Abstract
C15H10O3, orthorhombic, Pca21 (no. 29), a = 10.2949(9) Å, b = 11.1616(9) Å, c = 19.2902(18) Å, V = 2216.6(3) Å3, Z = 8, Rgt(F) = 0.0302, wRref(F2) = 0.0783, T = 100(2) K.
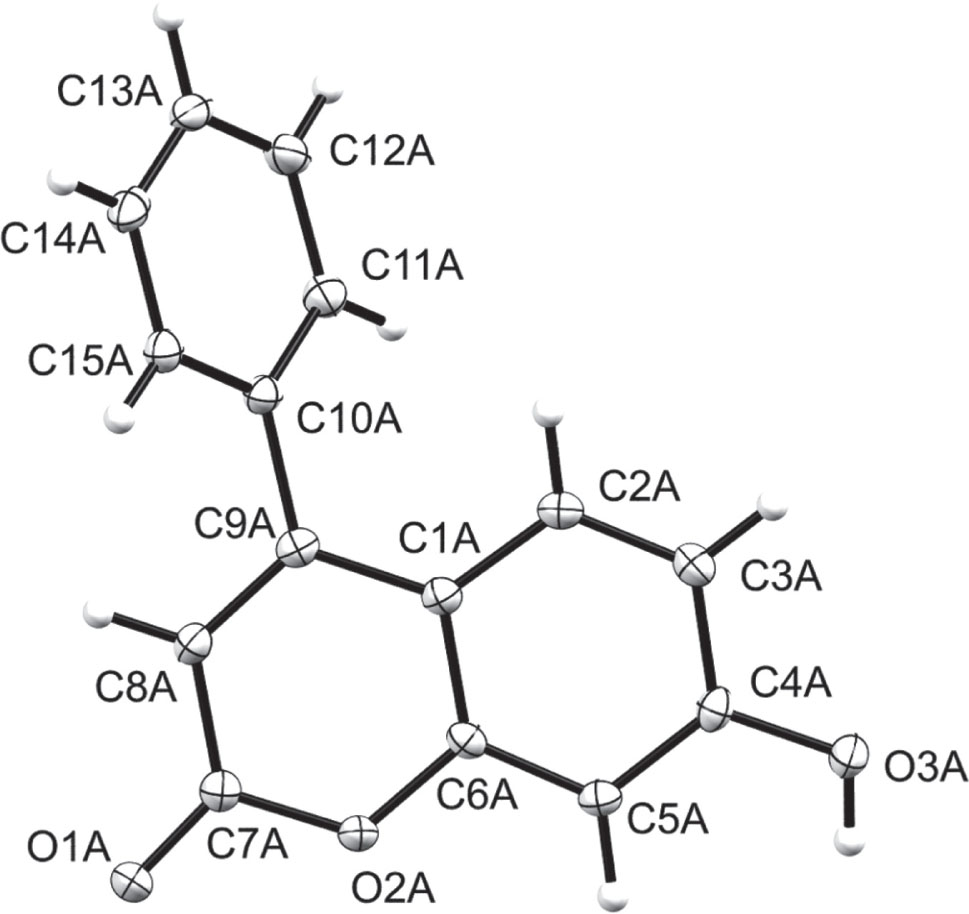
One of two crystallographically independent molecules is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Yellow cube |
| Size: | 0.34 × 0.19 × 0.13 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.10 mm−1 |
| Diffractometer, scan mode: | Bruker SMART APEX-II, φ and ω |
| θmax, completeness: | 27.1°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 16505, 4870, 0.020 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 4398 |
| N(param)refined: | 327 |
| Programs: | Bruker [1], SHELX [2], [4], Mercury [3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| O1A | 0.40529(15) | 1.06797(14) | 0.52290(8) | 0.0230(4) |
| O2A | 0.47075(14) | 0.89724(14) | 0.47707(8) | 0.0183(3) |
| O3A | 0.63608(16) | 0.53720(15) | 0.38100(9) | 0.0246(4) |
| H3A | 0.693104 | 0.549236 | 0.411357 | 0.037* |
| C1A | 0.3389(2) | 0.78939(19) | 0.39366(11) | 0.0173(4) |
| C2A | 0.3327(2) | 0.6905(2) | 0.34834(11) | 0.0197(5) |
| H2A | 0.258401 | 0.680286 | 0.319770 | 0.024* |
| C3A | 0.4319(2) | 0.6085(2) | 0.34465(11) | 0.0210(5) |
| H3AA | 0.425025 | 0.542019 | 0.314133 | 0.025* |
| C4A | 0.5435(2) | 0.6225(2) | 0.38576(11) | 0.0191(4) |
| C5A | 0.5551(2) | 0.7210(2) | 0.42967(11) | 0.0185(4) |
| H5A | 0.631009 | 0.732419 | 0.456843 | 0.022* |
| C6A | 0.4532(2) | 0.80177(19) | 0.43274(11) | 0.0170(4) |
| C7A | 0.3796(2) | 0.9864(2) | 0.48301(12) | 0.0183(5) |
| C8A | 0.2616(2) | 0.9743(2) | 0.44417(12) | 0.0192(4) |
| H8A | 0.197321 | 1.035026 | 0.447996 | 0.023* |
| C9A | 0.2385(2) | 0.8791(2) | 0.40210(11) | 0.0173(4) |
| C10A | 0.1115(2) | 0.8686(2) | 0.36569(11) | 0.0190(5) |
| C11A | 0.0375(2) | 0.7639(2) | 0.37068(13) | 0.0238(5) |
| H11A | 0.068924 | 0.697459 | 0.396477 | 0.029* |
| C12A | −0.0826(2) | 0.7578(3) | 0.33756(14) | 0.0282(5) |
| H12A | −0.133961 | 0.687401 | 0.341524 | 0.034* |
| C13A | −0.1273(2) | 0.8538(2) | 0.29888(12) | 0.0284(6) |
| H13A | −0.208029 | 0.848101 | 0.275301 | 0.034* |
| C14A | −0.0552(2) | 0.9579(2) | 0.29435(12) | 0.0262(5) |
| H14A | −0.086689 | 1.023911 | 0.268177 | 0.031* |
| C15A | 0.0637(2) | 0.9657(2) | 0.32830(12) | 0.0212(5) |
| H15A | 0.112627 | 1.037774 | 0.325950 | 0.025* |
| O1B | 0.09122(16) | 0.95727(15) | 0.61974(9) | 0.0250(4) |
| H1B | 0.035010 | 0.946572 | 0.588788 | 0.038* |
| O2B | 0.25957(14) | 0.59916(14) | 0.52180(8) | 0.0188(3) |
| O3B | 0.32652(16) | 0.42945(15) | 0.47533(9) | 0.0235(4) |
| C1B | 0.3889(2) | 0.7064(2) | 0.60645(11) | 0.0176(5) |
| C2B | 0.3936(2) | 0.8030(2) | 0.65274(12) | 0.0213(5) |
| H2B | 0.467603 | 0.812589 | 0.681663 | 0.026* |
| C3B | 0.2937(2) | 0.8843(2) | 0.65724(12) | 0.0214(5) |
| H3B | 0.298956 | 0.948811 | 0.689232 | 0.026* |
| C4B | 0.1835(2) | 0.8722(2) | 0.61459(11) | 0.0198(5) |
| C5B | 0.1732(2) | 0.7752(2) | 0.56971(11) | 0.0182(4) |
| H5B | 0.098217 | 0.764690 | 0.541682 | 0.022* |
| C6B | 0.2754(2) | 0.6941(2) | 0.56689(11) | 0.0175(4) |
| C7B | 0.3514(2) | 0.5109(2) | 0.51589(12) | 0.0189(5) |
| C8B | 0.4691(2) | 0.5234(2) | 0.55523(12) | 0.0195(5) |
| H8B | 0.533922 | 0.463224 | 0.551519 | 0.023* |
| C9B | 0.4906(2) | 0.6183(2) | 0.59739(11) | 0.0188(5) |
| C10B | 0.6187(2) | 0.6341(2) | 0.63195(11) | 0.0195(5) |
| C11B | 0.6904(2) | 0.7391(2) | 0.62150(12) | 0.0218(5) |
| H11B | 0.656365 | 0.801301 | 0.593236 | 0.026* |
| C12B | 0.8118(2) | 0.7524(2) | 0.65249(12) | 0.0231(5) |
| H12B | 0.861447 | 0.822698 | 0.644253 | 0.028* |
| C13B | 0.8600(2) | 0.6634(2) | 0.69527(12) | 0.0247(5) |
| H13B | 0.941709 | 0.673889 | 0.717389 | 0.030* |
| C14B | 0.7899(2) | 0.5590(2) | 0.70602(13) | 0.0256(5) |
| H14B | 0.823459 | 0.497996 | 0.735326 | 0.031* |
| C15B | 0.6700(2) | 0.5438(2) | 0.67374(12) | 0.0225(5) |
| H15B | 0.622867 | 0.471478 | 0.680223 | 0.027* |
Source of material
The titled compound was prepared by mixing resorcinol (2.0 g) and methyl benzoylacetate (3.2 g) and then added conc. H2SO4 (8 mL, 75%). The temperature of the stirred mixture was increased to 35 °C. After stirring for five hours, the mixture was poured into crushed ice and neutralized with a NaOH solution. The mixture was filtered under vacuum and the residue was washed with plenty of water. The resulting product was purified by silica gel column chromatography with 70% ethylacetate in hexane as eluent and the product was obtained as a yellow solid (4.1 g, 96%) and then recrystallized by ethanol slow evaporation to obtain titled compound as light-yellow crystals. m.p.: 247–249 °C.
Experimental details
A Bruker Smart APEX2 diffractometer with Mo Kα radiation was used for crystal evaluation and data collection [1]. The structure was solved by the direct method using the SHELXS [2] program and refined. The visual crystal structure information was performed using Mercury [3] system software. All C—Haromatic and O—H bond distances were restrained to 0.95 Å, 0.98 Å and 0.99 Å with Uiso(Haromatic) = 1.2Ueq and Uiso(Hhydroxyl) = 1.5Ueq of parent atom, respectively.
Comment
Coumarins are a class of natural compounds with potential benefits on human nature as they play a vital role as food and cosmetics constituents [5], dye-sensitized solar cells [6] and cigarettes additives [7]. In addition, coumarins possess anti-bacterial [8], anti-inflammatory [9], anti-oxidant [10], [11], anticoagulant [8], anti-tumor [12], hepatoprotective, anti-carcinogenic, anti-viral and anti-thrombotic activities [13].
Two crystallographically independent molecules exist in the asymmetric unit of the title compound. Each molecule has a coumarinyl and a phenyl moiety with a measured dihedral angle of 122.67(6)° and 127.38(6)° between the moieties, respectively. All bond distances and angles appear to be normal relative to closely related compounds in literature [14], [15], [16], [17], [18], [19], [20]. Classical, intermolecular O—H⋯O hydrogen bonding were observed between the hydroxyl and carbonyl groups. These links form chains which extends along the crystallographic b axis.
Acknowledgements
We wish to extend our gratitude to the University of KwaZulu-Natal for financial support and an enabling environment for research.
References
1. Bruker. APEXII. Bruker AXS Inc, Madison, WI, USA (2009).Suche in Google Scholar
2. Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Suche in Google Scholar PubMed
3. Macrae, C. F.; Bruno, I. J.; Chisholm, J. A.; Edgington, P. R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P. A.: Mercury CSD 2.0 – new features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 41 (2008) 466–470.10.1107/S0021889807067908Suche in Google Scholar
4. Sheldrick, G. M.: Crystal structure refinement with SHELXL. Acta Crystallogr. C71 (2015) 3–8.10.1107/S2053229614024218Suche in Google Scholar PubMed PubMed Central
5. O’Kennedy, R.; Thornes, R. D.: Coumarins: biology, applications and mode of action. John Wiley & Sons, Chichester (1997).Suche in Google Scholar
6. Hara, K.; Sayama, K.; Ohga, Y.; Shinpo, A.; Suga, S.; Arakawa, H.: A coumarin-derivative dye sensitized nanocrystalline TiO2 solar cell having a high solar-energy conversion efficiency up to 5.6%. Chem. Commun. 37 (2001) 569–570.10.1039/b010058gSuche in Google Scholar
7. Givel, M.: A comparison of US and Norwegian regulation of coumarin in tobacco products. Tob. Control 12 (2003) 401–405.10.1136/tc.12.4.401Suche in Google Scholar PubMed PubMed Central
8. Basanagouda, M.; Kulkarni, M. V.; Sharma, D.; Gupta, V. K.; Sandhyarani, P.; Sasal, V. P.: Synthesis of some new 4-aryloxmethylcoumarins and examination of their antibacterial and antifungal activities. J. Chem. Sci. 121 (2009) 485–495.10.1007/s12039-009-0058-zSuche in Google Scholar
9. Emmanuel-Giota, A. A.; Fylaktakidou, K. C.; Hadjipavlou-Litina, D. J.; Litinas, K. E.; Nicolaides, D. N.: Synthesis and biological evaluation of several 3-(coumarin-4-yl)tetrahydroisoxazole and 3-(coumarin-4-yl)dihydropyrazole derivatives. J. Heterocycl. Chem. 38 (2001) 717–722.10.1002/jhet.5570380329Suche in Google Scholar
10. Vukovic, N.; Sukdolak, S.; Solujic, S.; Niciforovic, N.: An efficient synthesis and antioxidant properties of novel imino and amino derivatives of 4-hydroxy coumarins. Arch. Pharm. Res. 33 (2010) 5–15.10.1007/s12272-010-2220-zSuche in Google Scholar PubMed
11. Hamdi, N.; Dixneuf, P. H.: In topics in heterocyclic chemistry. Springer-Verlag, Berlin Heidelberg (2007).Suche in Google Scholar
12. Marchenko, M. M.; Kopyl’chuk, G. P.; Shmarakov, I. A.; Ketsa, O. V.; Kushnir, V. N.: Synthesis and antitumor activity of 5-(5′,6′-benzocoumaro-3′-yl)methylaminouracil hydrobromide and its liposomal medicinal form. Pharm. Chem. J. 40 (2006) 296–297.10.1007/s11094-006-0113-8Suche in Google Scholar
13. Jung, K.; Park, Y. J.; Ryu, J. S.: Scandium(III) triflate–catalyzed coumarin synthesis. Synth. Commun. 38 (2008) 4395–4406.10.1080/00397910802369513Suche in Google Scholar
14. Zhong, H.; Ruan, J.-L.; Yao, Q.-Q.: Two new 4-arylcoumarins from the seeds of Calophyllum polyanthum. J. Asian Nat. Prod. Res. 12 (2010) 562–568.10.1080/10286020.2010.484806Suche in Google Scholar PubMed
15. Khalymbadzha, I. A.; Chupakhin, O. N.; Fatykhov, R. F.; Charushin, V. N.; Schepochkin, A. V.; Kartsev, V. G.: Transition-metal-free cross-dehydrogenative coupling of triazines with 5,7-dihydroxycoumarins. Synlett 27 (2016) 2606–2610.10.1055/s-0035-1562794Suche in Google Scholar
16. Sandjo, L. P.; Foster, A. J.; Rheinheimer, J.; Anke, H.; Opatz, T.; Thines, E.: Coumarin derivatives from Pedilanthus tithymaloides as inhibitors of conidial germination in Magnaporthe oryzae. Tetrahedron Lett. 53 (2012) 2153–2156.10.1016/j.tetlet.2012.02.056Suche in Google Scholar
17. Wang, Z.; Li, X.; Wang, L.; Li, P.: Photoinduced cyclization of alkynoates to coumarins with N-iodosuccinimide as a free-radical initiator under ambient and metal-free conditions. Tetrahedron 75 (2019) 1044–1051.10.1016/j.tet.2019.01.013Suche in Google Scholar
18. Chan, G.; Awang, K.; Hadi, A. H. A.; Ng, S. W.: 6-[(E)-3,7-Dimethyl-octa-2,6-dien-yl]-5,7-dihydr-oxy-8-(2-methyl- butano-yl)-4-phenyl-2H-chromen-2-one from Mesua kunstleri King (Kosterm). Acta Crystallogr. E64 (2008) o1332.10.1107/S1600536808018151Suche in Google Scholar PubMed PubMed Central
19. De, A.: Crystal structure and conformational aspects of an optically inactive bitter antibiotic Mesuol from Mesua ferrea Linn. J. Crystallogr. Spectrosc. Res. 21 (1991) 97–103.10.1007/BF01158983Suche in Google Scholar
20. Chan, G.; Awang, K.; Ismail, N. H.; Ng, S. W.; Tiekink, E. R. T.: 6-[(2E)-3,7-Dimethylocta-2,6-dien-1-yl]-5,7-dihydroxy-8-(2-methylbutanoyl)-4-phenyl-2H-chromen-2-one–6-[(2E)-3,7-dimethylocta-2,6-dien-1-yl]-5,7-dihydroxy-8-(3-methylbutanoyl)-4-phenyl-2H-chromen-2-one (1/1) from Mesua elegans. Acta Crystallogr. E68 (2012) o939–o940.10.1107/S1600536812008628Suche in Google Scholar PubMed PubMed Central
©2020 Vuyisa Mzozoyana et al., published by De Gruyter.
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Artikel in diesem Heft
- Frontmatter
- Crystal structure of catena-poly[(μ2-3-(benzo[d]thiazol-2-yl)-5-carboxybenzoato-κ2N:O)silver(I)], C15H8AgNO4S
- Crystal structure of bis(4-phenylpiperazin-1-ium) bis(2-(4-phenylpiperazin-1-yl)succinato-κ2O,O′)copper(II) tetrahydrate, C48H70CuN8O12, [C10H14N2]2[Cu(C14H17N2O4)2] ⋅ 4 H2O
- Crystal structure of triaqua-bis(2-(6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylenedecahydronaphthalen-1-yl)-1-(2-oxo-2,5-dihydrofuran-3-yl)ethane-1-sulfonato-κ2O,O′)calcium(II) – ethanol (1/2), C44H76CaO19S2
- The crystal structure of ethyl 5-(4-(diphenylamino)phenyl)thiophene-2-carboxylate, C25H21NO2S
- The crystal structure of 5-bromo-2-(2-methyl-2H-tetrazol-5-yl)pyridine, C7H6BrN5
- The crystal structure of (E)-5-chloro-2-hydroxy-N′-(2-hydroxy-4-methoxybenzylidene)benzohydrazide, C15H13ClN2O4
- The crystal structure of (2Z,2′Z)-N′,N′′′′-(pyridine-2,6-dicarbonyl)dipicolinohydrazonamide, C19H17N9O2
- Photochromic properties and crystal structure of 3,3′-(perfluorocyclopent-1-ene-1,2-diyl)bis(5-(4-(azidomethyl)phenyl)-2-methylthiophene), C29H20F6N6S2
- Crystal structure of aqua-dichlorido-(4-(((3-ethoxy-2-oxidobenzylidene)hydrazono)(oxido)methyl)pyridin-1-ium-κ3N,O,O′)iron(III), C15H16Cl2N3O4Fe
- Crystal structure of catena-poly[diaqua-(μ2-4,4′-bipyridine-κ2N:N′)-bis(2,3,4,5-tetrabromo-6-carboxybenzoato-κ1O)-nickel(II)], C26H14Br8NiN2O10
- Crystal structure of diethanol-κ1O-bis(μ2-N-((2-oxidonaphthalen-1-yl)methylene)pyrazine-2-carbohydrazonato-κ5N,O,O′:O′:N′)-bis(nitrato-κ2O,O′)dieuropium(III), C36H32N10O12Eu2
- The crystal structure of 2-aminoisophthalic acid, C8H7NO4
- Crystal structure of (E)-2-(4-((3,4-difluorobenzyl)oxy)styryl)-4,6-dimethoxybenzaldehyde, C24H20F2O4
- Crystal structure of 2-benzoylpyrene, C23H14O
- Crystal structure of chlorido-(η6-p-cymene)-(N-(2-fluorophenyl)-1-(pyridin-2-yl)methanimine-κ2N,N′)ruthenium(II) – acetone (1/1), C22H23ClN2F7OPRu
- The crystal structure of 2-bromoisonicotinic acid, C6H4BrNO2
- Crystal structure of 1,3,5,7-tetraphenyl-8-(N-phenylformamido)-2-oxa-5-azabicyclo[4.2.0]oct -3-en-7-yl benzoate, C44H34N2O4
- Synthesis and crystal structure of 4-(3-acetyl-5-(thiophen-2-yl)-2,3-dihydro-1,3,4-oxadiazol-2-yl)-7-(diethylamino)-2H-chromen-2-one, C21H21N3O4S
- Crystal structure of poly[diaqua-(μ2-4,4′-bipyridine-κ2N:N′)-(μ2-3,4,5,6-tetrafluorophthalato-κ2O:O′)nickel(II)], C18H12F4NiN2O6
- Crystal structure of 4-hydroxynaphtho[2,3-b]benzofuran-6,11-dione, C16H8O4
- The crystal structure of 3,10-bis(4-methoxyphenyl)-6,12-dibenzyl-2,9-acetyl-6,12-diazapentacyclo[6.3.1.02,7.04,11.05,9]dodecane – acetone (1/1), C45H48N2O5
- The crystal structure of (E)-2-(((2-(1H-indol-3-yl)ethyl)iminio)methyl)-6-bromophenolate, C17H15N2BrO
- Crystal structure of catena-poly[diaqua-(μ2-oxalyl dihydrazide-κ4N,O:N′,O′)-bis(μ2-pyridine-2,3-dicarboxylato-κ3N,O,O′)dicadmium(II)] hexahydrate, C16H28O18N6Cd2
- Crystal structure of poly[tetra-(μ4-naphthalene-1,8-dicarboxylato-κ4O:O,O′: O′′:O′′,O′′′)-(μ4-oxo-κ4O:O:O:O) penta-lead(II)], C48H24O17Pb5
- Crystal structure of 5H-dibenzo[c,f][1,5]oxabismocin-12 (7H)-yl acetate, C16H15O3Bi
- The crystal structure of 2-(4-chloro-6-nitrophenyl)-1-(4-chloro-3-nitrophenyl)diazene 1-oxide, C12H6Cl2N4O5
- Crystal structure of bis(3-methyl-N-(1-(pyrazin-2-yl)ethylidene)benzohydrazonato-κ3O,N,N′)nickel(II), C28H26N8O2Ni
- Crystal structure of 3,10-bis(4-chlorophenyl)-6,12-dibenzyl-2,9-acetyl-6,12-diazapentacyclo[6.3.1.02,7.04,11.05,9]-dodecane, C40H36Cl2N2O2
- Crystal structure of bis[(μ2-4⋯O,O′:O′)-(4-hydroxybenzoato-κ2O,O′)-(1,10-phenanthroline-κ2N,N′)]-di-lead(II)μ-4-hydroxybenzoato-κ3O,O′:O′;κ3O,O′:O′-bis-[(4-hydroxybenzoato-κ2O,O′)bis(1,10-phenanthroline-κ2N,N′)di-lead(II)] monohydrate, C52H36N4O12Pb2 ⋅ H2O
- Crystal structure of poly[diaqua-(μ3-3,4,5,6-tetrafluoro-phthalato-κ3O:O′:O′′)-(μ2-1,2-bis(4-pyridyl)ethene-κ2N:N′)cobalt(II)], C14H9CoF4NO6
- Crystal structure of 7-hydroxy-4-phenyl-2H-chromen-2-one, C15H10O3
- Crystal structure of 3,7-dimethyl-1-(5-oxohexyl)-3,7-dihydro-1H-purine-2,6-dione 4-hydroxybenzoic acid, C20H24N4O6
- Crystal structure of catena-poly[(μ2-1,5-dimethyl-2-phenyl-4-((pyridin-4-ylmethylene)amino)-1,2-dihydro-3H-pyrazol-3-one-κ2N:O)-bis(nitrato-κ1O)zinc(II)], C17H16N6O7Zn
- The crystal structure of diaqua-bis(6-aminopicolinato-κ2N,O)magnesium(II), C12H14O6N4Mg
- Crystal structure of (pyridine-2-carboxamide-κ2N,O)-[tris((1H-benzo[d]imidazol-2-yl)methyl)amine-κ4N,N′,N′′,N′′′]nickel(II) diperchlorate — methanol (1/3), C33H39Cl2N9NiO12
- Crystal structure of catena-poly[diaqua-bis(3-(4-trifluoromethyl-phenyl)-acrylato-κO1)-(μ2-1,4-bis(1-imidazolyl)benzene-κ2N3:N3′)cobalt(II)], C32H26CoF6N4O6
- Crystal structure of (E)-3-(2-(2-hydroxy-4-methoxystyryl)-3,3-dimethyl-3H-indol-1-ium-1-yl)propane-1-sulfonate monohydrate, C22H25NO5S⋅H2O
- The crystal structure of bis(N-oxy-2-(1H-tetrazol-1-yl) acetamide κ2O,O′)-diaqua-zinc(II), C6H12ZnN10O6
- Crystal structure of (E)-4-((4-chlorophenylimino)methyl)pyridinium 3,5-dinitrobenzoate, C19H13ClN4O6
- Crystal structure of dichlorido-bis((E)-2-((pyridin-4-ylmethylene)amino)phenol)zinc(II), C24H20Cl2N4O2Zn
- Crystal structure of cyclo-[tetrachlorido-bis(μ2-p-xylylenediamine-κ2N:N′)dipalladium(II)] dimethyl sulfoxide solvate, C20H36Cl4N4O2Pd2S2
- Crystal structure of 4-(3-fluorophenyl)-7-hydroxy-2H-chromen-2-one, C15H9FO3
- Crystal structure of (E)-2-((2-(pyrimidin-2-yl)hydrazono)methyl)quinolin-1-ium perchlorate – methanol (1/1), C15H16N5O5Cl
- The crystal structure of bis(N-(amino(pyridin-2-yl)methylene)-5-chloro-2-hydroxybenzohydrazonato-κ3N,N′,O)zinc(II) – methanol (2/5), C57H60Cl2N16O13Zn2
- Synthesis and crystal structure of 4,4′-di(4-pyridyl)-6,6′-di(tert-butyl)-2,2′-[propylenedioxybis(nitrilomethylidyne)]diphenol, C35H40N4O4
- Crystal structure of (3E,3′E)-3,3′-((1,3,4-thiadiazole-2,5-diyl)bis(sulfanediyl))bis(4-hydroxy-4-phenylbut-3-en-2-one), C22H18N2O4S3
- Crystal structure of (N-benzyl-N-methyl-dithiocarbamato-κ2S,S′)di(4-chlorobenzyl)chloridotin(IV), C23H22Cl3NS2Sn
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ6O6) sodium bromide hydrate, [Na(18-crown-6)]Br ⋅ H2O, C12H26BrNaO7
- Crystal structure of 7-ethoxyl-6,8-difluoro-4-oxo-1-phenyl-1,4-dihydro-quinoline-3-carboxylic acid, C18H13F2N1O4
- Crystal structure of chlorido (2-(4-ethylphenyl)pyrimidine-k2C,N)(triphenylphosphane-kP) palladium(II), C30H26ClN2PPd
- Crystal structure of 18-crown-6 – 1,4-diiodotetrafluorobenzene – acetonitrile (1/1/2), C22H30F4I2N2O6
- Crystal structure of diisobutyl 2,5-dihydroxycyclohexa-1,4-diene-1,4-dicarboxylate, C16H24O6
- Crystal structure of poly[[tris(μ2-cis-1,2-cyclohexanedicarboxylato)-κ2O, O′]-bis[μ3-1,3,5-tris[(1H-imidazol-1-yl)methyl]benzene-κ3N, N′,N′′]-trizinc(II)] – water (1/20), C60H106N12O32Zn3
- The synthesis and crystal structure of 5-amino-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-((trifluoromethyl)sulfinyl)-1H-pyrazole-3-carboxamide–tetrahydrofuran (1/1), C16H14N4Cl2F6O3S
- Crystal structure of dimethylbis(diisopropyldithiocarbamato-κ2S,S′)tin(IV), C16H34N2S4Sn
- Crystal structure of diisopropyl 2,5-dihydroxycyclohexa-1,4-diene-1,4-dicarboxylate, C14H20O6
- The synthesis and crystal structure of ethyl (E)-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-5-((2-methoxybenzylidene)amino)-4-((trifluoromethyl)sulfinyl)-1H-pyrazole-3-carboxylate, C22H15N3Cl2F6O4S
- The crystal structure of a matrine derivative, 13-(methylamine-1-yl) carbodithioate matrine, C17H27N3OS2
- Crystal structure of bis(2-hydroxy-6-((phenylimino)methyl)phenolato-κ2N,O)copper(II), C26H20CuN2O4
- The crystal structure of 2-p-fluorophenyl-5-dihydroxymethyl-1,3,4-oxadiazole, C9H7FN2O3
- Crystal structure of dichloridobis(4-chlorophenyl-κC1)(1,10-phenanthroline-κ2N,N′)tin(IV), C24H16Cl4N2Sn
- Crystal structure of bis{bromido-triphenyltin(IV)}(μ2-[4,4′-bipyridine]1,1′-dioxide-κ2O:O′), C46H38Br2N2O2Sn2
- Crystal structure of 2-(5-chloro-quinolin-8-yloxy)-N-quinolin-8-yl-acetamide, C20H14N3O2Cl
- Crystal structure of bis(N-(1-(3-ethylpyrazin-2-yl)ethylidene)-3-hydroxy-2-naphthohydrazonato-κ3N,N′,O)cobalt(II) — dimethylformamide (1/1), C41H41N9O5Co
- Crystal structure of bis[2-(1-(3-ethylpyrazin-2-yl)ethylidene)-1-tosylhydrazin-1-ido-κ3-N,N′,O]copper(II), C30H34N8O4S2Cu
- Crystal structure of (2-p-tolylpyrimidine-κ2C,N)(triphenylphosphane-κP) palladium(II), C29H24ClN2PPd
- Halogen bonding in crystal structure of bis(1,4,7,10-tetraoxacyclododecane-κ4O,O′,O′′,O′′′)cesium triiodide, C16H32CsI3O8
- The synthesis and crystal structure of N-(3-cyano-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-(trifluoromethylsulfinyl)-1H-pyrazol-5-yl)-2-phenylacetamide, C20H10N4Cl2F6O2S
- The crystal structure of 4-(trifluoromethyl)nicotinic acid, C7H4F3NO2
- Crystal structure of 3-(2-methylbenzyl)thiazolidin-2-one, C11H13ONS
- The crystal structure of 2,2,2-trifluoro-1-(isoquinolin-1-yl)ethane-1,1-diol, C11H8F3NO2
- The crystal structure of 3-bromoisonicotinic acid, C6H4BrNO2
- The crystal structure of 5-nitropicolinic acid monohydrate, C6H6N2O5
- The crystal structure of 3-(4-hydroxybenzyl)-1,5-dioxaspiro[5.5]undecane-2,4-dione, C16H18O5
- Crystal structure of [[Mo3Se7(S2CNEt2)3]2(μ-Se)] ⋅ 2(C6H4Cl2), C42H68Cl4Mo6N6S12Se15
- Crystal structure of (E)-4-hydroxy-3-((5-phenyl-1,3,4-oxadiazol-2-yl)thio)pent-3-en-2-one, C13H12N2O3S
- The crystal structure of (2,3-dioxo-5,6:13,14-dibenzo-9,10-benzo-1,4,8,11-7, 11-diene-κ4N,N′,N′′,N′′′)-nickel(II), Ni(C22H14N4O2)
- Crystal structure of 3-(1-benzyl-2-ethyl-4-nitro-1H-imidazol-5-ylthio)-propanoic acid, C15H17N3O4S
- The crystal structure of dichlorobis(2-(dicyclohexylphosphino)-2′,4′,6′-tri-i-propyl-1,1′-biphenyl) palladium(II)-dichloroform, C68H100Cl8P2Pd
- Crystal structure and antimicrobial properties of (1,4,7,10-tetraoxacyclododecane-κ4O,O′,O′′,O′′′)cesium(I) pentaiodide, C16H32CsI5O8
Artikel in diesem Heft
- Frontmatter
- Crystal structure of catena-poly[(μ2-3-(benzo[d]thiazol-2-yl)-5-carboxybenzoato-κ2N:O)silver(I)], C15H8AgNO4S
- Crystal structure of bis(4-phenylpiperazin-1-ium) bis(2-(4-phenylpiperazin-1-yl)succinato-κ2O,O′)copper(II) tetrahydrate, C48H70CuN8O12, [C10H14N2]2[Cu(C14H17N2O4)2] ⋅ 4 H2O
- Crystal structure of triaqua-bis(2-(6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylenedecahydronaphthalen-1-yl)-1-(2-oxo-2,5-dihydrofuran-3-yl)ethane-1-sulfonato-κ2O,O′)calcium(II) – ethanol (1/2), C44H76CaO19S2
- The crystal structure of ethyl 5-(4-(diphenylamino)phenyl)thiophene-2-carboxylate, C25H21NO2S
- The crystal structure of 5-bromo-2-(2-methyl-2H-tetrazol-5-yl)pyridine, C7H6BrN5
- The crystal structure of (E)-5-chloro-2-hydroxy-N′-(2-hydroxy-4-methoxybenzylidene)benzohydrazide, C15H13ClN2O4
- The crystal structure of (2Z,2′Z)-N′,N′′′′-(pyridine-2,6-dicarbonyl)dipicolinohydrazonamide, C19H17N9O2
- Photochromic properties and crystal structure of 3,3′-(perfluorocyclopent-1-ene-1,2-diyl)bis(5-(4-(azidomethyl)phenyl)-2-methylthiophene), C29H20F6N6S2
- Crystal structure of aqua-dichlorido-(4-(((3-ethoxy-2-oxidobenzylidene)hydrazono)(oxido)methyl)pyridin-1-ium-κ3N,O,O′)iron(III), C15H16Cl2N3O4Fe
- Crystal structure of catena-poly[diaqua-(μ2-4,4′-bipyridine-κ2N:N′)-bis(2,3,4,5-tetrabromo-6-carboxybenzoato-κ1O)-nickel(II)], C26H14Br8NiN2O10
- Crystal structure of diethanol-κ1O-bis(μ2-N-((2-oxidonaphthalen-1-yl)methylene)pyrazine-2-carbohydrazonato-κ5N,O,O′:O′:N′)-bis(nitrato-κ2O,O′)dieuropium(III), C36H32N10O12Eu2
- The crystal structure of 2-aminoisophthalic acid, C8H7NO4
- Crystal structure of (E)-2-(4-((3,4-difluorobenzyl)oxy)styryl)-4,6-dimethoxybenzaldehyde, C24H20F2O4
- Crystal structure of 2-benzoylpyrene, C23H14O
- Crystal structure of chlorido-(η6-p-cymene)-(N-(2-fluorophenyl)-1-(pyridin-2-yl)methanimine-κ2N,N′)ruthenium(II) – acetone (1/1), C22H23ClN2F7OPRu
- The crystal structure of 2-bromoisonicotinic acid, C6H4BrNO2
- Crystal structure of 1,3,5,7-tetraphenyl-8-(N-phenylformamido)-2-oxa-5-azabicyclo[4.2.0]oct -3-en-7-yl benzoate, C44H34N2O4
- Synthesis and crystal structure of 4-(3-acetyl-5-(thiophen-2-yl)-2,3-dihydro-1,3,4-oxadiazol-2-yl)-7-(diethylamino)-2H-chromen-2-one, C21H21N3O4S
- Crystal structure of poly[diaqua-(μ2-4,4′-bipyridine-κ2N:N′)-(μ2-3,4,5,6-tetrafluorophthalato-κ2O:O′)nickel(II)], C18H12F4NiN2O6
- Crystal structure of 4-hydroxynaphtho[2,3-b]benzofuran-6,11-dione, C16H8O4
- The crystal structure of 3,10-bis(4-methoxyphenyl)-6,12-dibenzyl-2,9-acetyl-6,12-diazapentacyclo[6.3.1.02,7.04,11.05,9]dodecane – acetone (1/1), C45H48N2O5
- The crystal structure of (E)-2-(((2-(1H-indol-3-yl)ethyl)iminio)methyl)-6-bromophenolate, C17H15N2BrO
- Crystal structure of catena-poly[diaqua-(μ2-oxalyl dihydrazide-κ4N,O:N′,O′)-bis(μ2-pyridine-2,3-dicarboxylato-κ3N,O,O′)dicadmium(II)] hexahydrate, C16H28O18N6Cd2
- Crystal structure of poly[tetra-(μ4-naphthalene-1,8-dicarboxylato-κ4O:O,O′: O′′:O′′,O′′′)-(μ4-oxo-κ4O:O:O:O) penta-lead(II)], C48H24O17Pb5
- Crystal structure of 5H-dibenzo[c,f][1,5]oxabismocin-12 (7H)-yl acetate, C16H15O3Bi
- The crystal structure of 2-(4-chloro-6-nitrophenyl)-1-(4-chloro-3-nitrophenyl)diazene 1-oxide, C12H6Cl2N4O5
- Crystal structure of bis(3-methyl-N-(1-(pyrazin-2-yl)ethylidene)benzohydrazonato-κ3O,N,N′)nickel(II), C28H26N8O2Ni
- Crystal structure of 3,10-bis(4-chlorophenyl)-6,12-dibenzyl-2,9-acetyl-6,12-diazapentacyclo[6.3.1.02,7.04,11.05,9]-dodecane, C40H36Cl2N2O2
- Crystal structure of bis[(μ2-4⋯O,O′:O′)-(4-hydroxybenzoato-κ2O,O′)-(1,10-phenanthroline-κ2N,N′)]-di-lead(II)μ-4-hydroxybenzoato-κ3O,O′:O′;κ3O,O′:O′-bis-[(4-hydroxybenzoato-κ2O,O′)bis(1,10-phenanthroline-κ2N,N′)di-lead(II)] monohydrate, C52H36N4O12Pb2 ⋅ H2O
- Crystal structure of poly[diaqua-(μ3-3,4,5,6-tetrafluoro-phthalato-κ3O:O′:O′′)-(μ2-1,2-bis(4-pyridyl)ethene-κ2N:N′)cobalt(II)], C14H9CoF4NO6
- Crystal structure of 7-hydroxy-4-phenyl-2H-chromen-2-one, C15H10O3
- Crystal structure of 3,7-dimethyl-1-(5-oxohexyl)-3,7-dihydro-1H-purine-2,6-dione 4-hydroxybenzoic acid, C20H24N4O6
- Crystal structure of catena-poly[(μ2-1,5-dimethyl-2-phenyl-4-((pyridin-4-ylmethylene)amino)-1,2-dihydro-3H-pyrazol-3-one-κ2N:O)-bis(nitrato-κ1O)zinc(II)], C17H16N6O7Zn
- The crystal structure of diaqua-bis(6-aminopicolinato-κ2N,O)magnesium(II), C12H14O6N4Mg
- Crystal structure of (pyridine-2-carboxamide-κ2N,O)-[tris((1H-benzo[d]imidazol-2-yl)methyl)amine-κ4N,N′,N′′,N′′′]nickel(II) diperchlorate — methanol (1/3), C33H39Cl2N9NiO12
- Crystal structure of catena-poly[diaqua-bis(3-(4-trifluoromethyl-phenyl)-acrylato-κO1)-(μ2-1,4-bis(1-imidazolyl)benzene-κ2N3:N3′)cobalt(II)], C32H26CoF6N4O6
- Crystal structure of (E)-3-(2-(2-hydroxy-4-methoxystyryl)-3,3-dimethyl-3H-indol-1-ium-1-yl)propane-1-sulfonate monohydrate, C22H25NO5S⋅H2O
- The crystal structure of bis(N-oxy-2-(1H-tetrazol-1-yl) acetamide κ2O,O′)-diaqua-zinc(II), C6H12ZnN10O6
- Crystal structure of (E)-4-((4-chlorophenylimino)methyl)pyridinium 3,5-dinitrobenzoate, C19H13ClN4O6
- Crystal structure of dichlorido-bis((E)-2-((pyridin-4-ylmethylene)amino)phenol)zinc(II), C24H20Cl2N4O2Zn
- Crystal structure of cyclo-[tetrachlorido-bis(μ2-p-xylylenediamine-κ2N:N′)dipalladium(II)] dimethyl sulfoxide solvate, C20H36Cl4N4O2Pd2S2
- Crystal structure of 4-(3-fluorophenyl)-7-hydroxy-2H-chromen-2-one, C15H9FO3
- Crystal structure of (E)-2-((2-(pyrimidin-2-yl)hydrazono)methyl)quinolin-1-ium perchlorate – methanol (1/1), C15H16N5O5Cl
- The crystal structure of bis(N-(amino(pyridin-2-yl)methylene)-5-chloro-2-hydroxybenzohydrazonato-κ3N,N′,O)zinc(II) – methanol (2/5), C57H60Cl2N16O13Zn2
- Synthesis and crystal structure of 4,4′-di(4-pyridyl)-6,6′-di(tert-butyl)-2,2′-[propylenedioxybis(nitrilomethylidyne)]diphenol, C35H40N4O4
- Crystal structure of (3E,3′E)-3,3′-((1,3,4-thiadiazole-2,5-diyl)bis(sulfanediyl))bis(4-hydroxy-4-phenylbut-3-en-2-one), C22H18N2O4S3
- Crystal structure of (N-benzyl-N-methyl-dithiocarbamato-κ2S,S′)di(4-chlorobenzyl)chloridotin(IV), C23H22Cl3NS2Sn
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ6O6) sodium bromide hydrate, [Na(18-crown-6)]Br ⋅ H2O, C12H26BrNaO7
- Crystal structure of 7-ethoxyl-6,8-difluoro-4-oxo-1-phenyl-1,4-dihydro-quinoline-3-carboxylic acid, C18H13F2N1O4
- Crystal structure of chlorido (2-(4-ethylphenyl)pyrimidine-k2C,N)(triphenylphosphane-kP) palladium(II), C30H26ClN2PPd
- Crystal structure of 18-crown-6 – 1,4-diiodotetrafluorobenzene – acetonitrile (1/1/2), C22H30F4I2N2O6
- Crystal structure of diisobutyl 2,5-dihydroxycyclohexa-1,4-diene-1,4-dicarboxylate, C16H24O6
- Crystal structure of poly[[tris(μ2-cis-1,2-cyclohexanedicarboxylato)-κ2O, O′]-bis[μ3-1,3,5-tris[(1H-imidazol-1-yl)methyl]benzene-κ3N, N′,N′′]-trizinc(II)] – water (1/20), C60H106N12O32Zn3
- The synthesis and crystal structure of 5-amino-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-((trifluoromethyl)sulfinyl)-1H-pyrazole-3-carboxamide–tetrahydrofuran (1/1), C16H14N4Cl2F6O3S
- Crystal structure of dimethylbis(diisopropyldithiocarbamato-κ2S,S′)tin(IV), C16H34N2S4Sn
- Crystal structure of diisopropyl 2,5-dihydroxycyclohexa-1,4-diene-1,4-dicarboxylate, C14H20O6
- The synthesis and crystal structure of ethyl (E)-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-5-((2-methoxybenzylidene)amino)-4-((trifluoromethyl)sulfinyl)-1H-pyrazole-3-carboxylate, C22H15N3Cl2F6O4S
- The crystal structure of a matrine derivative, 13-(methylamine-1-yl) carbodithioate matrine, C17H27N3OS2
- Crystal structure of bis(2-hydroxy-6-((phenylimino)methyl)phenolato-κ2N,O)copper(II), C26H20CuN2O4
- The crystal structure of 2-p-fluorophenyl-5-dihydroxymethyl-1,3,4-oxadiazole, C9H7FN2O3
- Crystal structure of dichloridobis(4-chlorophenyl-κC1)(1,10-phenanthroline-κ2N,N′)tin(IV), C24H16Cl4N2Sn
- Crystal structure of bis{bromido-triphenyltin(IV)}(μ2-[4,4′-bipyridine]1,1′-dioxide-κ2O:O′), C46H38Br2N2O2Sn2
- Crystal structure of 2-(5-chloro-quinolin-8-yloxy)-N-quinolin-8-yl-acetamide, C20H14N3O2Cl
- Crystal structure of bis(N-(1-(3-ethylpyrazin-2-yl)ethylidene)-3-hydroxy-2-naphthohydrazonato-κ3N,N′,O)cobalt(II) — dimethylformamide (1/1), C41H41N9O5Co
- Crystal structure of bis[2-(1-(3-ethylpyrazin-2-yl)ethylidene)-1-tosylhydrazin-1-ido-κ3-N,N′,O]copper(II), C30H34N8O4S2Cu
- Crystal structure of (2-p-tolylpyrimidine-κ2C,N)(triphenylphosphane-κP) palladium(II), C29H24ClN2PPd
- Halogen bonding in crystal structure of bis(1,4,7,10-tetraoxacyclododecane-κ4O,O′,O′′,O′′′)cesium triiodide, C16H32CsI3O8
- The synthesis and crystal structure of N-(3-cyano-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-(trifluoromethylsulfinyl)-1H-pyrazol-5-yl)-2-phenylacetamide, C20H10N4Cl2F6O2S
- The crystal structure of 4-(trifluoromethyl)nicotinic acid, C7H4F3NO2
- Crystal structure of 3-(2-methylbenzyl)thiazolidin-2-one, C11H13ONS
- The crystal structure of 2,2,2-trifluoro-1-(isoquinolin-1-yl)ethane-1,1-diol, C11H8F3NO2
- The crystal structure of 3-bromoisonicotinic acid, C6H4BrNO2
- The crystal structure of 5-nitropicolinic acid monohydrate, C6H6N2O5
- The crystal structure of 3-(4-hydroxybenzyl)-1,5-dioxaspiro[5.5]undecane-2,4-dione, C16H18O5
- Crystal structure of [[Mo3Se7(S2CNEt2)3]2(μ-Se)] ⋅ 2(C6H4Cl2), C42H68Cl4Mo6N6S12Se15
- Crystal structure of (E)-4-hydroxy-3-((5-phenyl-1,3,4-oxadiazol-2-yl)thio)pent-3-en-2-one, C13H12N2O3S
- The crystal structure of (2,3-dioxo-5,6:13,14-dibenzo-9,10-benzo-1,4,8,11-7, 11-diene-κ4N,N′,N′′,N′′′)-nickel(II), Ni(C22H14N4O2)
- Crystal structure of 3-(1-benzyl-2-ethyl-4-nitro-1H-imidazol-5-ylthio)-propanoic acid, C15H17N3O4S
- The crystal structure of dichlorobis(2-(dicyclohexylphosphino)-2′,4′,6′-tri-i-propyl-1,1′-biphenyl) palladium(II)-dichloroform, C68H100Cl8P2Pd
- Crystal structure and antimicrobial properties of (1,4,7,10-tetraoxacyclododecane-κ4O,O′,O′′,O′′′)cesium(I) pentaiodide, C16H32CsI5O8


