matos, M. J., Garrido, J., Uriarte, E., Chromone, B F. A valid scaffold in medicinal chemistry. Chem. Rev. 2014, 114, 4960–4992; https://doi.org/10.1021/cr400265z.Suche in Google Scholar PubMed
16. Sharma, K. S., Kumar, S., Chand, K., Kathuria, A., Gupta, A., Jain, R. An update on natural occurrence and biological activity of chromones. Curr. Med. Chem. 2011, 18, 3825–3852; https://doi.org/10.2174/092986711803414359.Suche in Google Scholar PubMed
17. Ramprasad, J., Nayak, N., Dalimba, U. Design of new phenothiazine-thiadiazole hybrids via molecular hybridization approach for the development of potent antitubercular agents. Eur. J. Med. Chem. 2015, 106, 75–84; https://doi.org/10.1016/j.ejmech.2015.10.035.Suche in Google Scholar PubMed
18. Li, W.-W., Gu, Y.-Z., Cao, L. Synthesis and crystal structure of tert- butyl (2′R,3R,3′R,4a′R,9a′S)-1-acetyl-5-chloro-3″-methyl-2,5″,9′-trioxo-1″-phenyl-1″,4a′,5″, 9a′-tetrahydro-1′H,3′H,9′H-dispiro[indoline-3,4′-xanthene-2′,4″-pyrazole]-3′-carboxylate, C36H32ClN3O7. Z. Kristallogr. NCS 2021, 236, 77–80; https://doi.org/10.1515/ncrs-2020-0408.Suche in Google Scholar
19. Liu, X.-L., Zuo, X., Wang, J.-X., Chang, S.-q., Wei, Q.-D., Zhou, Y. A bifunctional pyrazolone-chromone synthon directed organocatalytic double Michael cascade reaction: forging five stereocenters in structurally diverse hexahydroxanthones. Org. Chem. Front. 2019, 6, 1485–1490; https://doi.org/10.1039/c9qo00265k.Suche in Google Scholar
© 2021 Wu-Wu Li et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of [aqua-(4-iodopyridine-2,6-dicarboxylato-κ3 O,N,O′)-(1,10-phenanothroline-κ2 N,N′)copper(II)] dihydrate, C19H16O7N3CuI
- The crystal structure of tetrakis(1-isopropyl-1H-imidazolium) octamolybdate, C24H44Mo8N8O26
- Crystal structure of catena-poly[bis(µ2-3,5-bis(1-imidazolyl)pyridine-κ2 N:N′)-(µ2-3-nitrophthalato-k3 O,O′:O″)cadmium(II)] dihydrate, C30H25N11O8Cd
- The crystal structure of diaqua-bis(2-(3-(1H-pyrazol-4-yl)-1H-1,2,4-triazol-5-yl)pyridine-κ2 N:N′)-bis(3,5-dicarboxybenzoato-κ1 O)cobalt(II), C38H30CoN12O14
- Crystal structure of the nickel(II) complex aqua-(2,6-di(pyrazin-2-yl)-4,4′-bipyridine-κ3 N,N′,N′′)-(phthalato-κ2 O,O′)nickel(II) tetrahydrate, C26H26N6O9Ni
- The crystal structure of 1-[5-(2-fluorophenyl)-1-(pyridine-3-sulfonyl)-1H-pyrrol-3-yl]-N-methylmethanaminium 3-carboxyprop-2-enoate, C21H20FN3O6S
- The crystal structure of 1,2-bis(4-pyridyl)ethane - 4,4-dihydroxydiphenylmethane (1/1), C25H21N2O2
- Crystal structure of bis(2-((E)-5-chloro-2-hydroxybenzylidene)hydrazineyl)methaniminium trifluoroacetate dihydrate, C34H36Cl4N10O12
- Crystal structure of 1-cyclopropyl-7-ethoxy-6,8-difluoro-4-oxo-1,4-dihydro-quinoline-3-carboxylic acid, C15H13F2NO4
- Crystal structure of methyl 3-(1H-naphtho[1,8-de][1,3,2]diazaborinin-2(3H)-yl)benzoate, C18H15BN2O2
- Crystal structure of (E)-N′-(2-chloro-6-hydroxybenzylidene)-2-hydroxybenzohydrazide, C14H11ClN2O3
- Crystal structure of Al-rich fluorophlogopite, K1.0(Mg2.8Al0.2)(Si2.8Al1.2)O10F2
- The crystal structure of 4,5-diiodo-1,3-dimesityl-1H-1,2,3-triazol-3-ium hexafluoridoantimonate(V), C20H22F6I2N3Sb
- Crystal structure of tris(3-iodopyridin-1-ium) catena-poly[(hexachlorido-κ1 Cl)-(μ2-trichlorido-κ2 Cl:Cl)diantimony(III)], C15H15Cl9I3N3Sb2
- Crystal structure of methyl 2-(1H-naphtho[1,8-de][1.3.2]diazaborinin-2(3H-yl)benzoate C18H15BN2O2
- The crystal structure of 1,8-bis(4-methoxybenzoyl)naphthalene-2,7-diyl dibenzoate, C40H28O8
- Crystal structure of 2-bromo-1,3,6,8-tetramethylBOPHY (BOPHY = bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl)methylene)hydrazine), C14H15B2BrF4N4
- The crystal structure of (E)-3-chloro-2-(2-(2-fluorobenzylidene)hydrazinyl)pyridine, C12H9ClFN3
- Crystal structure of bis(µ2- 4-iodopyridine-2,6-dicarboxylato-κ3O:N:O′)-bis(4-iodopyridine-2,6-dicarboxylato-κ3O:N:O′)-bis(µ2-1-(4-pyridyl)piperazine-κ2N:N′)-hexa-aqua-tetra-copper(II), C46H46Cu4I4N10O22
- Crystal structure of poly[diaqua-(μ2-2,5-dihydroxyterephthalato-κ2O:O′)(μ2-bis(4-pyridylformyl)piperazine-κ2N:N′)cadmium(II)] dihydrate, C24H28CdN4O12
- Crystal structure of poly[aqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)-(μ3-2,3,5,6-tetrafluoroterephthalato-κ3O:O′:O′′)cadmium(II)], C17H14N4O5F4Cd
- Crystal structure of 6-(quinolin-8-yl)benzo[a]phenanthridin-5(6H)-one, C26H16N2O
- The crystal structure of aqua-bis(6-chloropicolinato-κ2N,O)copper(II), C12H8Cl2N2O5Cu
- Crystal structure of catena-poly[diaqua-bis(μ2-4,4′-bipyridyl-κ2N:N′) disilver(I)] 4-oxidopyridine-3-sulfonate trihydrate, C25H29Ag2N5O9S
- The crystal structure of 4-(3-bromophenyl)pyrimidin-2-amine, C10H8BrN3
- Crystal structure of 6-oxo-4-phenyl-1-propyl-1,6-dihydropyridine-3-carbonitrile, C15H14N2O
- Crystal structure of 4-(2,2-difluoroethyl)-2,4-dimethyl-6-(trifluoromethyl)isoquinoline-1,3(2H,4H)-dione, C14H12F5NO2
- Crystal structure of dibromido-(1-methyl-1H-imidazole-κ1N)-(3-(3-methyl-1H-imidazol-3-ium-1-yl)propanoato-κ1O)zinc(II), C11H16Br2N4O2Zn
- The crystal structure of 1,1′-(((2 (dimethylamino)ethyl)azanediyl)bis(methylene)) bis(naphthalen-2-olato-κ4 N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)- titanium(IV) ─ dichloromethane (2/1), C33H29N3O6Ti
- The layered crystal structure of bis(theophyllinium) hexachloridostannate (IV), C14H18N8O8SnCl6
- The crystal structre of 3-(1-ethenyl-1H-imidazol-3-ium-3-yl)propane-1-sulfonate, C8H12N2O3S
- Synthesis and crystal structure of di-tert-butyl 1″-acetyl-2,2″,9′-trioxo-4a′,9a′-dihydro-1′H,3′H,9′H-dispiro[indoline-3,2′-xanthene-4′,3″-indoline]-1,3′-dicarboxylate, C39H38N2O9
- The crystal structure of 4-chloro-2-(quinolin-8-yl)isoindoline-1,3-dione, C17H9ClN2O2
- The crystal structure of 1-fluoro-4-(p-tolylethynyl)benzene, C15H11F
- The crystal structure of bis[4-bromo-2-(1H-pyrazol-3-yl) phenolato-κ2N,O] copper(II), C18H12Br2CuN4O2
- The crystal structure of poly[(μ 3-imidazolato-κ 3 N:N:N′)(tetrahydrofuran- κ 1 O)lithium(I)], C7H11LiN2O
- Crystal structure of N′,N′′′-((1E,1′E)-(propane-2,2-diylbis(1H-pyrrole-5,2diyl))bis(methaneylylidene))di(nicotinohydrazide) pentahydrate, C25H24N8O2·5H2O
- Crystal structure of 3-(2-ethoxy-2-oxoethyl)-1-ethyl-1H-imidazol-3-ium hexafluoridophos-phate(V), C9H15F6N2O2P
- Crystal structure of (1,10-phenanthroline-κ2N,N′)-bis(3-thiophenecarboxylato-κ2O,O′)copper(II), C22H14N2O4S2Cu
- The crystal structure of 2-amino-3-carboxypyridin-1-ium iodide hemihydrate, C6H8IN2O2.5
- Crystal structure of (E)-7-methoxy-2-((6-methoxypyridin-2-yl)methylene)-tetralone, C18H17NO3
- The crystal structure of [μ-hydroxido-bis[(5,5′-dimethyl-2,2′-bipyridine-κ2N,N′)-tricarbonylrhenium(I)] bromide hemihydrate, C30H26N4O9Re2Br
- The crystal structure of 2,5-bis(3,5-dimethylphenyl)thiazolo[5,4-d]thiazole, C20H18N2S2
- The crystal structure of 5-benzoyl-1-[(E)-(4-fluorobenzylidene)amino]-4-phenylpyrimidin-2(1H)-one, C24H16FN3O2
- Crystal structure of monocarbonyl(N-nitroso-N-oxido-phenylamine-κ 2 O,O′)(tricyclohexylphosphine-κP)rhodium(I), C25H39N2O3PRh
- Crystal structure of poly[bis[μ3-1,3,5-tris[(1H-imidazol-1-yl)methyl]benzene-κ3N:N′:N″]nickel(II)] hexafluorosilicate, C36H36N12NiSiF6
- The crystal structure of 13-(pyrazole-1-yl-4-carbonitrile)-matrine, C19H25N5O
- Crystal structure of 3,5-bis((E)-4-methoxy-2-(trifluoromethyl)benzylidene)-1-methylpiperidin-4-one, C24H21F6NO3
- The crystal structure of N,N′-(Disulfanediyldi-2,1-phenylene)di(6′-methylpyridine)-2-carboxamide, C26H22N4O2S2
- Crystal structure of (E)-7-fluoro-2-(4-methoxy-2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C19H14F4O2
- Crystal structure of ethyl 1-(4-fluorophenyl)-4-phenyl-1H-pyrrole-3-carboxylate, C19H16FNO2
- The crystal structure of cis-diaqua-bis (N-butyl-N-(pyridin-2-yl)pyridin-2-amine-κ2N,N′)cobalt(II)] dichloride trihydrate, C28H44Cl2N6O5Co
- Crystal structure of (E)-7-methoxy-2-((6-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C18H17NO3
- Crystal structure of (E)-2-((3-fluoropyridin-4-yl)methylene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- The crystal structure of 6-bromohexanoic acid, C6H11BrO2
- The crystal structure of 4-chloro-thiophenol, C6H5ClS
- The crystal structure of 4-bromobenzyl chloride, C7H6BrCl
- The crystal structure of di-tert-butyl dicarbonate, C10H18O5
- The crystal structure of (2-(4-chlorophenyl)-5-methyl-1,3-dioxan-5-yl)methanol, C12H15ClO3
- The crystal structure of the co-crystal: 2-hydroxybenzoic acid – N′-(butan-2-ylidene)pyridine-4-carbohydrazide, C10H13N3O·C7H6O3
- Crystal structure and anti-inflammatory activity of (E)-7-fluoro-2-((5-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- Crystal structure of (E)-7-fluoro-2-((6-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- Crystal structure of 1,1′-(butane-1,4-diyl)bis(3-propyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C32H56F24N8P4
- The crystal structure of dichlorido-bis(3-methyl-3-imidazolium-1-ylpropionato-κ2)-cadmium(II), C14H20CdCl2N4O4
- Crystal structure of 1-(2-cyanobenzyl)-3-cyano-4-phenyl-4-(2-cyanobenzyl)-1,4-dihydropyridine monohydrate, C56H42N8O
- The crystal structure of 3-(carboxymethyl)-1-ethenyl-1H-imidazol-3-ium chloride, C7H9N2O2Cl
- The crystal structure of adamantylmethoxydiphenylsilane, C23H28OSi
- Redetermination of the crystal structure of (2E,4Z,13E,15Z)-3,5,14,16-tetramethyl-2,6,13,17-tetraazatricyclo[16.4.0.07,12]docosa-1(22),2,4,7,9,11,13,15,18,20-decaene, C22H24N4
- Crystal structure of (E)-7-hydroxy-2-((6-methoxypyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H15NO3
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2 N:N′)cobalt(II)] dinitrate, C18H28N10O8Co
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of [aqua-(4-iodopyridine-2,6-dicarboxylato-κ3 O,N,O′)-(1,10-phenanothroline-κ2 N,N′)copper(II)] dihydrate, C19H16O7N3CuI
- The crystal structure of tetrakis(1-isopropyl-1H-imidazolium) octamolybdate, C24H44Mo8N8O26
- Crystal structure of catena-poly[bis(µ2-3,5-bis(1-imidazolyl)pyridine-κ2 N:N′)-(µ2-3-nitrophthalato-k3 O,O′:O″)cadmium(II)] dihydrate, C30H25N11O8Cd
- The crystal structure of diaqua-bis(2-(3-(1H-pyrazol-4-yl)-1H-1,2,4-triazol-5-yl)pyridine-κ2 N:N′)-bis(3,5-dicarboxybenzoato-κ1 O)cobalt(II), C38H30CoN12O14
- Crystal structure of the nickel(II) complex aqua-(2,6-di(pyrazin-2-yl)-4,4′-bipyridine-κ3 N,N′,N′′)-(phthalato-κ2 O,O′)nickel(II) tetrahydrate, C26H26N6O9Ni
- The crystal structure of 1-[5-(2-fluorophenyl)-1-(pyridine-3-sulfonyl)-1H-pyrrol-3-yl]-N-methylmethanaminium 3-carboxyprop-2-enoate, C21H20FN3O6S
- The crystal structure of 1,2-bis(4-pyridyl)ethane - 4,4-dihydroxydiphenylmethane (1/1), C25H21N2O2
- Crystal structure of bis(2-((E)-5-chloro-2-hydroxybenzylidene)hydrazineyl)methaniminium trifluoroacetate dihydrate, C34H36Cl4N10O12
- Crystal structure of 1-cyclopropyl-7-ethoxy-6,8-difluoro-4-oxo-1,4-dihydro-quinoline-3-carboxylic acid, C15H13F2NO4
- Crystal structure of methyl 3-(1H-naphtho[1,8-de][1,3,2]diazaborinin-2(3H)-yl)benzoate, C18H15BN2O2
- Crystal structure of (E)-N′-(2-chloro-6-hydroxybenzylidene)-2-hydroxybenzohydrazide, C14H11ClN2O3
- Crystal structure of Al-rich fluorophlogopite, K1.0(Mg2.8Al0.2)(Si2.8Al1.2)O10F2
- The crystal structure of 4,5-diiodo-1,3-dimesityl-1H-1,2,3-triazol-3-ium hexafluoridoantimonate(V), C20H22F6I2N3Sb
- Crystal structure of tris(3-iodopyridin-1-ium) catena-poly[(hexachlorido-κ1 Cl)-(μ2-trichlorido-κ2 Cl:Cl)diantimony(III)], C15H15Cl9I3N3Sb2
- Crystal structure of methyl 2-(1H-naphtho[1,8-de][1.3.2]diazaborinin-2(3H-yl)benzoate C18H15BN2O2
- The crystal structure of 1,8-bis(4-methoxybenzoyl)naphthalene-2,7-diyl dibenzoate, C40H28O8
- Crystal structure of 2-bromo-1,3,6,8-tetramethylBOPHY (BOPHY = bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl)methylene)hydrazine), C14H15B2BrF4N4
- The crystal structure of (E)-3-chloro-2-(2-(2-fluorobenzylidene)hydrazinyl)pyridine, C12H9ClFN3
- Crystal structure of bis(µ2- 4-iodopyridine-2,6-dicarboxylato-κ3O:N:O′)-bis(4-iodopyridine-2,6-dicarboxylato-κ3O:N:O′)-bis(µ2-1-(4-pyridyl)piperazine-κ2N:N′)-hexa-aqua-tetra-copper(II), C46H46Cu4I4N10O22
- Crystal structure of poly[diaqua-(μ2-2,5-dihydroxyterephthalato-κ2O:O′)(μ2-bis(4-pyridylformyl)piperazine-κ2N:N′)cadmium(II)] dihydrate, C24H28CdN4O12
- Crystal structure of poly[aqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)-(μ3-2,3,5,6-tetrafluoroterephthalato-κ3O:O′:O′′)cadmium(II)], C17H14N4O5F4Cd
- Crystal structure of 6-(quinolin-8-yl)benzo[a]phenanthridin-5(6H)-one, C26H16N2O
- The crystal structure of aqua-bis(6-chloropicolinato-κ2N,O)copper(II), C12H8Cl2N2O5Cu
- Crystal structure of catena-poly[diaqua-bis(μ2-4,4′-bipyridyl-κ2N:N′) disilver(I)] 4-oxidopyridine-3-sulfonate trihydrate, C25H29Ag2N5O9S
- The crystal structure of 4-(3-bromophenyl)pyrimidin-2-amine, C10H8BrN3
- Crystal structure of 6-oxo-4-phenyl-1-propyl-1,6-dihydropyridine-3-carbonitrile, C15H14N2O
- Crystal structure of 4-(2,2-difluoroethyl)-2,4-dimethyl-6-(trifluoromethyl)isoquinoline-1,3(2H,4H)-dione, C14H12F5NO2
- Crystal structure of dibromido-(1-methyl-1H-imidazole-κ1N)-(3-(3-methyl-1H-imidazol-3-ium-1-yl)propanoato-κ1O)zinc(II), C11H16Br2N4O2Zn
- The crystal structure of 1,1′-(((2 (dimethylamino)ethyl)azanediyl)bis(methylene)) bis(naphthalen-2-olato-κ4 N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)- titanium(IV) ─ dichloromethane (2/1), C33H29N3O6Ti
- The layered crystal structure of bis(theophyllinium) hexachloridostannate (IV), C14H18N8O8SnCl6
- The crystal structre of 3-(1-ethenyl-1H-imidazol-3-ium-3-yl)propane-1-sulfonate, C8H12N2O3S
- Synthesis and crystal structure of di-tert-butyl 1″-acetyl-2,2″,9′-trioxo-4a′,9a′-dihydro-1′H,3′H,9′H-dispiro[indoline-3,2′-xanthene-4′,3″-indoline]-1,3′-dicarboxylate, C39H38N2O9
- The crystal structure of 4-chloro-2-(quinolin-8-yl)isoindoline-1,3-dione, C17H9ClN2O2
- The crystal structure of 1-fluoro-4-(p-tolylethynyl)benzene, C15H11F
- The crystal structure of bis[4-bromo-2-(1H-pyrazol-3-yl) phenolato-κ2N,O] copper(II), C18H12Br2CuN4O2
- The crystal structure of poly[(μ 3-imidazolato-κ 3 N:N:N′)(tetrahydrofuran- κ 1 O)lithium(I)], C7H11LiN2O
- Crystal structure of N′,N′′′-((1E,1′E)-(propane-2,2-diylbis(1H-pyrrole-5,2diyl))bis(methaneylylidene))di(nicotinohydrazide) pentahydrate, C25H24N8O2·5H2O
- Crystal structure of 3-(2-ethoxy-2-oxoethyl)-1-ethyl-1H-imidazol-3-ium hexafluoridophos-phate(V), C9H15F6N2O2P
- Crystal structure of (1,10-phenanthroline-κ2N,N′)-bis(3-thiophenecarboxylato-κ2O,O′)copper(II), C22H14N2O4S2Cu
- The crystal structure of 2-amino-3-carboxypyridin-1-ium iodide hemihydrate, C6H8IN2O2.5
- Crystal structure of (E)-7-methoxy-2-((6-methoxypyridin-2-yl)methylene)-tetralone, C18H17NO3
- The crystal structure of [μ-hydroxido-bis[(5,5′-dimethyl-2,2′-bipyridine-κ2N,N′)-tricarbonylrhenium(I)] bromide hemihydrate, C30H26N4O9Re2Br
- The crystal structure of 2,5-bis(3,5-dimethylphenyl)thiazolo[5,4-d]thiazole, C20H18N2S2
- The crystal structure of 5-benzoyl-1-[(E)-(4-fluorobenzylidene)amino]-4-phenylpyrimidin-2(1H)-one, C24H16FN3O2
- Crystal structure of monocarbonyl(N-nitroso-N-oxido-phenylamine-κ 2 O,O′)(tricyclohexylphosphine-κP)rhodium(I), C25H39N2O3PRh
- Crystal structure of poly[bis[μ3-1,3,5-tris[(1H-imidazol-1-yl)methyl]benzene-κ3N:N′:N″]nickel(II)] hexafluorosilicate, C36H36N12NiSiF6
- The crystal structure of 13-(pyrazole-1-yl-4-carbonitrile)-matrine, C19H25N5O
- Crystal structure of 3,5-bis((E)-4-methoxy-2-(trifluoromethyl)benzylidene)-1-methylpiperidin-4-one, C24H21F6NO3
- The crystal structure of N,N′-(Disulfanediyldi-2,1-phenylene)di(6′-methylpyridine)-2-carboxamide, C26H22N4O2S2
- Crystal structure of (E)-7-fluoro-2-(4-methoxy-2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C19H14F4O2
- Crystal structure of ethyl 1-(4-fluorophenyl)-4-phenyl-1H-pyrrole-3-carboxylate, C19H16FNO2
- The crystal structure of cis-diaqua-bis (N-butyl-N-(pyridin-2-yl)pyridin-2-amine-κ2N,N′)cobalt(II)] dichloride trihydrate, C28H44Cl2N6O5Co
- Crystal structure of (E)-7-methoxy-2-((6-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C18H17NO3
- Crystal structure of (E)-2-((3-fluoropyridin-4-yl)methylene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- The crystal structure of 6-bromohexanoic acid, C6H11BrO2
- The crystal structure of 4-chloro-thiophenol, C6H5ClS
- The crystal structure of 4-bromobenzyl chloride, C7H6BrCl
- The crystal structure of di-tert-butyl dicarbonate, C10H18O5
- The crystal structure of (2-(4-chlorophenyl)-5-methyl-1,3-dioxan-5-yl)methanol, C12H15ClO3
- The crystal structure of the co-crystal: 2-hydroxybenzoic acid – N′-(butan-2-ylidene)pyridine-4-carbohydrazide, C10H13N3O·C7H6O3
- Crystal structure and anti-inflammatory activity of (E)-7-fluoro-2-((5-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- Crystal structure of (E)-7-fluoro-2-((6-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- Crystal structure of 1,1′-(butane-1,4-diyl)bis(3-propyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C32H56F24N8P4
- The crystal structure of dichlorido-bis(3-methyl-3-imidazolium-1-ylpropionato-κ2)-cadmium(II), C14H20CdCl2N4O4
- Crystal structure of 1-(2-cyanobenzyl)-3-cyano-4-phenyl-4-(2-cyanobenzyl)-1,4-dihydropyridine monohydrate, C56H42N8O
- The crystal structure of 3-(carboxymethyl)-1-ethenyl-1H-imidazol-3-ium chloride, C7H9N2O2Cl
- The crystal structure of adamantylmethoxydiphenylsilane, C23H28OSi
- Redetermination of the crystal structure of (2E,4Z,13E,15Z)-3,5,14,16-tetramethyl-2,6,13,17-tetraazatricyclo[16.4.0.07,12]docosa-1(22),2,4,7,9,11,13,15,18,20-decaene, C22H24N4
- Crystal structure of (E)-7-hydroxy-2-((6-methoxypyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H15NO3
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2 N:N′)cobalt(II)] dinitrate, C18H28N10O8Co


