Abstract
C21H20FN3O6S, monoclinic, P21/n (no. 14), a = 7.8502(4) Å, b = 7.9892(4) Å, c = 34.9707(19) Å, β = 92.500(1)°, V = 2191.2(2) Å3, Z = 4, R gt (F) = 0.0502, wR ref(F 2) = 0.1361, T = 296(2) K.
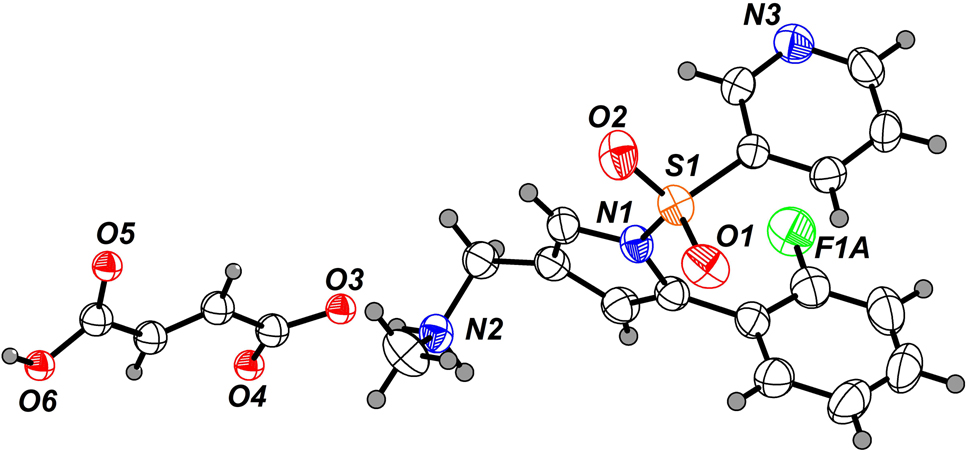
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colorless block |
| Size: | 0.22 × 0.20 × 0.18 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.20 mm−1 |
| Diffractometer, scan mode: | Xcalibur, ω |
| θ max, completeness: | 25.3°, >99% |
| N(hkl)measured, N(hkl)unique, R int: | 13924, 3987, 0.029 |
| Criterion for I obs, N(hkl)gt: | I obs > 2 σ(I obs), 3246 |
| N(param)refined: | 309 |
| Programs: | CrysAlisPRO [1], SHELX [2], [3], Diamond [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| F1Aa | 0.0874 (6) | −0.1222 (6) | 0.12937 (14) | 0.0684 (17) |
| F1Bb | −0.1513 (4) | 0.2482 (4) | 0.21207 (9) | 0.0730 (12) |
| C1 | −0.0984 (4) | 0.0956 (4) | 0.20479 (9) | 0.0522 (7) |
| H1a | −0.1327 | 0.2033 | 0.2109 | 0.063* |
| C2 | −0.1182 (4) | −0.0301 (5) | 0.23108 (10) | 0.0667 (9) |
| H2 | −0.1632 | −0.0071 | 0.2547 | 0.080* |
| C3 | −0.0709 (4) | −0.1886 (5) | 0.22208 (11) | 0.0694 (10) |
| H3 | −0.0837 | −0.2743 | 0.2397 | 0.083* |
| C4 | −0.0041 (4) | −0.2238 (4) | 0.18700 (11) | 0.0649 (9) |
| H4 | 0.0265 | −0.3327 | 0.1808 | 0.078* |
| C5 | 0.0164 (4) | −0.0955 (4) | 0.16141 (9) | 0.0501 (7) |
| H5b | 0.0623 | −0.1188 | 0.1379 | 0.060* |
| C6 | −0.0296 (3) | 0.0681 (3) | 0.16969 (8) | 0.0401 (6) |
| C7 | −0.0062 (3) | 0.2029 (3) | 0.14155 (7) | 0.0375 (6) |
| C8 | −0.0934 (3) | 0.2358 (3) | 0.10806 (8) | 0.0427 (6) |
| H8 | −0.1891 | 0.1780 | 0.0986 | 0.051* |
| C9 | −0.0153 (3) | 0.3728 (3) | 0.08967 (7) | 0.0382 (6) |
| C10 | 0.1214 (3) | 0.4203 (3) | 0.11206 (7) | 0.0395 (6) |
| H10 | 0.1973 | 0.5056 | 0.1067 | 0.047* |
| C11 | 0.3870 (3) | 0.1348 (3) | 0.17531 (7) | 0.0329 (5) |
| C12 | 0.3723 (3) | 0.0148 (3) | 0.20328 (7) | 0.0423 (6) |
| H12 | 0.3117 | 0.0356 | 0.2250 | 0.051* |
| C13 | 0.4506 (4) | −0.1366 (4) | 0.19783 (9) | 0.0500 (7) |
| H13 | 0.4448 | −0.2212 | 0.2160 | 0.060* |
| C14 | 0.5377 (4) | −0.1608 (4) | 0.16506 (9) | 0.0522 (7) |
| H14 | 0.5875 | −0.2649 | 0.1615 | 0.063* |
| C15 | 0.4795 (3) | 0.1017 (3) | 0.14341 (8) | 0.0434 (6) |
| H15 | 0.4890 | 0.1849 | 0.1250 | 0.052* |
| C16 | −0.0695 (3) | 0.4493 (3) | 0.05203 (7) | 0.0428 (6) |
| H16A | −0.0869 | 0.3612 | 0.0332 | 0.051* |
| H16B | 0.0207 | 0.5215 | 0.0436 | 0.051* |
| C17 | −0.2140 (4) | 0.6948 (4) | 0.07991 (10) | 0.0656 (9) |
| H17A | −0.1875 | 0.6577 | 0.1056 | 0.098* |
| H17B | −0.3197 | 0.7554 | 0.0792 | 0.098* |
| H17C | −0.1246 | 0.7666 | 0.0717 | 0.098* |
| C18 | 0.3860 (3) | 1.3032 (3) | 0.04141 (7) | 0.0371 (6) |
| C19 | 0.3044 (4) | 1.1353 (4) | 0.03467 (9) | 0.0521 (7) |
| H19 | 0.1954 | 1.1310 | 0.0232 | 0.063* |
| C20 | 0.3786 (4) | 0.9975 (4) | 0.04408 (9) | 0.0518 (7) |
| H20 | 0.4849 | 1.0030 | 0.0568 | 0.062* |
| C21 | 0.3038 (4) | 0.8278 (4) | 0.03573 (9) | 0.0543 (7) |
| N1 | 0.1284 (3) | 0.3191 (3) | 0.14459 (6) | 0.0372 (5) |
| N2 | −0.2299 (3) | 0.5488 (3) | 0.05427 (6) | 0.0393 (5) |
| H1N | −0.316 (3) | 0.481 (3) | 0.0606 (8) | 0.047* |
| H2N | −0.256 (3) | 0.584 (3) | 0.0306 (6) | 0.047* |
| N3 | 0.5555 (3) | −0.0451 (3) | 0.13799 (7) | 0.0532 (6) |
| O1 | 0.2070 (3) | 0.3366 (3) | 0.21468 (5) | 0.0558 (5) |
| O2 | 0.3938 (3) | 0.4586 (2) | 0.16699 (6) | 0.0564 (5) |
| O3 | 0.3126 (2) | 1.4208 (2) | 0.02315 (5) | 0.0409 (4) |
| O4 | 0.5120 (2) | 1.3194 (2) | 0.06391 (5) | 0.0479 (5) |
| O5 | 0.1548 (3) | 0.8072 (3) | 0.02459 (9) | 0.0834 (8) |
| O6 | 0.4121 (3) | 0.7116 (3) | 0.04125 (8) | 0.0721 (7) |
| H1O | 0.368 (5) | 0.613 (3) | 0.0348 (11) | 0.087* |
| S1 | 0.28475 (8) | 0.32843 (8) | 0.178744 (18) | 0.03953 (19) |
-
aOccupancy: 0.410(5), bOccupancy: 0.590(5).
Source of material
All chemicals, reagents and solvents are of analytical grade and are commercially available. Preparation of [5-(2-fluorophenyl)-1-(pyridine-3-sulfonyl)-1H-pyrrol-3-yl]-N-methylmethanaminium 3-carboxyprop-2-enoate: 10.08 g (30.5 mmol) of 5-(2-fluorophenyl)-1-(pyridin-3-yl-sulfonyl)-1H-pyrrole-3-formaldehyde was dissolved in 150 ml methanol, and 4.7 g (45.8 mmol) of 30% methylamine methanol solution was slowly added at room temperature. 1.2 g (30.5 mmol) NaBH4 was added under cooling with an ice bath for 3 h at room temperature, 1 N hydrochloric acid (about 30 ml) was added to adjust pH to 6–7. The methanol solvent was removed, 30 ml ethyl acetate was added, and then ammonia water (about 10 ml) was added to adjust pH to 12, stratification was carried out, 10 ml ethyl acetate was added to the water layer for stripping once. The organic layer was combined, washed with brine and dried with anhydrous sodium sulfate. Add 10 ml DMF, stir, add 3.543 g (30.5 mmol) fumaric acid, stir at 60 °C for 30 min, cool to room temperature, stir for 2 h, filter, wash the filter cake with 5 ml ethyl acetate: DMF = 1:2. Then wash with 2 ml ethyl acetate, dry to obtain about 8.87 g of a white solid, with a yield of 67.1%. 1H NMR (600 MHz, DMSO) δ 10.420 (s, 2H), 8.885 (dd, J = 4.8, 1.3 Hz, 1H), 8.565 (d, J = 2.3 Hz, 1H), 7.888 (dt, J = 8.3, 1.8 Hz, 1H), 7.776 (s, 1H), 7.615 (dd, J = 8.2, 4.9 Hz, 1H), 7.528 (m, 1H), 7.240 (d, J = 8.4 Hz, 1H), 7.216 (d, J = 6.6 Hz, 1H), 7.103 (td, J = 7.8, 1.8 Hz, 1H), 6.508 (d, J = 1.8 Hz, 1H), 6.487 (s, 2H), 3.916 (s, 2H), 2.459 (s, 3H). Crystals were grown in methanol and water (7:3) at room temperature.
Experimental details
Hydrogen atoms were placed in their geometrically idealized positions and constrained to ride on their parent atoms. The fluoro substituent is disordered over two positions (Table 2).
Comment
1-[5-(2-Fluorophenyl)-1-(pyridine-3-sulfonyl)-1H-pyrrol-3-yl]-N-methylmethanaminium 3-carboxyprop-2-enoate (Vonoprazan fumarate) is a novel proton pump inhibitor (PPI), potassium competitive acid blocker (P–CAB) by competitive inhibition of proton pump (H+, K+–ATPase) and in the function of K+ is a reversible antagonist [5–7]. Different from traditional PPIs, the effect of Vonoprazan fumarate is independent of proton pump activation conditions. P–CABs can significantly reduce the occurrence of nocturnal acid breakthrough [8–10]. The structure of the title compound was elucidated by spectroscopic methods (NMR) and X-ray diffraction. All bond lengths and angles in the crystal structure are within the normal range [11]. Intermolecular hydrogen bonds are found through the chain structure of the anions (fumarate) oxygen atoms [O6⃛O3: 2.523(2) Å].
Funding source: Heilongjiang Natural Science Foundation
Award Identifier / Grant number: YQ2020H002
Funding source: Cultivating Young Innovative Talents in Heilongjiang Province
Award Identifier / Grant number: UNPYSCT-2020056
Funding source: Provincial Basic Scientific Research Operating Expenses Project of Heilongjiang Province
Award Identifier / Grant number: 2018–KYYWF-0950
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work was supported by the excellent youth project of Heilongjiang Natural Science Foundation (YQ2020H002), and the project of Cultivating Young Innovative Talents in Heilongjiang Province (UNPYSCT-2020056), and the Provincial Basic Scientific Research Operating Expenses Project of Heilongjiang Province (2018–KYYWF-0950).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Oxford Diffraction Ltd. CrysAlisPRO; Oxford Diffraction Ltd.: Abingdon, Oxfordshire, England, 2006.Suche in Google Scholar
2. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Suche in Google Scholar PubMed PubMed Central
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar
4. Brandenburg, K. DIAMOND. Visual Crystal Structure Information System. Ver. 4.0; Crystal Impact: Bonn, Germany, 2015.Suche in Google Scholar
5. Wang, Y., Wang, C., Wang, S., Zhou, Q., Dai, D., Shi, J., Xu, X., Luo, Q. Cytochrome P450-based drug-drug interactions of vonoprazan in vitro and in vivo. Front. Pharmacol. 2020, 11, 53–62; https://doi.org/10.3389/fphar.2020.00053.Suche in Google Scholar PubMed PubMed Central
6. Marabotto, E., Ziola, S., Savarino, V., Giannini, E. G., Furnari, M., Bodini, G., Zingone, F., Ghisa, M., Barberio, B., Zentilin, P., Savarino, E. Vonoprazan fumarate for the treatment of gastric ulcers: a short review on emerging data. Clin. Exp. Gastroenterol. 2020, 13, 99–104; https://doi.org/10.2147/ceg.s228352.Suche in Google Scholar PubMed PubMed Central
7. Suzuki, N., Hiraga, J., Takagi, Y., Tsuzuki, T., Uematsu, N., Kagami, Y. Immune thrombocytopenia induced by vonoprazan fumarate: a single center retrospective study. Ann. Hematol. 2018, 97, 741–742; https://doi.org/10.1007/s00277-017-3206-4.Suche in Google Scholar PubMed
8. Luo, Z., Liu, A., Liu, Y., Wang, G., Chen, X., Wang, H., Li, M., Zhang, H., Qiu, Y., Zhai, H. Development of a stability-indicating HPLC method for simultaneous determination of ten related substances in vonoprazan fumarate drug substance. J. Pharmaceut. Biomed. Anal. 2018, 149, 133–142; https://doi.org/10.1016/j.jpba.2017.11.011.Suche in Google Scholar PubMed
9. Sugano, K. Vonoprazan fumarate, a novel potassium-competitive acid blocker, in the management of gastroesophageal reflux disease: safety and clinical evidenceto date. Ther. Adv. Gastroenterol. 2018, 11, 1–14; https://doi.org/10.1177/1756283x17745776.Suche in Google Scholar PubMed PubMed Central
10. Masaoka, T., Kameyama, H., Yamane, T., Yamamoto, Y., Takeuchi, H., Suzuki, H., Kitagawa, Y., Kanai, T. pathophysiology of potassium-competitive acid blocker- refractory gastroesophageal reflux and the potential of potassium-competitive acid blocker test. J. Neurogastroenterol. Motil. 2018, 24, 577–583; https://doi.org/10.5056/jnm18036.Suche in Google Scholar PubMed PubMed Central
11. Nishida, H., Arikawa, Y., Hirase, K., Imaeda, T., Inatomi, N., Hori, Y., Matsukawa, J., Fujioka, Y., Hamada, T., Iida, M., Nishitani, M., Imanishi, A., Fukui, H., Itoh, F., Kajino, M. Identification of a novel fluoropyrrole derivative as a potassium-competitive acid blocker with long duration of action. Bioorg. Med. Chem. 2017, 25, 3298–3314; https://doi.org/10.1016/j.bmc.2017.04.014.Suche in Google Scholar PubMed
© 2021 Yi-Xia Gong et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of [aqua-(4-iodopyridine-2,6-dicarboxylato-κ3 O,N,O′)-(1,10-phenanothroline-κ2 N,N′)copper(II)] dihydrate, C19H16O7N3CuI
- The crystal structure of tetrakis(1-isopropyl-1H-imidazolium) octamolybdate, C24H44Mo8N8O26
- Crystal structure of catena-poly[bis(µ2-3,5-bis(1-imidazolyl)pyridine-κ2 N:N′)-(µ2-3-nitrophthalato-k3 O,O′:O″)cadmium(II)] dihydrate, C30H25N11O8Cd
- The crystal structure of diaqua-bis(2-(3-(1H-pyrazol-4-yl)-1H-1,2,4-triazol-5-yl)pyridine-κ2 N:N′)-bis(3,5-dicarboxybenzoato-κ1 O)cobalt(II), C38H30CoN12O14
- Crystal structure of the nickel(II) complex aqua-(2,6-di(pyrazin-2-yl)-4,4′-bipyridine-κ3 N,N′,N′′)-(phthalato-κ2 O,O′)nickel(II) tetrahydrate, C26H26N6O9Ni
- The crystal structure of 1-[5-(2-fluorophenyl)-1-(pyridine-3-sulfonyl)-1H-pyrrol-3-yl]-N-methylmethanaminium 3-carboxyprop-2-enoate, C21H20FN3O6S
- The crystal structure of 1,2-bis(4-pyridyl)ethane - 4,4-dihydroxydiphenylmethane (1/1), C25H21N2O2
- Crystal structure of bis(2-((E)-5-chloro-2-hydroxybenzylidene)hydrazineyl)methaniminium trifluoroacetate dihydrate, C34H36Cl4N10O12
- Crystal structure of 1-cyclopropyl-7-ethoxy-6,8-difluoro-4-oxo-1,4-dihydro-quinoline-3-carboxylic acid, C15H13F2NO4
- Crystal structure of methyl 3-(1H-naphtho[1,8-de][1,3,2]diazaborinin-2(3H)-yl)benzoate, C18H15BN2O2
- Crystal structure of (E)-N′-(2-chloro-6-hydroxybenzylidene)-2-hydroxybenzohydrazide, C14H11ClN2O3
- Crystal structure of Al-rich fluorophlogopite, K1.0(Mg2.8Al0.2)(Si2.8Al1.2)O10F2
- The crystal structure of 4,5-diiodo-1,3-dimesityl-1H-1,2,3-triazol-3-ium hexafluoridoantimonate(V), C20H22F6I2N3Sb
- Crystal structure of tris(3-iodopyridin-1-ium) catena-poly[(hexachlorido-κ1 Cl)-(μ2-trichlorido-κ2 Cl:Cl)diantimony(III)], C15H15Cl9I3N3Sb2
- Crystal structure of methyl 2-(1H-naphtho[1,8-de][1.3.2]diazaborinin-2(3H-yl)benzoate C18H15BN2O2
- The crystal structure of 1,8-bis(4-methoxybenzoyl)naphthalene-2,7-diyl dibenzoate, C40H28O8
- Crystal structure of 2-bromo-1,3,6,8-tetramethylBOPHY (BOPHY = bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl)methylene)hydrazine), C14H15B2BrF4N4
- The crystal structure of (E)-3-chloro-2-(2-(2-fluorobenzylidene)hydrazinyl)pyridine, C12H9ClFN3
- Crystal structure of bis(µ2- 4-iodopyridine-2,6-dicarboxylato-κ3O:N:O′)-bis(4-iodopyridine-2,6-dicarboxylato-κ3O:N:O′)-bis(µ2-1-(4-pyridyl)piperazine-κ2N:N′)-hexa-aqua-tetra-copper(II), C46H46Cu4I4N10O22
- Crystal structure of poly[diaqua-(μ2-2,5-dihydroxyterephthalato-κ2O:O′)(μ2-bis(4-pyridylformyl)piperazine-κ2N:N′)cadmium(II)] dihydrate, C24H28CdN4O12
- Crystal structure of poly[aqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)-(μ3-2,3,5,6-tetrafluoroterephthalato-κ3O:O′:O′′)cadmium(II)], C17H14N4O5F4Cd
- Crystal structure of 6-(quinolin-8-yl)benzo[a]phenanthridin-5(6H)-one, C26H16N2O
- The crystal structure of aqua-bis(6-chloropicolinato-κ2N,O)copper(II), C12H8Cl2N2O5Cu
- Crystal structure of catena-poly[diaqua-bis(μ2-4,4′-bipyridyl-κ2N:N′) disilver(I)] 4-oxidopyridine-3-sulfonate trihydrate, C25H29Ag2N5O9S
- The crystal structure of 4-(3-bromophenyl)pyrimidin-2-amine, C10H8BrN3
- Crystal structure of 6-oxo-4-phenyl-1-propyl-1,6-dihydropyridine-3-carbonitrile, C15H14N2O
- Crystal structure of 4-(2,2-difluoroethyl)-2,4-dimethyl-6-(trifluoromethyl)isoquinoline-1,3(2H,4H)-dione, C14H12F5NO2
- Crystal structure of dibromido-(1-methyl-1H-imidazole-κ1N)-(3-(3-methyl-1H-imidazol-3-ium-1-yl)propanoato-κ1O)zinc(II), C11H16Br2N4O2Zn
- The crystal structure of 1,1′-(((2 (dimethylamino)ethyl)azanediyl)bis(methylene)) bis(naphthalen-2-olato-κ4 N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)- titanium(IV) ─ dichloromethane (2/1), C33H29N3O6Ti
- The layered crystal structure of bis(theophyllinium) hexachloridostannate (IV), C14H18N8O8SnCl6
- The crystal structre of 3-(1-ethenyl-1H-imidazol-3-ium-3-yl)propane-1-sulfonate, C8H12N2O3S
- Synthesis and crystal structure of di-tert-butyl 1″-acetyl-2,2″,9′-trioxo-4a′,9a′-dihydro-1′H,3′H,9′H-dispiro[indoline-3,2′-xanthene-4′,3″-indoline]-1,3′-dicarboxylate, C39H38N2O9
- The crystal structure of 4-chloro-2-(quinolin-8-yl)isoindoline-1,3-dione, C17H9ClN2O2
- The crystal structure of 1-fluoro-4-(p-tolylethynyl)benzene, C15H11F
- The crystal structure of bis[4-bromo-2-(1H-pyrazol-3-yl) phenolato-κ2N,O] copper(II), C18H12Br2CuN4O2
- The crystal structure of poly[(μ 3-imidazolato-κ 3 N:N:N′)(tetrahydrofuran- κ 1 O)lithium(I)], C7H11LiN2O
- Crystal structure of N′,N′′′-((1E,1′E)-(propane-2,2-diylbis(1H-pyrrole-5,2diyl))bis(methaneylylidene))di(nicotinohydrazide) pentahydrate, C25H24N8O2·5H2O
- Crystal structure of 3-(2-ethoxy-2-oxoethyl)-1-ethyl-1H-imidazol-3-ium hexafluoridophos-phate(V), C9H15F6N2O2P
- Crystal structure of (1,10-phenanthroline-κ2N,N′)-bis(3-thiophenecarboxylato-κ2O,O′)copper(II), C22H14N2O4S2Cu
- The crystal structure of 2-amino-3-carboxypyridin-1-ium iodide hemihydrate, C6H8IN2O2.5
- Crystal structure of (E)-7-methoxy-2-((6-methoxypyridin-2-yl)methylene)-tetralone, C18H17NO3
- The crystal structure of [μ-hydroxido-bis[(5,5′-dimethyl-2,2′-bipyridine-κ2N,N′)-tricarbonylrhenium(I)] bromide hemihydrate, C30H26N4O9Re2Br
- The crystal structure of 2,5-bis(3,5-dimethylphenyl)thiazolo[5,4-d]thiazole, C20H18N2S2
- The crystal structure of 5-benzoyl-1-[(E)-(4-fluorobenzylidene)amino]-4-phenylpyrimidin-2(1H)-one, C24H16FN3O2
- Crystal structure of monocarbonyl(N-nitroso-N-oxido-phenylamine-κ 2 O,O′)(tricyclohexylphosphine-κP)rhodium(I), C25H39N2O3PRh
- Crystal structure of poly[bis[μ3-1,3,5-tris[(1H-imidazol-1-yl)methyl]benzene-κ3N:N′:N″]nickel(II)] hexafluorosilicate, C36H36N12NiSiF6
- The crystal structure of 13-(pyrazole-1-yl-4-carbonitrile)-matrine, C19H25N5O
- Crystal structure of 3,5-bis((E)-4-methoxy-2-(trifluoromethyl)benzylidene)-1-methylpiperidin-4-one, C24H21F6NO3
- The crystal structure of N,N′-(Disulfanediyldi-2,1-phenylene)di(6′-methylpyridine)-2-carboxamide, C26H22N4O2S2
- Crystal structure of (E)-7-fluoro-2-(4-methoxy-2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C19H14F4O2
- Crystal structure of ethyl 1-(4-fluorophenyl)-4-phenyl-1H-pyrrole-3-carboxylate, C19H16FNO2
- The crystal structure of cis-diaqua-bis (N-butyl-N-(pyridin-2-yl)pyridin-2-amine-κ2N,N′)cobalt(II)] dichloride trihydrate, C28H44Cl2N6O5Co
- Crystal structure of (E)-7-methoxy-2-((6-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C18H17NO3
- Crystal structure of (E)-2-((3-fluoropyridin-4-yl)methylene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- The crystal structure of 6-bromohexanoic acid, C6H11BrO2
- The crystal structure of 4-chloro-thiophenol, C6H5ClS
- The crystal structure of 4-bromobenzyl chloride, C7H6BrCl
- The crystal structure of di-tert-butyl dicarbonate, C10H18O5
- The crystal structure of (2-(4-chlorophenyl)-5-methyl-1,3-dioxan-5-yl)methanol, C12H15ClO3
- The crystal structure of the co-crystal: 2-hydroxybenzoic acid – N′-(butan-2-ylidene)pyridine-4-carbohydrazide, C10H13N3O·C7H6O3
- Crystal structure and anti-inflammatory activity of (E)-7-fluoro-2-((5-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- Crystal structure of (E)-7-fluoro-2-((6-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- Crystal structure of 1,1′-(butane-1,4-diyl)bis(3-propyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C32H56F24N8P4
- The crystal structure of dichlorido-bis(3-methyl-3-imidazolium-1-ylpropionato-κ2)-cadmium(II), C14H20CdCl2N4O4
- Crystal structure of 1-(2-cyanobenzyl)-3-cyano-4-phenyl-4-(2-cyanobenzyl)-1,4-dihydropyridine monohydrate, C56H42N8O
- The crystal structure of 3-(carboxymethyl)-1-ethenyl-1H-imidazol-3-ium chloride, C7H9N2O2Cl
- The crystal structure of adamantylmethoxydiphenylsilane, C23H28OSi
- Redetermination of the crystal structure of (2E,4Z,13E,15Z)-3,5,14,16-tetramethyl-2,6,13,17-tetraazatricyclo[16.4.0.07,12]docosa-1(22),2,4,7,9,11,13,15,18,20-decaene, C22H24N4
- Crystal structure of (E)-7-hydroxy-2-((6-methoxypyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H15NO3
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2 N:N′)cobalt(II)] dinitrate, C18H28N10O8Co
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of [aqua-(4-iodopyridine-2,6-dicarboxylato-κ3 O,N,O′)-(1,10-phenanothroline-κ2 N,N′)copper(II)] dihydrate, C19H16O7N3CuI
- The crystal structure of tetrakis(1-isopropyl-1H-imidazolium) octamolybdate, C24H44Mo8N8O26
- Crystal structure of catena-poly[bis(µ2-3,5-bis(1-imidazolyl)pyridine-κ2 N:N′)-(µ2-3-nitrophthalato-k3 O,O′:O″)cadmium(II)] dihydrate, C30H25N11O8Cd
- The crystal structure of diaqua-bis(2-(3-(1H-pyrazol-4-yl)-1H-1,2,4-triazol-5-yl)pyridine-κ2 N:N′)-bis(3,5-dicarboxybenzoato-κ1 O)cobalt(II), C38H30CoN12O14
- Crystal structure of the nickel(II) complex aqua-(2,6-di(pyrazin-2-yl)-4,4′-bipyridine-κ3 N,N′,N′′)-(phthalato-κ2 O,O′)nickel(II) tetrahydrate, C26H26N6O9Ni
- The crystal structure of 1-[5-(2-fluorophenyl)-1-(pyridine-3-sulfonyl)-1H-pyrrol-3-yl]-N-methylmethanaminium 3-carboxyprop-2-enoate, C21H20FN3O6S
- The crystal structure of 1,2-bis(4-pyridyl)ethane - 4,4-dihydroxydiphenylmethane (1/1), C25H21N2O2
- Crystal structure of bis(2-((E)-5-chloro-2-hydroxybenzylidene)hydrazineyl)methaniminium trifluoroacetate dihydrate, C34H36Cl4N10O12
- Crystal structure of 1-cyclopropyl-7-ethoxy-6,8-difluoro-4-oxo-1,4-dihydro-quinoline-3-carboxylic acid, C15H13F2NO4
- Crystal structure of methyl 3-(1H-naphtho[1,8-de][1,3,2]diazaborinin-2(3H)-yl)benzoate, C18H15BN2O2
- Crystal structure of (E)-N′-(2-chloro-6-hydroxybenzylidene)-2-hydroxybenzohydrazide, C14H11ClN2O3
- Crystal structure of Al-rich fluorophlogopite, K1.0(Mg2.8Al0.2)(Si2.8Al1.2)O10F2
- The crystal structure of 4,5-diiodo-1,3-dimesityl-1H-1,2,3-triazol-3-ium hexafluoridoantimonate(V), C20H22F6I2N3Sb
- Crystal structure of tris(3-iodopyridin-1-ium) catena-poly[(hexachlorido-κ1 Cl)-(μ2-trichlorido-κ2 Cl:Cl)diantimony(III)], C15H15Cl9I3N3Sb2
- Crystal structure of methyl 2-(1H-naphtho[1,8-de][1.3.2]diazaborinin-2(3H-yl)benzoate C18H15BN2O2
- The crystal structure of 1,8-bis(4-methoxybenzoyl)naphthalene-2,7-diyl dibenzoate, C40H28O8
- Crystal structure of 2-bromo-1,3,6,8-tetramethylBOPHY (BOPHY = bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl)methylene)hydrazine), C14H15B2BrF4N4
- The crystal structure of (E)-3-chloro-2-(2-(2-fluorobenzylidene)hydrazinyl)pyridine, C12H9ClFN3
- Crystal structure of bis(µ2- 4-iodopyridine-2,6-dicarboxylato-κ3O:N:O′)-bis(4-iodopyridine-2,6-dicarboxylato-κ3O:N:O′)-bis(µ2-1-(4-pyridyl)piperazine-κ2N:N′)-hexa-aqua-tetra-copper(II), C46H46Cu4I4N10O22
- Crystal structure of poly[diaqua-(μ2-2,5-dihydroxyterephthalato-κ2O:O′)(μ2-bis(4-pyridylformyl)piperazine-κ2N:N′)cadmium(II)] dihydrate, C24H28CdN4O12
- Crystal structure of poly[aqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)-(μ3-2,3,5,6-tetrafluoroterephthalato-κ3O:O′:O′′)cadmium(II)], C17H14N4O5F4Cd
- Crystal structure of 6-(quinolin-8-yl)benzo[a]phenanthridin-5(6H)-one, C26H16N2O
- The crystal structure of aqua-bis(6-chloropicolinato-κ2N,O)copper(II), C12H8Cl2N2O5Cu
- Crystal structure of catena-poly[diaqua-bis(μ2-4,4′-bipyridyl-κ2N:N′) disilver(I)] 4-oxidopyridine-3-sulfonate trihydrate, C25H29Ag2N5O9S
- The crystal structure of 4-(3-bromophenyl)pyrimidin-2-amine, C10H8BrN3
- Crystal structure of 6-oxo-4-phenyl-1-propyl-1,6-dihydropyridine-3-carbonitrile, C15H14N2O
- Crystal structure of 4-(2,2-difluoroethyl)-2,4-dimethyl-6-(trifluoromethyl)isoquinoline-1,3(2H,4H)-dione, C14H12F5NO2
- Crystal structure of dibromido-(1-methyl-1H-imidazole-κ1N)-(3-(3-methyl-1H-imidazol-3-ium-1-yl)propanoato-κ1O)zinc(II), C11H16Br2N4O2Zn
- The crystal structure of 1,1′-(((2 (dimethylamino)ethyl)azanediyl)bis(methylene)) bis(naphthalen-2-olato-κ4 N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)- titanium(IV) ─ dichloromethane (2/1), C33H29N3O6Ti
- The layered crystal structure of bis(theophyllinium) hexachloridostannate (IV), C14H18N8O8SnCl6
- The crystal structre of 3-(1-ethenyl-1H-imidazol-3-ium-3-yl)propane-1-sulfonate, C8H12N2O3S
- Synthesis and crystal structure of di-tert-butyl 1″-acetyl-2,2″,9′-trioxo-4a′,9a′-dihydro-1′H,3′H,9′H-dispiro[indoline-3,2′-xanthene-4′,3″-indoline]-1,3′-dicarboxylate, C39H38N2O9
- The crystal structure of 4-chloro-2-(quinolin-8-yl)isoindoline-1,3-dione, C17H9ClN2O2
- The crystal structure of 1-fluoro-4-(p-tolylethynyl)benzene, C15H11F
- The crystal structure of bis[4-bromo-2-(1H-pyrazol-3-yl) phenolato-κ2N,O] copper(II), C18H12Br2CuN4O2
- The crystal structure of poly[(μ 3-imidazolato-κ 3 N:N:N′)(tetrahydrofuran- κ 1 O)lithium(I)], C7H11LiN2O
- Crystal structure of N′,N′′′-((1E,1′E)-(propane-2,2-diylbis(1H-pyrrole-5,2diyl))bis(methaneylylidene))di(nicotinohydrazide) pentahydrate, C25H24N8O2·5H2O
- Crystal structure of 3-(2-ethoxy-2-oxoethyl)-1-ethyl-1H-imidazol-3-ium hexafluoridophos-phate(V), C9H15F6N2O2P
- Crystal structure of (1,10-phenanthroline-κ2N,N′)-bis(3-thiophenecarboxylato-κ2O,O′)copper(II), C22H14N2O4S2Cu
- The crystal structure of 2-amino-3-carboxypyridin-1-ium iodide hemihydrate, C6H8IN2O2.5
- Crystal structure of (E)-7-methoxy-2-((6-methoxypyridin-2-yl)methylene)-tetralone, C18H17NO3
- The crystal structure of [μ-hydroxido-bis[(5,5′-dimethyl-2,2′-bipyridine-κ2N,N′)-tricarbonylrhenium(I)] bromide hemihydrate, C30H26N4O9Re2Br
- The crystal structure of 2,5-bis(3,5-dimethylphenyl)thiazolo[5,4-d]thiazole, C20H18N2S2
- The crystal structure of 5-benzoyl-1-[(E)-(4-fluorobenzylidene)amino]-4-phenylpyrimidin-2(1H)-one, C24H16FN3O2
- Crystal structure of monocarbonyl(N-nitroso-N-oxido-phenylamine-κ 2 O,O′)(tricyclohexylphosphine-κP)rhodium(I), C25H39N2O3PRh
- Crystal structure of poly[bis[μ3-1,3,5-tris[(1H-imidazol-1-yl)methyl]benzene-κ3N:N′:N″]nickel(II)] hexafluorosilicate, C36H36N12NiSiF6
- The crystal structure of 13-(pyrazole-1-yl-4-carbonitrile)-matrine, C19H25N5O
- Crystal structure of 3,5-bis((E)-4-methoxy-2-(trifluoromethyl)benzylidene)-1-methylpiperidin-4-one, C24H21F6NO3
- The crystal structure of N,N′-(Disulfanediyldi-2,1-phenylene)di(6′-methylpyridine)-2-carboxamide, C26H22N4O2S2
- Crystal structure of (E)-7-fluoro-2-(4-methoxy-2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C19H14F4O2
- Crystal structure of ethyl 1-(4-fluorophenyl)-4-phenyl-1H-pyrrole-3-carboxylate, C19H16FNO2
- The crystal structure of cis-diaqua-bis (N-butyl-N-(pyridin-2-yl)pyridin-2-amine-κ2N,N′)cobalt(II)] dichloride trihydrate, C28H44Cl2N6O5Co
- Crystal structure of (E)-7-methoxy-2-((6-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C18H17NO3
- Crystal structure of (E)-2-((3-fluoropyridin-4-yl)methylene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- The crystal structure of 6-bromohexanoic acid, C6H11BrO2
- The crystal structure of 4-chloro-thiophenol, C6H5ClS
- The crystal structure of 4-bromobenzyl chloride, C7H6BrCl
- The crystal structure of di-tert-butyl dicarbonate, C10H18O5
- The crystal structure of (2-(4-chlorophenyl)-5-methyl-1,3-dioxan-5-yl)methanol, C12H15ClO3
- The crystal structure of the co-crystal: 2-hydroxybenzoic acid – N′-(butan-2-ylidene)pyridine-4-carbohydrazide, C10H13N3O·C7H6O3
- Crystal structure and anti-inflammatory activity of (E)-7-fluoro-2-((5-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- Crystal structure of (E)-7-fluoro-2-((6-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- Crystal structure of 1,1′-(butane-1,4-diyl)bis(3-propyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C32H56F24N8P4
- The crystal structure of dichlorido-bis(3-methyl-3-imidazolium-1-ylpropionato-κ2)-cadmium(II), C14H20CdCl2N4O4
- Crystal structure of 1-(2-cyanobenzyl)-3-cyano-4-phenyl-4-(2-cyanobenzyl)-1,4-dihydropyridine monohydrate, C56H42N8O
- The crystal structure of 3-(carboxymethyl)-1-ethenyl-1H-imidazol-3-ium chloride, C7H9N2O2Cl
- The crystal structure of adamantylmethoxydiphenylsilane, C23H28OSi
- Redetermination of the crystal structure of (2E,4Z,13E,15Z)-3,5,14,16-tetramethyl-2,6,13,17-tetraazatricyclo[16.4.0.07,12]docosa-1(22),2,4,7,9,11,13,15,18,20-decaene, C22H24N4
- Crystal structure of (E)-7-hydroxy-2-((6-methoxypyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H15NO3
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2 N:N′)cobalt(II)] dinitrate, C18H28N10O8Co

