The nutritional, phytochemical composition, and utilisation of different parts of maize: A comparative analysis
-
Pratikantam Swati
Abstract
Maize (Zea mays L.) is known to be one of the current crops with wide adaptability and the potential to grow in various agroecological zones. It has been titled as “queen of cereals” group owing to its high genetic yield capability and abundance among the cereal crop. This study highlights the nutritive composition, phytochemical composition, pharmaceutical properties, and the unconventional use (like ethanol production) of maize plant parts such as the husk, silk, and cob, along with their utilisation in the food sector and pharmaceutical industries. Apart from the kernels, bulk of the harvest, if not used as manure, is majorly treated as waste and is usually discarded. Maize can be incorporated and utilised in the waste management of crop residues. The industrial significance of the maize crop is unmatched when compared to other cereal crops and it is used as a raw material for over 3,000 products in various sectors namely, sweeteners, cosmetics, textiles, gum, alcoholic beverages, films, package, and paper industries. Each part of the maize plant is rich in macronutrients (carbohydrates and proteins) and micronutrients (vitamins and minerals) along with other phytochemical constituents due to which it has an immense scope to be used in value-added products providing various pharmacological properties.
1 Introduction
The world’s most widely grown cereal, maize (Zea mays L.), was domesticated in Central America [1]. The spread of maize from its centre of origin in Mexico to various parts of the world has been rapid with respect to its evolution as a crop and its use in a variety of food products [2]. Maize is one of the most adaptable crops cultivated in various agro-climatic zones [3]. It is also known as the poor man’s nutri-cereal. It serves as a food crop for roughly 4.5 billion people in 94 developing nations, supplying about 30% of their daily caloric needs. In addition to being a significant source of oil, starch, biofuel, etc., 63% of maize grown worldwide is utilised as animal feed [4].
It has become a globally important crop by virtue of its multiple applications in the field of food and fodder, alongside its industrial usage [5]. Aside from that, maize is a crucial industrial raw material with a lot of scope for value addition. Maize is a highly evolved higher organism with well-formed morphological structures, each of which performs specific biological and physiological activities. As a result, maize can now be used as a model organism in scientific research [6].
The various parts of the maize plant are shown in Figure 1. A cob is the centre core of an ear of corn where the kernels develop, often known as a cob of the corn. The ear is commonly called the “cob” or “pole” of the plant. Stigma maydis, the corn silk, is a cluster of silky fibres that emerges from the apex of the ear of the plant. Husks encase the ear. An extended style is linked to each ovary by each fibre. Corn silk is a valuable provider of proteins, vitamins, carbohydrates, minerals, as well as fibre to humans [7]. It is one of the major by-products of maize often discarded. The husk constitutes 20–30% of the maize plant. Corn husks are majorly used in waste utilisation projects. It is used in high-voltage supercapacitors [8].
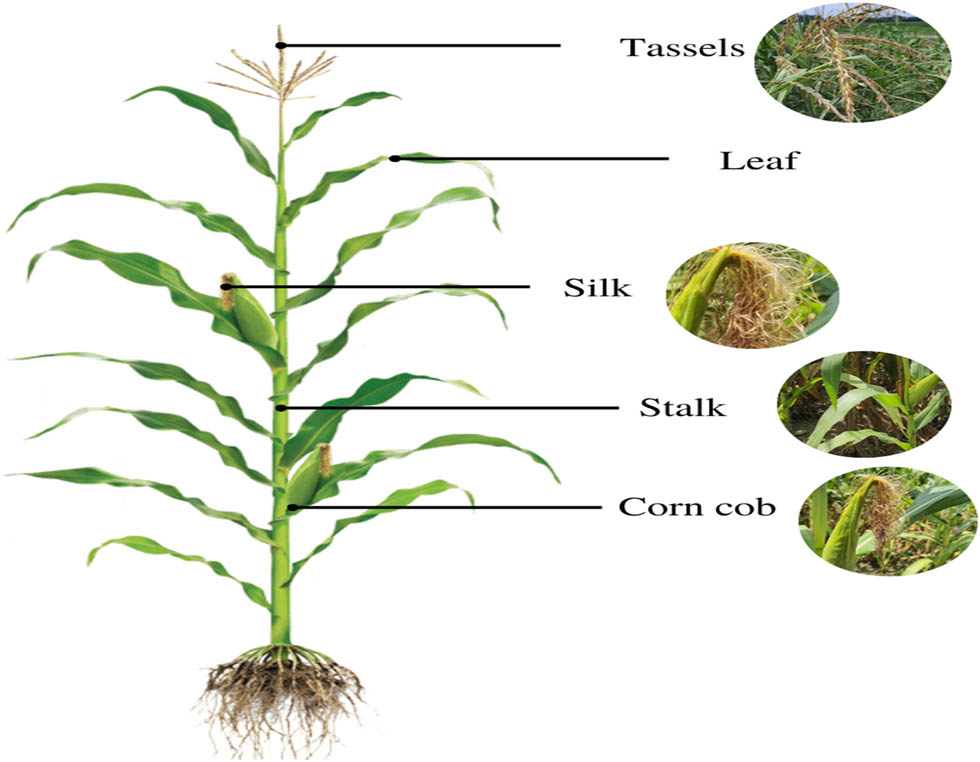
Parts of a maize plant. Source: The author.
Based on the properties of the kernel, there are five broad categories of corn namely dent corn, flint corn, popcorn, floury corn, and sweet corn. The majority of commercial maize is of the dent kind, so-called because of the recessed crown. The flint type of corn has a spherical head and the toughest seed because it has a significant amount of vitreous endosperm, also known as corneous or horny endosperm. Popcorn is a little flint corn variety that prevails in Argentina, selected regions of Italy as well as Africa [9]. The first generation of corn containing desirable levels of lysine and tryptophan, commonly known as the quality protein maize (QPM), has an intermediary glasslike endosperm that is mostly a floury endosperm. Sweet corn has high sugar content. Unlike other varieties, which are harvested when the kernels are dry and mature, sweet corn is harvested while still immature and eaten as a vegetable rather than a grain [10].
2 Production and consumption of maize
Maize is a globally prominent crop cultivated with a production of around 44 billion tonnes of grain in the years 2022–2023. Based on the current trend, by 2030, maize production is projected to surpass that of wheat [11]. The United States of America produces half of the world’s maize, with Asia producing a third (32%) and Europe and Africa producing the majority of the rest (11 and 7.4%, respectively). Eight nations – the United States, Brazil, China, Ukraine, Argentina, India, Mexico, and Indonesia – deliver more than 25 MT per year, accounting for a total of 881 MT, or 75%, of the world’s maize production (77.4%) [12]. In the year 2020, one-third of the farms worldwide cultivated maize [11].
Despite abundant domestic corn supplies, the main factor driving massive imports is the current considerable discount for imported feed grains, especially at ports in South China. As of late June 2024, FAS/Beijing reports that imported Brazilian corn was $285 per ton, while local China corn was $348 per ton. From October to May of 2024, 20.7 million tons of corn was imported, of which 14.8 million tons were from Brazil. Brazil has a competitive edge due to its abundant harvest and favourable seasonal timing, which makes its maize an extremely attractive origin for Chinese consumers. What remained, 5.9 million tons, mostly came from Trade Data Monitor, based in the US and Ukraine [13,14].
Within the cereals group, maize has one of the highest production [14]. It is a fast-growing cash crop, and it now accounts for the majority of the global coarse grain trade. Meeting the global food exigencies and desire for consumption, maize has come up as one of the top crops for developing countries. Maize was initially in demand for its use in poultry feed and for the starch derived from it. The demand for starch is high and is increasing at a rate of 10–12% yearly concerning the increasing expenditure in the food and pharmaceutical industry. Starch derived from maize has been successfully utilised in textile, food, pharmaceutical, and paper industries [15].
The prepotent bulk maize grown in India is utilised as chicken feed. The remainder of the bulk is distributed for fodder, the food industry, industrial purposes, the starch industry, pre-packaged food, and for exportation or other menial uses [14].
Maize is the primary food source for the majority of indigenous groups. Due to its high productivity, maize has the highest cost–benefit ratio compared to other cereal crop, and its economics of cultivation are practically identical to those of sorghum and wheat. Since many small-scale farmers cultivate maize, it provides a cost-effective source of food for those who live in rural areas [4].
3 Cultivation and development of maize
Maize is an adaptable cereal that can grow in diverse agroecologies in temperate and tropical climates from 58° North to 30° South and can even thrive at higher altitudes of up to 4,000 masl. With a photoperiod of 12.5 h and relative humidity of 85–100%, it is a short-day plant [16]. Maize performs badly in saline soils, particularly during blooming. Temperatures between 17 and 33°C are ideal for optimal maize production, and a minimum soil temperature of 12°C is required for germination. Maize with its broad scope of adaptations has great output potential [6].
The plant has separate male and female parts, and this aids in the disciplined pollination imperative in genetic analysis. A single pollinated ear can produce hundreds of seeds each of which is a fruit of single seeds making it a suitable product for phenotypic analysis [17].
The kernel consists of an embryo, from which the rest of the plant commences, i.e., the neighbouring maternal tissues and endosperm. The peripheral maternal tissue consists of the nutritive nucleus. With an increase in the size of the endosperm, nucleus degenerates. The seeds are the nutrient storehouse of the plant. They also initiate the next sporophytic procreation of the plant [18].
Maize being an angiosperm undergoes the process of double fertilisation where the two genetically identical male gametes (sperm cells) are discharged into the embryo sac upon fertilisation. The nucleus of the first sperm and the egg cell integrate to produce a diploid embryo. The nucleus of the second sperm combines with the dual polar nuclei present in the embryo sac which produces a triploid endosperm [19].
The three specific kernel sections are the endosperm, the embryo, and the maternal tissues. These undergo a securely organised development through three major phases: early development, the filling phase, and final maturation [20].
Within the initial 15 days following pollination, the endosperm and embryo grow and develop promptly from a single cell into profoundly distinct tissue. Following this is the second phase, the filling stage which occurs between 12 and 35 days following pollination. The embryo’s scutellum along with the central domain of the endosperm concentrates essential reserves. The maternal tissues carry the necessary nutrients. After pollination, the kernel goes through a 35–50-day maturation period during which it loses moisture and enters a quiescent phase before dispersing [21].
There are two stages in the lifecycle of maize plant, namely, the vegetative phase commonly called the V phase and the reproductive phase called the R phase. The primary stage in the V phase is emergence. This is succeeded by the V1 stage and goes up to the V21 stage. This suggests the number of leaf blades developed by the plant at that particular stage. An immature maize plant can curl a new leaf from the whorl at a rate of 3–4 days. Maize plants can develop around 20 leaves throughout [22]. Maize plants embark on the reproductive growth phase after successful tassel emergence. The reproductive stage can be perceived by seed development [23]. Once the vegetative phase is complete, the plant enters the first stage of the reproductive phase (R1), which involves silk formation. This is a crucial stage as the plant is sensitive to water and nutrient stress, which can lead to strained growth of the cobs. After R1 is the R2 stage, also called blister stage. In this phase, the kernels are small and watery. The plant reaches its physiological maturity at the R6 stage as shown in Table 1. This signifies the break in the flow of water and nutrients between the kernel and the shank [24].
Vegetative and reproductive growth stages of maize [22–24]
| Development stages of maize | Characteristics |
|---|---|
| Vegetative stage | |
| VE | This is the emergence stage. The coleoptile breaks through the soil surface and grows |
| V1–V2 | At this stage, the first and the second leaf collars are visible |
| V3 | The third leaf collar is visible, plant begins to photosynthesise and rely on nodal root system |
| V4 | The fourth leaf collar is visible |
| V5–V6 | The fifth and sixth leaf collars are visible, growing point is above the soil surface, critical period of nitrogen uptake begins, and kernel row numbers are determined |
| V7–Vn | The seventh to nth leaf collars are visible, period of very rapid growth |
| Vt | Tasselling occurs at this stage. Emergence of tassel indicates transition to the reproductive phase |
| Reproductive stage | |
| R1 Silking | Silking is the stage at which the silks are visible. Physiological maturity can be estimated by adding 50–55 days to the silking date |
| R2 Blistering | About 12 days after silking, silks darken and dry out. Kernels are white and form a small blister containing clear fluid. Each kernel develops an embryo. Stress at this stage can reduce yield potential by causing kernel abortion |
| R3 Milking | About 20 days after silking, kernels are yellow and clear fluid turns milky white as starch accumulates |
| R4 Dough | About 26 days after silking, the starchy liquid inside the kernels has a dough-like consistency. Kernels contain about 70% moisture and begin to dent at the top. At this stage they have accumulated close to 50% of their maximum dry weight |
| R5 Dent | About 38 days after silking, nearly all kernels are dented and contain about 55% moisture. Cob has distinct colour. Colour depends on its variety |
| R6 (Full maturity) | About 60 days after silking, physiological maturity is reached, and kernels have attained maximum dry weight at 30–35% moisture |
4 Nutritive composition of maize
It is reported that most maize genotypes have approximately 67–72% starch, 8% protein, 2–3% fibre, 12–15% moisture, 2–4% fat, and 1.5% of minerals (Table 2) [4]. Saponin, allantoin, sterol, stigmasterol, alkaloids, hordenine, and polyphenols are phytochemical secondary metabolites that have been found in the leaves, kernels, and corn silk.
Nutritive value of different parts of maize plant
| Nutrient | Corn cob | Corn silk | Corn husk | Corn tassels | References |
|---|---|---|---|---|---|
| Carbohydrates (%) | 74.51 | 65.5–74.3 | 73.41 | 68.73 | [29,30,16,31] |
| Protein (%) | 3.42 | 9.42–17.6 | 12.41 | 4.06 | [16,29,32] |
| Fat (%) | 9.55 | 0.26–4.74 | 8.09 | 2.6 | [16,30] |
| Total fibre (%) | 7.3 | 7.34 | 22.17 | 8.55 | [16,26,30,32] |
| Ash (%) | 4.41 | 1.2–3.91 | 17.90 | 2.134 | [16,29,30,32] |
| Moisture (%) | 5.43 | 9.65–10.4 | 9.06 | 4.51 | [16,29,30] |
| Calcium (mg/100 g) | 0.025 | 63.2 ± 1.1 | 1.019 | 1.0 | [29–31] |
| Magnesium (mg/100 g) | 0.031 | 88.6 ± 8.9 | 26.03 | 13 | [30,31,16] |
| Potassium (mg/100 g) | 0.241 | 1656.2 ± 89.4 | 596 | 197.3 | [30,31] |
| Sodium (mg/100 g) | 0.041 | 25.6 ± 0.2 | 25.98 | 18.4 | [30,31] |
| Iron (mg/100 g) | 0.0024 | 0.005 | 0.85 | [16,30] | |
| Zinc (mg/100 g) | 0.0018 | 0.0615 | 12.6 | [16,29,30] |
The availability of various vitamins namely vitamin A, vitamin C, and vitamin K, as well as β-carotene and selenium, aids in thyroid gland and immune system function. Corn contains all the B-complex vitamins – thiamine, niacin, riboflavin, and pantothenic acid – which have been proven to be beneficial to the heart, hair, skin, digestion, and brain. They also aid in the maintenance of a healthy immune system [25]. Baby corn is richer in ascorbic acid and β-carotene when compared to other vegetables [26].
Two important amino acids are lacking in the protein content of maize endosperm. Due to this, conventional maize cultivars have low biological value and poor net protein utilisation. However, it has been noted that high-QPM contains a higher quantity of tryptophan as well as lysine in the endosperm protein [27].
Maize is an abundant provider of dietary fibre, majorly accumulated in the endosperm. The amount of fibre present in the maize kernels varies between 61 and 86%. This depends on the variety of the maize plant. Alongside fibre, the kernel also consists of non-starch polysaccharides like cellulose, hemicellulose, and small amounts of lignin, which is positioned in the bran [28].
5 Phytochemical composition of maize
Phytochemicals are produced through the primary or secondary metabolism of plants. They are essential for plant development and oppose competitors, pathogens, and predators. Maize contains a range of phytochemicals. Of these phytochemicals, some of them work as antioxidants that neutralise free radicals and eliminate their potential to cause harm. The most extensively studied phytochemicals are carotenoids and flavonoids for their antioxidant potential. They boost immune system, combat the risk of specific types of cancers, and promote cardiovascular, vision, bone, and neurocognitive health [33].
The health properties of maize phytochemicals are chiefly credited to their significant antioxidant content and their antiradical activity, and their anti-mutagenesis, anti-carcinogenesis, anti-inflammatory, and enzyme inhibitory activity. Unfortunately, most of the phytochemicals are degradable and lost during the process of milling, processing, and storage of the produce [34].
The simplest and most common phenolic compound is phenolic acid, which is usually found in whole grains. In the case of maize, the prominently found phenolic acids are coumaric, caffeic, protocatechuic, hydroxybenzoic, vanillic, syringic, sinapic, ferulic acids, and gallic acid as shown in Table 3. The total phenolic acids present in maize average around 255 mg/100 g, of which ferulic acid is the main compound accounting for 70% of the overall amount [35].
Phytochemical value of maize
| List of nutrients | Kernel | Silk | Husk | Tassel | Cob | References |
|---|---|---|---|---|---|---|
| Total phenolic content | 21.38 GAE/100 g | 97.8 lg GAE/g | 13.34 mg/g | 6.75 ± 0.11 mg/g | 50.19 ± 1.80 mg/g | [42–46] |
| Total anthocyanin content (mg/g) | 29.93 mg CGE/100 g | 256.50 mg CGE/100 g | 21.89 ± 1.23 | 23.88 ± 1.23 | [44,45] | |
| Total carotenoid content | — | 11.3 mg CGE/100 g | 36.1 μg/g | [47,48] | ||
| Chlorogenic acid (mg/100 g) | — | — | 10.15 | [49] | ||
| Ferulic acid (mg/100 g) | — | — | 1293.41 | 1,164 ± 10 | [49,50] | |
| p-Coumaric acid mg/100 g | — | — | 573.87 | 933.1 ± 11.7 | [49,50] | |
| p-Hydroxybenzoic acid | — | — | 15.38 mg/100 g | 10.52 μg/L | 32.63 ± 0.34 | [49–51] |
| Total flavonoid (mg CE/100 g) | — | 264.2 ± 0.9 | 192.4 ± 0.7 | 198.3 ± 0.7 | [50] | |
| Vanillic acid (μg/L) | — | — | 1046.26 | [51] | ||
| Fumaric acid (μg/L) | — | — | 151.51 | [51] | ||
| Acetohydroxamic acid (μg/L) | — | — | 145.64 | [51] |
GAE: gallic acid equivalent; CGE: cyanidin 3-glucoside equivalent, CE: catechin equivalent.
In vitro, ferulic acid played a role in hindering the multiplication and spread of human lung cancer cells [36], whereas gallic acid reduces the growth of new blood vessels in ovarian cancer cells [37].
Maize, the magic golden grain, is abundant in phytochemicals and phytosterols. The phytochemicals bear an antioxidant tendency. The tender maize silk contains many bioactive compounds proving great medicinal benefits. Some water-soluble vitamins, flavonoids, anthocyanins, phenolic acids, carotenoids, polyphenols, and glycosides are found in maize. Prevention of various diseases, management of oxidative stress in the body, and the remedy to cancer are all facilitated by the oxidant potential of maize. In addition, consumption of maize has also proven to diminish the risk of developing threatening diseases [28].
Flavonoids have potent antioxidant properties which enable them to scavenge free radicals and prevent protein glycation. Rats were found to be protected against ischemia-reperfusion injury by anthocyanins. Also, it has been discovered that they exhibit antioxidant and antiradical properties, which are linked to several preventive properties like anti-cancerous, anti-inflammatory, anti-obesity, anti-diabetic, cardioprotective, and hepatoprotective [38]. Bioactive phytochemicals like flavonoids, steroids, phenolic acids, alkaloids, tannins, carotenoids, saponins, anthocyanins, and other phenolic compounds are abundantly found in silk, seed, stem, leaves, roots, and other sections of maize plant. Maize seeds contain phytochemicals of pharmacological significance [16]. Maize silk holds a significant amount of bioactive phytochemical compounds like polysaccharides, vitamins, terpenoids, steroids, alkaloids, tannins, saponins, volatile oils, and sugars. Studies have proven that flavonoids present in corn silk had a positive impact against stress due to oxidation and exhibited fatigue-preventing activity in mice. Each part of maize plant is a valuable source of phytochemical compounds [39]. The extraction of these phytochemicals from biomass improves its properties and suitability for biological conversion to energy sources, such as biogas or bioethanol [40,41].
6 Pharmacological properties of maize
Maize has been seen as a remedy for various health issues such as emaciation, eating disorders, and haemorrhoids since ancient times. They also possess analgesic property and are helpful for sexual well-being, especially for men suffering from erectile dysfunction. It also helps in stimulating the production of sex-related hormones. Maize silk is also seen as a remedy for the treatment of urinary tract infections and kidney stones across multiple nations. In China, maize silk is used as a remedy for the retention of fluid in the liver as well as jaundice. It also lowers blood pressure and the proper functioning of the liver and the production of bile. For bladder symptoms, a decoction of leaves, roots, and silk is used for the treatment. A tea decoction of the cob is used for gut ailments. It also acts as an excellent emollient for wounds and ulcers, as well as for oedema [25].
Simple phenolic acids have been shown to elevate glucose uptake and increase the synthesis of glycogen exhibiting an anti-diabetic effect. This improves the lipid and glucose profiles about obesity and other cardiovascular diseases [52]. They decrease the levels of glucose present in the blood and the activity of glucose-6-phosphatase and phosphoenolpyruvate carboxykinase, glucose and peroxidation of the lipids while increasing the levels of glycogen as well as insulin [53].
In vitro, it has been proven that peptides which are derived from maize exhibit multiple biological effects like antioxidant, anti-cancer, anti-inflammatory, hepatoprotective, facilitation of alcohol metabolism, antimicrobial, cell penetration action, inhibition of dipeptidyl peptidase IV, and anti-hypertensive [54].
Anthocyanins which are found in dietary plants exhibit high activity levels against oxidative stress. They play a curative, defensive, or complementary role in controlling chronic diseases [28]. Corn silk has the potential to have a mild stimulating effect, increase urine production, and relieve inflammation. Its demulcent potency plays a vital role in cases of cystitis and irritation of the bladder due to uric acid and phosphate stones. Chinese medicine utilises maize silk for the treatment of oedema. Studies on the phytochemicals found in corn silk brought to light the presence of chlorogenic acid, ferulic acid, saponins, phytosterols, resin, and sugars [55].
The mechanisms of the different properties of maize are given in Figure 2.
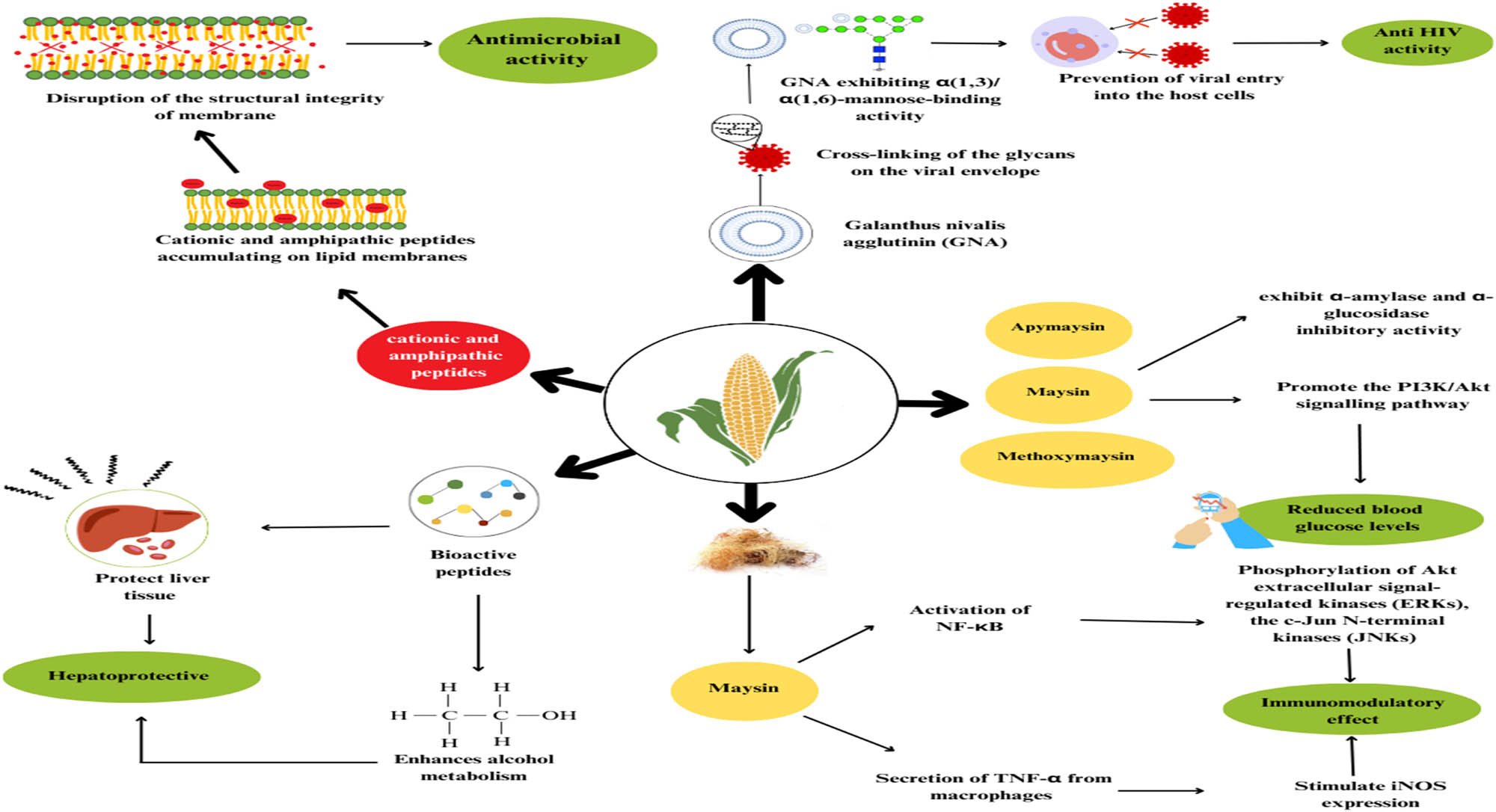
Mechanisms of pharmacological properties of the different parts of maize plant. Source: The author.
6.1 Anti-HIV activity
Maize has an anti-HIV activity compound named Galanthus nivalis agglutinin (GNA), which is very useful against HIV AIDS disease. Maize is believed to be a good treatment for HIV 1.
The compound found in maize silk – GNA – acts solely on mannose and distinctly identify the ending mannose compounds found in high-mannose N-glycans. Currently, GNA is taken as the ideal lectin and portrays a major body of GNA-related lectins having an inclination for mannose and mannose-containing oligosaccharides [55]. The numerous carbohydrate-binding sites found on tetrameric lectins like GNA facilitate a sturdy attachment to and the cross-linkage of the glycans on the viral envelope. GNA exerts α[1,3]/α[1,6]-mannose-binding potential. Thus, they inhibit the entry of viruses into the host along with other lectins, hence acting as anti-viral components. This usually takes place via selective interactions between the lectins and the exposed glycans present on the glycoproteins on the surface of the virus [56].
6.2 Anti-diabetic activity
Maize and its parts have been shown to have anti-diabetic properties. They do so by exhibiting ɑ-amylase and ɑ-glucosidase inhibitory action and by promoting the phosphatidylinositol 3-kinase (PI3K/Akt) signalling pathway. The polysaccharides in corn silk are majorly responsible for the effect [57].
Usually, inhibition of the α‐glucosidases present in the small intestine can be done by anti-diabetic botanicals. These enzymes are needed for converting oligosaccharides and polysaccharides into conveniently absorbed monosaccharides. When α‐glucosidases are inhibited, the rise in the amount of glucose in the plasma after a meal is blunted by the lowering of blood glucose levels [58]. α‐Glucosidase inhibitors, like acarbose, miglitol, and voglibose, are routinely used for type 2 diabetes [59]. Maize silk phenolics have been shown to prevent carbohydrate‐hydrolysing enzyme activity. Like with other anti-diabetic plants, hypoglycaemia caused due to the consumption of corn silk extracts may thus be partly attributed to the suppression of glucosidases in the intestine. Apramycin, maysin, and methoxymaysin are the maize silk chemicals potentially mainly accountable for the inhibition of glucosidase, as determined by the molecular docking simulation [60].
A study by Thiraphatthanavong et al. [61] demonstrated that Purple waxy maize is a food that may help prevent diabetic cataracts. The primary significant enzyme antioxidants in the lens of diabetic cataracts, superoxide dismutase and catalase, are inhibited by oxidative stress. The reduction in antioxidant enzymes seems to be a key factor in the increase in oxidative stress and cataractogenesis associated with diabetic cataracts. The fundamental mechanism of this seems to be dose-dependent. High doses of the seed extracts work by suppressing the rate-limiting enzyme, aldose reductase, involved in the polyol pathway, whereas medium doses of the seed extract reduce oxidative stress. Also, it has been demonstrated that a polysaccharide derived from maize silk polysaccharides (PCS2) has the potential to be a medication for the treatment of type 2 diabetes mellitus [62].
6.3 Antimicrobial activity
According to a study by Nessa et al. [63] examining the preventive capacity for microbial growth of maize extract, it has been proven that maize silk extracts can shield the body from a variety of disease states brought on by pathogenic organisms. In vitro, the cationic and amphipathic peptides exhibit a membrane-destabilising action. They accumulate on lipid membranes and cause the membrane’s structural integrity to be compromised by the formation of pores or by exhibiting the action of a detergent and synchronously eradicating the membrane [64].
6.4 Reduction in the risk of heart diseases
The fat and cholesterol content of maize is minimal. Cereals are known to be rich in complex carbohydrates, vitamins, and minerals along with fibre. Whole grain fibre lowers the chance of developing diabetes and heart disease [15]. The fibre helps in pushing out the cholesterol from the body and hence reduces the chances of hypercholesterolemia and other heart diseases.
An elevation in systemic blood pressure (SBP) is a defining feature of hypertension. A number of physiological systems regulate SBP, including the renin–angiotensin–aldosterone system (RAAS), which also plays a role in the aetiology of hypertension. The conversion of angiotensin I into angiotensin II through decarboxylation is facilitated by an angiotensin-converting enzyme in the RAAS. By stimulating proximal tubule cell hypertrophy, angiotensin II causes salt to be reabsorbed into the kidney, raising blood volume and SBP [65]. The precise biochemical mechanisms by which bioactive peptides work on the basis of the presence of amino acids like arginine which acts as a precursor of vasodilatory nitric acid and acts as an angiotensin-converting enzyme inhibitor [66].
6.5 Anticancer activity
As the genes determining how our cells grow and multiply change, the risk of cancer develops [67]. For anti-cancer action, residues of glycine and arginine are deemed essential. Glycine residues largely play a structural role, due to their capacity for cyclisation and their involvement in the creation of β-turns, and arginine residues play a part in the treatment of cancer. Moreover, anti-cancer peptides contain cysteine, glycine, isoleucine, lysine, and tryptophan residues at various locations; nevertheless, it is uncertain what these residues do [68].
According to a study, corn silk polysaccharides (CSP) effectively slowed the formation of tumours and increased the lifespan of mice carrying the H22 hepatocellular carcinoma. The CSP had dose-dependent anticancer efficacy and showed no damage to the liver, kidney, or body weight. Moreover, improvements were made in the thymus index, peripheral white blood cells count, and spleen index of patients with H22 tumour. Additionally, giving corn silk to mice with the H22 tumour might considerably boost IL-2 (Interleukins), IL-6, and tumour necrosis factor (TNF) serum levels [69].
6.6 Hepatoprotective and alcohol metabolism-facilitating activity
During liver illness, hepatic cells are subjected to a variety of stressors. For instance, in chronic liver disease, the liver tissue deteriorates along with inflammation and parenchyma renewal, which causes fibrosis and cirrhosis [70]. Maize bioactive peptides have been shown to preserve liver tissue and improve ethanol metabolism in rats when used in vivo. According to certain studies, in animal models, it was observed that peptides made from corn gluten meal reduce the amount of cell damage indicators. For instance, a study showed that the pentapeptide QLLPF decreased the blood alcohol level in mice. The same pentapeptide inhibited apoptosis of hepatocytes with a combination of corn peptides [71]. These in vivo results, according to the scientists, point to maize peptides having a hepatoprotective impact. Nevertheless, no research on humans has been done yet to support these conclusions.
6.7 Immunomodulatory activity
Maysin dose-dependently, up to 100 g/mL, exhibits the stimulation of iNOS expression and the activation of macrophages. This causes them to release TNF and produce nitrogen oxide. The same compound, maysin, also enhanced the phosphorylation of the upstream signalling elements, Akt, extracellular signal-regulated kinase (ERK), and c-Jun N-terminal kinase (JNK), and activated NF-B. Results strongly suggested that maysin (a flavonoid) present in the corn silk stimulates the synthesis of TNF and production of iNOS via the NF-B and Akt signalling pathways. Activating NF-B, Akt, and MAPK (ERK and JNK) in RAW 264.7 macrophages causes maysin to have immunomodulatory effects by increasing TNF-secretion and iNOS production, proving maysin from corn silk to be an immunomodulator for early innate immunity [72].
7 Applications and utilisation of different parts of maize
Each part of the maize plant from the tassels to the roots shows high economic value. The seeds, stalk, husk, and cob can be incorporated into the production of various by-products. Maize grains have also proven to be favourable for the development of hydroponic fodder which is beneficial in hot climates [79].
Composting is an effective and economic approach for disposing agricultural solid waste in most agricultural countries [84]. The application of maize compost as an amendment to soils in the Mediterranean climate improves the incorporation of biomass to the soil and produces changes in the labile and recalcitrant soil organic matter fractions [85]. It has been proposed that maize crop residues may increase buildup of soil organic content [86]. A study by Gissi et al. [87] proved that achieving the regional target for biodiesel production will be impossible without having significant trade-offs with other soil-related eco-system services or causing land use change. These eco-system service trade-offs included habitat for soil organisms (supporting service), soil carbon storage (regulating service), groundwater quality protection (regulating service), and food crops (provisioning service).
Due to its high nutrient content, maize is considered to be a pauper’s nutria-cereal. Maize is utilised in the production of oil, starch, syrup, biscuits, flakes, puffs, etc., as shown in Table 4. Maize is not only limited to the food industry but also applicable in the non-food industries such as the production of toothpaste, detergent, paper, dyes, etc. It is an alternative raw material in the production of food containers, vitamin tablets, baby powder, plastic food packaging, diapers, candies, textile products, medicine, etc. One of the most important utilisations of maize is in the production of bioethanol as alternative to auto fuels internationally [88].
Utilisation of maize in the food and non-food sector
| Application | Product | Part of maize plant used | Composition and method of preparation | References |
|---|---|---|---|---|
| Food industry | Maize starch | Kernels | The wet starch obtained is dried at 40–45°C for 24 h. This is further used as a thickening agent | [73] |
| Corn syrup | Kernels | Corn starch is mixed with distilled water at 1:10 ratios to make a slurry and treated with α-amylase which is heated for 1 h at 95–100°C. The slurry is then saccharified with glucoamylase enzyme while heating and then it is filtered | [74] | |
| Soft drinks and alcohol | Kernels | The dry-grind ethanol process is used for preparation of beverages. It includes milling liquefaction at temperatures above 100°C, saccharification at 30°C, and fermentation | [75] | |
| Confectionaries, baking and jams | Kernels | Corn kernels are boiled and made into puree. To the puree jaggery, lemon, and pectin are added to prepare the maize jam | [76] | |
| Maize oil | Kernel, germ | Maize kernels are ground and the germ is separated. This is then air-dried for 18 h up to 5% (wb) moisture content. These are then steeped at 55°C for 18 h in steeping solution. Maize germ oil is then extracted using enzyme-assisted aqueous extraction | [77] | |
| Pharmaceutical industry | Pharmaceuticals | Kernels | The drug aspirin is covered with continuous film of an oxidised corn starch paste | [78] |
| Fodder industry | Livestock and poultry feed | Kernels, husk | Hydroponic maize is grown in a conventional greenhouse. The ambient temperature should be maintained between 15 and 32°C and that of relative humidity around 80–85% for appropriate growth | [79] |
| Biogas/biofuel | Biofuel | Cobs, husk | The lignocellulosic mass is gasified with syngas CO and H2. It then undergoes Fischer Tropsch synthesis to produce biodiesel | [80] |
| Plastic industry | Plastic, chemicals adhesive products | Kernels | Corn starch-based film production is achieved by solvent‐casting. This method involves solubilisation, casting, and drying. The starch is gelatinised, forming a solution which is poured onto Teflon plates. This is dried at room temperature | [81] |
| Electricals | Potassium ion battery | Corn husk | Corn husk is treated with concentrated solutions of HF and HCl for 24 h at room temperature. They are then washed to neutral pH and dried at 110°C. Subsequently, they are soaked in a KOH solution for 24 h and dried. Finally, the mixture is placed in a tube furnace (heating rate: 2 C min−1) and heated in an atmosphere at 800°C for 2 h. The powder is soaked in 1 M HCl and washed and dried to obtain the final product | [82] |
| Purification | Adsorbate | Tassels | The tassels are separated, grinded, and added to 200 mL of 97% H2SO4 for 24 h. This is then refluxed in a fume hood for 4 h. This mixture is filtered and cooled and drenched in 1% NaHCO3 solution to neutralize any remaining acid. The sample is rinsed using distilled water until the pH of the activated carbon is between 6 and 7, and then dried overnight in an oven at 120°C | [83] |
The green maize is boiled or roasted for consumption. Dried maize grains are used to make gruel soups and kinds of pasta due to their favourably high starchy content. The significance of maize as food is credited to the abundant nutrients packed into the kernels [89]. Products made from corn silks include corn silk teas and cosmetics which are retailed in countries such as Korea, Japan, the UK, China, and the USA [42].
The by-products of starch production, such as corn oil, corn steep liquor, and gluten, are value-added products with significant value. Within the paper and textile sectors, maize starch is widely utilised as a sizing ingredient. It is used in the food sector to make desserts like pies and puddings as well as confections and dressings. Maize starch can further be used for producing high fructose syrup when hydrolysed and enzymatically treated. Alcohol from grains of maize which is the traditional source of Bourbon whiskey is produced by fermentation and distillation [25]. Furthermore, the same process can also produce other alcohols such as glycerol, ethyl alcohol, acetic acid, propyl alcohol, butyl alcohol, acetone, acetaldehyde, lactic acid, and citric acid [89].
Dextrose and corn syrup are also made using maize starch. In many regions of the world, industrially produced cornmeal and flour are currently processed to create pre-cooked refined corn flour, dried nixtamalized flour, fermented corn flour, and other corn products. Assorted innate nutrient contents can be found in these items. Commercially available sweeteners are all manufactured from maize starch [15].
Around 40–75% of the feed rations for the poultry and livestock industries come from maize. The maize grain undergoes dry milling to produce three major products – corn meal, flour, and oil. Corn starch is extracted through the process of wet milling and it is used for cooking, as a laundry starch in the textile industry, and for paper sizing. The dextrins and adhesives produced from maize can be turned into gums used for stamps and envelopes [89]. Maize cob waste is available worldwide in high amounts, as maize is the most produced cereal in the world. It is generally left in the crop fields, but due to its low biodegradability, it has a negligible impact on soil fertility [90]. Instead, maize serves as a feedstock in the manufacturing of ethanol fuel. Typical sources of feedstock for biofuels include recycled organic materials, valueless wood, and non-edible crop wastes like maize stalks [80]. Some maize forms are also grown for ornamental use. Additional industrial applications of maize oil include soap, paint, textiles, nitroglycerin, pesticides, inks, and rust-proofing for metallic surfaces [88]. The need to produce more maize has been motivated by the high demand for ethanol [91].
8 Conclusion
Maize has usefulness in all the major sectors such as food, textile, industrial, and agriculture across the globe. Maize shows exceptional pharmacological properties proving to be beneficial to human health. There is a growing path toward the consumption of maize as a staple food, thereby improving the health of the population. Its medicinal qualities encourage it to be used in the health and medicine sector. The production of maize when compared to its overall utilisation is uneven. Despite the immense nutritive potential of its parts such as corn silk and tassels which are not traditionally consumed, corn kernels are the only part of the maize plant which have been strongly incorporated into the food industry. The cobs and husk have immense potential to be used in the paper, textile, and plastic industries. Apart from this, they are excellent biofuels and can be used as feedstock. Maize residue can be a good alternative to the extinguishable fuels on which most industries run.
-
Funding information: Authors state no funding involved.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and consented to its submission to the journal, reviewed all the results and approved the final version of the manuscript. PS: writing original draft; PR, JK, SK, and SE: editing and supervision, AA, RU, and ASA: literature review and supervision; and JS: conceptualisation and writing original draft. The authors hereby declare that the work presented in this article is original.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: Data will be available on request by the authors.
References
[1] Rouf Shah T, Prasad K, Kumar P. Maize – a potential source of human nutrition and health: a review. Cogent Food Agric. 2016 Dec;2(1):1166995.10.1080/23311932.2016.1166995Search in Google Scholar
[2] Azam MG, Sarker U, Uddin MS. Screening maize (Zea mays L.) genotypes for phosphorus deficiency at the seedling stage. Turk J Agric For. 2022;46(6):802–21.10.55730/1300-011X.3044Search in Google Scholar
[3] Azadi MS, Shokoohfar A, Mojdam M, Lak S, Fazel MA. Investigation of changes in corn hybrids grain protein, proline, and micronutrient content under the influence of drought and fertilizers. Turk J Agric For. 2022;46:413–23. 10.55730/1300-011X.3014.Search in Google Scholar
[4] Azadi MS, Shokoohfar A, Mojdam M, Lak S, Fazel MA. Investigation of changes in corn hybrids grain protein, proline, and micronutrient content under the influence of drought and fertilizers. Turk J Agric For. 2022;46(4):413–23.10.55730/1300-011X.3014Search in Google Scholar
[5] Hossain F, Muthusamy V, Bhat JS, Jha SK, Zunjare R, Das A, et al. Maize. In: Singh H, Kumar S, editors. Broadening the genetic base of grain cereals. New Delhi: Springer; 2016. p. 67–88. 10.1007/978-81-322-3613-9_4.Search in Google Scholar
[6] Awata LA, Tongoona P, Danquah E, Ifie BE, Mahabaleswara SL, Jumbo MB, et al. Understanding tropical maize (Zea mays L.): the major monocot in modernization and sustainability of agriculture in sub-Saharan Africa. Int J Adv Agric Res. 2018;7(2):32–77.Search in Google Scholar
[7] Choi DJ, Kim SL, Choi JW, Park YI. Neuroprotective effects of corn silk maysin via inhibition of H2O2-induced apoptotic cell death in SK-N-MC cells. Life Sci. 2014 Jul;109(1):57–64.10.1016/j.lfs.2014.05.020Search in Google Scholar PubMed
[8] Rani M, Nanaji K, Rao TN, Deshpande AS. Corn husk derived activated carbon with enhanced electrochemical performance for high-voltage supercapacitors. J Power Sources. 2020;471:228387.10.1016/j.jpowsour.2020.228387Search in Google Scholar
[9] Milind P, Isha D. Zea maize: a modern craze. Int Res J Pharm. 2013 May;4(6):39–43. 10.1016/j.jpowsour.2020.228387.Search in Google Scholar
[10] García-Lara S, Chuck-Hernandez C, Serna-Saldivar SO. Development and structure of the corn kernel. Corn. 2019 Jan;147–63. 10.1016/B978-0-12-811971-6.00006-1.Search in Google Scholar
[11] Erenstein O, Chamberlin J, Sonder K. Estimating the global number and distribution of maize and wheat farms. Glob Food Secur. 2021 Sep;30:100558. 10.1016/j.gfs.2021.100558.Search in Google Scholar
[12] Erenstein O, Jaleta M, Sonder K, Mottaleb K, Prasanna BM. Global maize production, consumption and trade: trends and R&D implications. Food Secur. 2022 Oct;14(5):1295–319. 10.1007/s12571-022-01288-7.Search in Google Scholar
[13] Food and Agriculture Organisation statistics; 2015. https://www.fao.org/fileadmin/user_upload/agns/pdf/WORKING_PAPER_AFLATOXIN_REPORTDJ10thOctober.pdf. Assessed on 30th March 2024.Search in Google Scholar
[14] USDA. Grain: World markets and trade [Assessed on 2024 July 20]. Available from https://apps.fas.usda.gov/psdonline/circulars/grain.pdf.Search in Google Scholar
[15] Murdia LK, Wadhwani R, Wadhawan N, Bajpai P, Shekhawat S. Maize utilization in India: an overview. Am J Food Nutr. 2016;4(6):169–76.Search in Google Scholar
[16] Choquette NE, Weldekidan T, Brewer J, Davis SB, Wisser RJ, Holland JB. Enhancing adaptation of tropical maize to temperate environments using genomic selection. G3: Genes Genom Genet. 2023;13(9):1–11. 10.1093/g3journal/jkad141.Search in Google Scholar PubMed PubMed Central
[17] Leroux BM, Goodyke AJ, Schumacher KI, Abbott CP, Clore AM, Yadegari R, et al. Maize early endosperm growth and development: from fertilization through cell type differentiation. Am J Bot. 2014 Aug;101(8):1259–74. 10.3732/ajb.1400083.Search in Google Scholar PubMed
[18] Consonni G, Castorina G, Varotto S. The Italian research on the molecular characterization of maize kernel development. Int J Mol Sci. 2022 Sep;23(19):11383. 10.3390/ijms231911383.Search in Google Scholar PubMed PubMed Central
[19] Dresselhaus T, Sprunck S, Wessel GM. Fertilization mechanisms in flowering plants. Curr Biol. 2016 Feb;26(3):R125–39. 10.1016/j.cub.2015.12.032.Search in Google Scholar PubMed PubMed Central
[20] Dai D, Ma Z, Song R. Maize kernel development. Mol Breed. 2021 Jan;41:1–33. 10.1007/s11032-020-01195-9.Search in Google Scholar PubMed PubMed Central
[21] Rousseau D, Widiez T, Di Tommaso S, Rositi H, Adrien J, Maire E, et al. Fast virtual histology using X-ray in-line phase tomography: application to the 3D anatomy of maize developing seeds. Plant Methods. 2015 Dec;11:1–10. 10.1186/s13007-015-0098-y.Search in Google Scholar PubMed PubMed Central
[22] Zhao X, Tong C, Pang X, Wang Z, Guo Y, Du F, et al. Functional mapping of ontogeny in flowering plants. Brief Bioinform. 2012 May;13(3):317–28. 10.1093/bib/bbr054.Search in Google Scholar PubMed
[23] Siebers MH, Slattery RA, Yendrek CR, Locke AM, Drag D, Ainsworth EA, et al. Simulated heat waves during maize reproductive stages alter reproductive growth but have no lasting effect when applied during vegetative stages. Agric Ecosyst Environ. 2017 Mar;240:162–70. 10.1016/j.agee.2016.11.008.Search in Google Scholar
[24] Li Y, Tao H, Zhang B, Huang S, Wang P. Timing of water deficit limits maize kernel setting in association with changes in the source-flow-sink relationship. Front Plant Sci. 2018 Oct;9:1326.10.3389/fpls.2018.01326Search in Google Scholar PubMed PubMed Central
[25] Kumar D, Jhariya AN. Nutritional, medicinal and economical importance of corn: a mini review. Res J Pharm Sci. 2013;2319:555X.Search in Google Scholar
[26] Hooda S, Kawatra A. Nutritional evaluation of baby corn (Zea mays). Nutr Food Sci. 2013 Feb;43(1):68–73. 10.1108/00346651311295932.Search in Google Scholar
[27] Bello OB, Olawuyi OJ, Ige SA, Mahamood J, Afolabi MS, Azeez MA, et al. Agro-nutritional variations of quality protein maize (Zea mays L.) in Nigeria. J Agric Sci (Belgrade). 2014;59(2):101–16. 10.2298/JAS1402101B.Search in Google Scholar
[28] Mann KK. Health benefits of maize phytochemicals. J Appl Soc Sci 1(2&3):107–14.Search in Google Scholar
[29] Agie JO, Yahya MD. Preparation and characterization of maize tassel fibre for adsorption processes. 2019. http://repository.futminna.edu.ng:8080/jspui/handle/123456789/5306.Search in Google Scholar
[30] Olagunju A, Onyike E, Muhammad A, Aliyu S, Abdullahi AS. Effects of fungal (Lachnocladium spp.) pretreatment on nutrient and antinutrient composition of corn cobs. Afr J Biochem Res. 2013 Dec;7(11):210–4.10.5897/AJBR2013.0715Search in Google Scholar
[31] Žilić S, Janković M, Basić Z, Vančetović J, Maksimović V. Antioxidant activity, phenolic profile, chlorophyll and mineral matter content of corn silk (Zea mays L): comparison with medicinal herbs. J Cereal Sci. 2016 May;69:363–70. 10.5897/AJBR2013.0715.Search in Google Scholar
[32] Cetinkaya N, Aykanat S, Ayaşan T, Celik C. Nutrient contents and in vitro digestibility of different parts of corn plant. South Afr J Anim Sci. 2020 Jan;50(2):302–9. 10.4314/sajas.v50i2.13.Search in Google Scholar
[33] Yeum KJ, Russell RM. Biological functions of plant pigment phytochemicals in humans. In: Laher I, editor. Systems biology of free radicals and antioxidants. Canada: Springer; 2014. p. 4023–45. 10.1007/978-3-642-30018-9_161.Search in Google Scholar
[34] Acosta-Estrada BA, Gutiérrez-Uribe JA, Serna-Saldivar SO. Minor constituents and phytochemicals of the kernel. In Elsevier eBooks; 2019. p. 369–403. 10.1016/B978-0-12-811971-6.00014-0.Search in Google Scholar
[35] Ndolo VU, Beta T. Distribution of carotenoids in endosperm, germ, and aleurone fractions of cereal grain kernels. Food Chem. 2013 Aug;139(1–4):663–71. 10.1016/j.foodchem.2013.01.014.Search in Google Scholar PubMed
[36] Fong Y, Tang CC, Hu HT, Fang HY, Chen BH, Wu CY, et al. Inhibitory effect of trans-ferulic acid on proliferation and migration of human lung cancer cells accompanied with increased endogenous reactive oxygen species and β-catenin instability. Chin Med. 2016 Dec;11:1–3. 10.1186/s13020-016-0116-7.Search in Google Scholar PubMed PubMed Central
[37] He Z, Chen AY, Rojanasakul Y, Rankin GO, Chen YC. Gallic acid, a phenolic compound, exerts anti-angiogenic effects via the PTEN/AKT/HIF-1α/VEGF signaling pathway in ovarian cancer cells. Oncol Rep. 2016 Jan;35(1):291–7. 10.3892/or.2015.4354.Search in Google Scholar PubMed PubMed Central
[38] Liu Y, Zhang L, Liang J. Activation of the Nrf2 defense pathway contributes to neuroprotective effects of phloretin on oxidative stress injury after cerebral ischemia/reperfusion in rats. J Neurol Sci. 2015 Apr;351(1–2):88–92. 10.1016/j.jns.2015.02.045.Search in Google Scholar PubMed
[39] Nawaz H, Muzaffar S, Aslam M, Ahmad S. Phytochemical composition: antioxidant potential and biological activities of corn. InTech eBooks; 2018. p. 49–67. 10.5772/intechopen.79648.Search in Google Scholar
[40] Zavala-López M, García-Lara S. An improved microscale method for extraction of phenolic acids from maize. Plant Methods. 2017 Dec;13(1):1–11. 10.1186/s13007-017-0235-x.Search in Google Scholar PubMed PubMed Central
[41] Oleszek M, Kowalska I, Oleszek W. Phytochemicals in bioenergy crops. Phytochem Rev. 2019 Jun;18:893–927. 10.1007/s11101-019-09639-7.Search in Google Scholar
[42] Sarepoua E, Tangwongchai R, Suriharn B, Lertrat K. Relationships between phytochemicals and antioxidant activity in corn silk. Int Food Res J. 2013 Sep;20(5):2073.Search in Google Scholar
[43] Duru CE. Mineral and phytochemical evaluation of Zea mays husk. Sci Afr. 2020 Mar;7:e00224. 10.1016/j.sciaf.2019.e00224.Search in Google Scholar
[44] Fernandez‐Aulis F, Hernandez‐Vazquez L, Aguilar‐Osorio G, Arrieta‐Baez D, Navarro‐Ocana A. Extraction and identification of anthocyanins in corn cob and corn husk from Cacahuacintle maize. J Food Sci. 2019 May;84(5):954–62. 10.1111/1750-3841.14589.Search in Google Scholar PubMed
[45] Simla S, Boontang S, Harakotr B. Anthocyanin content, total phenolic content, and antiradical capacity in different ear components of purple waxy corn at two maturation stages. Aust J Crop Sci. 2016 May;10(5):675–82. 10.21475/ajcs.2016.10.05.p7389.Search in Google Scholar
[46] Elsayed N, Marrez DA, Ali MA, El-Maksoud AA, Cheng W, Abedelmaksoud TG. Phenolic profiling and in-vitro bioactivities of corn (Zea mays L.) tassel extracts by combining enzyme-assisted extraction. Foods. 2022 Jul;11(14):2145. 10.3390/foods11142145.Search in Google Scholar PubMed PubMed Central
[47] Duangpapeng P, Ketthaisong D, Lomthaisong K, Lertrat K, Scott MP, Suriharn B. Corn tassel: a new source of phytochemicals and antioxidant potential for value-added product development in the agro-industry. Agronomy. 2018 Oct;8(11):242. 10.3390/agronomy8110242.Search in Google Scholar
[48] Lee J, Kim SL, Lee S, Chung MJ, Park YI. Immunostimulating activity of maysin isolated from corn silk in murine RAW 264.7 macrophages. BMB Rep. 2014 Jul;47(7):382. 10.5483/bmbrep.2014.47.7.191.Search in Google Scholar PubMed PubMed Central
[49] Galeana-López JA, Lizárraga-Velázquez CE, Hernández C, Leyva-López N, Heredia JB. Corn husk phenolics modulate hepatic antioxidant response in nile tilapia (Oreochromis niloticus) exposed to hypoxia. Molecules. 2021 Oct;26(20):6161. 10.3390/molecules26206161.Search in Google Scholar PubMed PubMed Central
[50] Yang T, Guang Hu J, Yu Y, Li G, Guo X, Li T, et al. Comparison of phenolics, flavonoids, and cellular antioxidant activities in ear sections of sweet corn (Zea mays L. saccharata Sturt). J Food Process Preserv. 2019 Jan;43(1):e13855. 10.1111/jfpp.13855.Search in Google Scholar
[51] Al-Khayri JM, Yüksel AK, Yüksel M, Işık M, Dikici E. Phenolic profile and antioxidant, anticholinergic, and antibacterial properties of corn tassel. Plants. 2022 Jul;11(15):1899. 10.3390/plants11151899.Search in Google Scholar PubMed PubMed Central
[52] Vinayagam R, Jayachandran M, Xu B. Antidiabetic effects of simple phenolic acids: a comprehensive review. Phytother Res. 2016 Feb;30(2):184–99. 10.1002/ptr.5528.Search in Google Scholar PubMed
[53] Senaphan K, Kukongviriyapan U, Sangartit W, Pakdeechote P, Pannangpetch P, Prachaney P, et al. Ferulic acid alleviates changes in a rat model of metabolic syndrome induced by high-carbohydrate, high-fat diet. Nutrients. 2015 Aug;7(8):6446–64. 10.3390/nu7085283.Search in Google Scholar PubMed PubMed Central
[54] Trinidad-Calderon PA, Acosta-Cruz E, Rivero-Masante MN, Diaz-Gomez JL, Garcia-Lara S, Lopez-Castillo LM. Maize bioactive peptides: from structure to human health. J Cereal Sci. 2021 Jul;100:103232. 10.1016/j.jcs.2021.103232.Search in Google Scholar
[55] Van Damme EJ. 35 years in plant lectin research: a journey from basic science to applications in agriculture and medicine. Glycoconj J. 2022 Feb;39(1):83–97. 10.1007/s10719-021-10015-x.Search in Google Scholar PubMed PubMed Central
[56] Gondim AC, da Silva SR, Mathys L, Noppen S, Liekens S, Sampaio AH, et al. Potent antiviral activity of carbohydrate-specific algal and leguminous lectins from the Brazilian biodiversity. MedChemComm. 2019;10(3):390–8. 10.1039/c8md00508g.Search in Google Scholar PubMed PubMed Central
[57] Guo Q, Chen Z, Santhanam RK, Xu L, Gao X, Ma Q, et al. Hypoglycemic effects of polysaccharides from corn silk (Maydis stigma) and their beneficial roles via regulating the PI3K/Akt signaling pathway in L6 skeletal muscle myotubes. Int J Biol Macromol. 2019 Jan;121:981–8. 10.1016/j.ijbiomac.2018.10.100.Search in Google Scholar PubMed
[58] Mata-Torres G, Andrade-Cetto A, Espinoza-Hernández FA, Cárdenas-Vázquez R. Hepatic glucose output inhibition by Mexican plants used in the treatment of type 2 diabetes. Front Pharmacol. 2020 Mar;11:215. 10.3389/fphar.2020.00215.Search in Google Scholar PubMed PubMed Central
[59] Wang PC, Zhao S, Yang BY, Wang QH, Kuang HX. Anti-diabetic polysaccharides from natural sources: a review. Carbohydr Polym. 2016 Sep;148:86–97. 10.1016/j.carbpol.2016.02.060.Search in Google Scholar PubMed
[60] Alvarado‐Díaz CS, Gutiérrez‐Méndez N, Mendoza‐López ML, Rodríguez‐Rodríguez MZ, Quintero‐Ramos A, Landeros‐Martínez LL, et al. Inhibitory effect of saccharides and phenolic compounds from maize silks on intestinal α‐glucosidases. J Food Biochem. 2019 Jul;43(7):e12896. 10.1111/jfbc.12896.Search in Google Scholar PubMed
[61] Thiraphatthanavong P, Wattanathorn J, Muchimapura S, Thukham-Mee W, Wannanon P, Tong Un T, et al. Preventive effect of Zea mays L.(purple waxy corn) on experimental diabetic cataract. BioMed Res Int. 2014;1–8. 10.1155/2014/507435.Search in Google Scholar PubMed PubMed Central
[62] Pan Y, Wang C, Chen Z, Li W, Yuan G, Chen H. Physicochemical properties and antidiabetic effects of a polysaccharide from corn silk in high-fat diet and streptozotocin-induced diabetic mice. Carbohydr Polym. 2017 May;164:370–8. 10.1016/j.carbpol.2017.01.092.Search in Google Scholar PubMed
[63] Nessa F, Ismail Z, Mohamed N. Antimicrobial activities of extracts and flavonoid glycosides of corn silk (Zea mays L). Int J Biotechnol Wellness Ind. 2012 Jun;1(2):115. 10.6000/1927-3037/2012.01.02.02.Search in Google Scholar
[64] Deslouches B, Di YP. Antimicrobial peptides with selective antitumor mechanisms: prospect for anticancer applications. Oncotarget. 2017 Jul;8(28):46635. 10.18632/oncotarget.16743.Search in Google Scholar PubMed PubMed Central
[65] Bhagani S, Kapil V, Lobo MD. Hypertension. Medicine. 2018;1–7. 10.1016/j.mpmed.2018.06.Search in Google Scholar
[66] Aluko RE. Structure and function of plant protein-derived antihypertensive peptides. Curr Op Food Sci. 2015 Aug;4:44–50. 10.1016/j.cofs.2015.05.002.Search in Google Scholar
[67] National Cancer Institute NCI; 2015. https://www.cancer.gov/about-cancer/causes-prevention/genetics#:∼:text=Genetic%20changes%20that%20cause%20cancer,that%20occur%20as%20cells%20divide.&text=Yes%2C%20cancer%20is%20a%20genetic,way%20cells%20grow%20and%20multiply. Assessed on 15 March 2024.Search in Google Scholar
[68] Trinidad-Calderón PA, Varela-Chinchilla CD, García-Lara S. Natural peptides inducing cancer cell death: mechanisms and properties of specific candidates for cancer therapeutics. Molecules. 2021 Dec;26(24):7453. 10.3390/molecules26247453.Search in Google Scholar PubMed PubMed Central
[69] Yang J, Li X, Xue Y, Wang N, Liu W. Anti-hepatoma activity and mechanism of corn silk polysaccharides in H22 tumor-bearing mice. Int J Biol Macromol. 2014 Mar;64:276–80. 10.1016/j.ijbiomac.2013.11.033.Search in Google Scholar PubMed
[70] Zhu B, He H, Hou T. A comprehensive review of corn protein‐derived bioactive peptides: production, characterization, bioactivities, and transport pathways. Compr Rev Food Sci Food Saf. 2019 Jan;18(1):329–45. 10.1111/1541-4337.12411.Search in Google Scholar PubMed
[71] Ma Z, Hou T, Shi W, Liu W, He H. Inhibition of hepatocyte apoptosis: an important mechanism of corn peptides attenuating liver injury induced by ethanol. Int J Mol Sci. 2015 Sep;16(9):22062–80. 10.3390/ijms160922062.Search in Google Scholar PubMed PubMed Central
[72] Lee J, Lee S, Kim SL, Choi JW, Seo JY, Choi DJ, et al. Corn silk maysin induces apoptotic cell death in PC-3 prostate cancer cells via mitochondria-dependent pathway. Life Sci. 2014 Dec;119(1–2):47–55. 10.1016/j.lfs.2014.10.012.Search in Google Scholar PubMed
[73] Agama-Acevedo E, Flores-Silva PC, Bello-Perez LA. Cereal starch production for food applications. In Elsevier eBooks. 2019; p. 71–102. 10.1016/B978-0-12-809440-2.00003-4.Search in Google Scholar
[74] Samaraweera SA, De Silva AB, Samaranayake MD, Gunawardhane KV, Herath HM. Potential application of locally grown Sri Lankan corn varieties to utilize in the food industry; corn starch and corn syrup. Int J Innov Res Technol Sci. 2018 Jul;4:17–22.Search in Google Scholar
[75] Mosier NS, Ileleji KE. How fuel ethanol is made from corn. In Elsevier eBooks; 2020. p. 539–44. 10.1016/b978-0-12-815497-7.00026-9.Search in Google Scholar
[76] Ahsan M, Asif R, Wasi N, Tariq S, Fawad S, Ejaz T, et al. Development and characterization of innovative nutraceutical corn jam. J Res (Sci). 2022 Jul;33(3):95–101.Search in Google Scholar
[77] Shende D, Sidhu GK. Response surface methodology to optimize enzyme-assisted aqueous extraction of maize germ oil. J Food Sci Technol. 2016 Aug;53:3282–95. 10.1007/s13197-016-2303-z.Search in Google Scholar PubMed PubMed Central
[78] Shikha K, Singh S. Maize utilization and value addition. Agric Food: e-newsletter. 2019 Sep;32:63.Search in Google Scholar
[79] Raghavendran VB, Alex Albert V, Tamilselvan N. Hydroponic maize fodder production—need for small and marginal farmers. Biotica Res Today. 2020;2(7):601–3.Search in Google Scholar
[80] Whalen J, Xu CC, Shen F, Kumar A, Eklund M, Yan J. Sustainable biofuel production from forestry, agricultural and waste biomass feedstocks. Appl Energy. 2017 Jul;198:281–3.10.1016/j.apenergy.2017.05.079Search in Google Scholar
[81] do Val Siqueira L, Arias CI, Maniglia BC, Tadini CC. Starch-based biodegradable plastics: methods of production, challenges and future perspectives. Curr Opin Food Sci. 2021 Apr;38:122–30. 10.1016/j.cofs.2020.10.020.Search in Google Scholar
[82] Wang Q, Gao C, Zhang W, Luo S, Zhou M, Liu Y, et al. Biomorphic carbon derived from corn husk as a promising anode materials for potassium ion battery. Electrochim Acta. 2019 Nov;324:134902. 10.1016/j.electacta.2019.134902.Search in Google Scholar
[83] Ebrahimi M, Bagheri S, Taleghani MM, Ghorabani M. Humic acids elimination from aqueous media utilizing derived activated carbon from raw maize tassel on equilibrium, thermodynamic and kinetics. Desalin Water Treat. 2019 Aug;1:10. 10.5004/dwt.2019.24336.Search in Google Scholar
[84] Waqas M, Hashim S, Humphries UW, Ahmad S, Noor R, Shoaib M, et al. Composting processes for agricultural waste management: a comprehensive review. Processes. 2023 Mar;11(3):731. 10.3390/pr11030731.Search in Google Scholar
[85] San-Emeterio M, De la Rosa L, Knicker JM, López-Núñez H, González-Pérez R, JA. Evolution of maize compost in a mediterranean agricultural soil: implications for carbon sequestration. Agronomy. 2023 Mar;13(3):769. 10.3390/agronomy13030769.Search in Google Scholar
[86] Mathew I, Shimelis H, Mutema M, Chaplot V. What crop type for atmospheric carbon sequestration: results from a global data analysis. Agric Ecosyst Environ. 2017 Jun;243:34–46. 10.1016/j.agee.2017.04.008.Search in Google Scholar
[87] Gissi E, Gaglio M, Aschonitis VG, Fano EA, Reho M. Soil-related ecosystem services trade-off analysis for sustainable biodiesel production. Biomass Bioenergy. 2018 Jul;114:83–99. 10.1016/j.biombioe.2017.08.028.Search in Google Scholar
[88] Saritha A, Ramanjaneyulu AV, Sainath N, Umarani E. Nutritional importance and value addition in maize. Biotica Res Today. 2020;2(9):974–7.Search in Google Scholar
[89] Badu-Apraku B, Fakorede MA. Advances in genetic enhancement of early and extra-early maize for sub-Saharan Africa. Springer; 2017. p. 427–52. 10.1007/978-3-319-64852-1.Search in Google Scholar
[90] Surra E, Bernardo M, Lapa N, Esteves IA, Fonseca I, Mota JP. Biomethane production through anaerobic co-digestion with maize cob waste based on a biorefinery concept: a review. J Environ Manag. 2019 Nov;249:109351.10.1016/j.jenvman.2019.109351Search in Google Scholar PubMed
[91] Lewandrowski J, Rosenfeld J, Pape D, Hendrickson T, Jaglo K, Moffroid K. The greenhouse gas benefits of corn ethanol–assessing recent evidence. Biofuels. 2019;1–15. 10.1080/17597269.2018.1546488.Search in Google Scholar
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Regular Articles
- Supplementation of P-solubilizing purple nonsulfur bacteria, Rhodopseudomonas palustris improved soil fertility, P nutrient, growth, and yield of Cucumis melo L.
- Yield gap variation in rice cultivation in Indonesia
- Effects of co-inoculation of indole-3-acetic acid- and ammonia-producing bacteria on plant growth and nutrition, soil elements, and the relationships of soil microbiomes with soil physicochemical parameters
- Impact of mulching and planting time on spring-wheat (Triticum aestivum) growth: A combined field experiment and empirical modeling approach
- Morphological diversity, correlation studies, and multiple-traits selection for yield and yield components of local cowpea varieties
- Participatory on-farm evaluation of new orange-fleshed sweetpotato varieties in Southern Ethiopia
- Yield performance and stability analysis of three cultivars of Gayo Arabica coffee across six different environments
- Biology of Spodoptera frugiperda (Lepidoptera: Noctuidae) on different types of plants feeds: Potency as a pest on various agricultural plants
- Antidiabetic activity of methanolic extract of Hibiscus sabdariffa Linn. fruit in alloxan-induced Swiss albino diabetic mice
- Bioinformatics investigation of the effect of volatile and non-volatile compounds of rhizobacteria in inhibiting late embryogenesis abundant protein that induces drought tolerance
- Nicotinamide as a biostimulant improves soybean growth and yield
- Farmer’s willingness to accept the sustainable zoning-based organic farming development plan: A lesson from Sleman District, Indonesia
- Uncovering hidden determinants of millennial farmers’ intentions in running conservation agriculture: An application of the Norm Activation Model
- Mediating role of leadership and group capital between human capital component and sustainability of horticultural agribusiness institutions in Indonesia
- Biochar technology to increase cassava crop productivity: A study of sustainable agriculture on degraded land
- Effect of struvite on the growth of green beans on Mars and Moon regolith simulants
- UrbanAgriKG: A knowledge graph on urban agriculture and its embeddings
- Provision of loans and credit by cocoa buyers under non-price competition: Cocoa beans market in Ghana
- Effectiveness of micro-dosing of lime on selected chemical properties of soil in Banja District, North West, Ethiopia
- Effect of weather, nitrogen fertilizer, and biostimulators on the root size and yield components of Hordeum vulgare
- Effects of selected biostimulants on qualitative and quantitative parameters of nine cultivars of the genus Capsicum spp.
- Growth, yield, and secondary metabolite responses of three shallot cultivars at different watering intervals
- Design of drainage channel for effective use of land on fully mechanized sugarcane plantations: A case study at Bone Sugarcane Plantation
- Technical feasibility and economic benefit of combined shallot seedlings techniques in Indonesia
- Control of Meloidogyne javanica in banana by endophytic bacteria
- Comparison of important quality components of red-flesh kiwifruit (Actinidia chinensis) in different locations
- Efficiency of rice farming in flood-prone areas of East Java, Indonesia
- Comparative analysis of alpine agritourism in Trentino, Tyrol, and South Tyrol: Regional variations and prospects
- Detection of Fusarium spp. infection in potato (Solanum tuberosum L.) during postharvest storage through visible–near-infrared and shortwave–near-infrared reflectance spectroscopy
- Forage yield, seed, and forage qualitative traits evaluation by determining the optimal forage harvesting stage in dual-purpose cultivation in safflower varieties (Carthamus tinctorius L.)
- The influence of tourism on the development of urban space: Comparison in Hanoi, Danang, and Ho Chi Minh City
- Optimum intra-row spacing and clove size for the economical production of garlic (Allium sativum L.) in Northwestern Highlands of Ethiopia
- The role of organic rice farm income on farmer household welfare: Evidence from Yogyakarta, Indonesia
- Exploring innovative food in a developing country: Edible insects as a sustainable option
- Genotype by environment interaction and performance stability of common bean (Phaseolus vulgaris L.) cultivars grown in Dawuro zone, Southwestern Ethiopia
- Factors influencing green, environmentally-friendly consumer behaviour
- Factors affecting coffee farmers’ access to financial institutions: The case of Bandung Regency, Indonesia
- Morphological and yield trait-based evaluation and selection of chili (Capsicum annuum L.) genotypes suitable for both summer and winter seasons
- Sustainability analysis and decision-making strategy for swamp buffalo (Bubalus bubalis carabauesis) conservation in Jambi Province, Indonesia
- Understanding factors affecting rice purchasing decisions in Indonesia: Does rice brand matter?
- An implementation of an extended theory of planned behavior to investigate consumer behavior on hygiene sanitation-certified livestock food products
- Information technology adoption in Indonesia’s small-scale dairy farms
- Draft genome of a biological control agent against Bipolaris sorokiniana, the causal phytopathogen of spot blotch in wheat (Triticum turgidum L. subsp. durum): Bacillus inaquosorum TSO22
- Assessment of the recurrent mutagenesis efficacy of sesame crosses followed by isolation and evaluation of promising genetic resources for use in future breeding programs
- Fostering cocoa industry resilience: A collaborative approach to managing farm gate price fluctuations in West Sulawesi, Indonesia
- Field investigation of component failures for selected farm machinery used in small rice farming operations
- Near-infrared technology in agriculture: Rapid, simultaneous, and non-destructive determination of inner quality parameters on intact coffee beans
- The synergistic application of sucrose and various LED light exposures to enhance the in vitro growth of Stevia rebaudiana (Bertoni)
- Weather index-based agricultural insurance for flower farmers: Willingness to pay, sales, and profitability perspectives
- Meta-analysis of dietary Bacillus spp. on serum biochemical and antioxidant status and egg quality of laying hens
- Biochemical characterization of trypsin from Indonesian skipjack tuna (Katsuwonus pelamis) viscera
- Determination of C-factor for conventional cultivation and soil conservation technique used in hop gardens
- Empowering farmers: Unveiling the economic impacts of contract farming on red chilli farmers’ income in Magelang District, Indonesia
- Evaluating salt tolerance in fodder crops: A field experiment in the dry land
- Labor productivity of lowland rice (Oryza sativa L.) farmers in Central Java Province, Indonesia
- Cropping systems and production assessment in southern Myanmar: Informing strategic interventions
- The effect of biostimulants and red mud on the growth and yield of shallots in post-unlicensed gold mining soil
- Effects of dietary Adansonia digitata L. (baobab) seed meal on growth performance and carcass characteristics of broiler chickens: A systematic review and meta-analysis
- Analysis and structural characterization of the vid-pisco market
- Pseudomonas fluorescens SP007s enhances defense responses against the soybean bacterial pustule caused by Xanthomonas axonopodis pv. glycines
- A brief investigation on the prospective of co-composted biochar as a fertilizer for Zucchini plants cultivated in arid sandy soil
- Supply chain efficiency of red chilies in the production center of Sleman Indonesia based on performance measurement system
- Investment development path for developed economies: Is agriculture different?
- Power relations among actors in laying hen business in Indonesia: A MACTOR analysis
- High-throughput digital imaging and detection of morpho-physiological traits in tomato plants under drought
- Converting compression ignition engine to dual-fuel (diesel + CNG) engine and experimentally investigating its performance and emissions
- Structuration, risk management, and institutional dynamics in resolving palm oil conflicts
- Spacing strategies for enhancing drought resilience and yield in maize agriculture
- Composition and quality of winter annual agrestal and ruderal herbages of two different land-use types
- Investigating Spodoptera spp. diversity, percentage of attack, and control strategies in the West Java, Indonesia, corn cultivation
- Yield stability of biofertilizer treatments to soybean in the rainy season based on the GGE biplot
- Evaluating agricultural yield and economic implications of varied irrigation depths on maize yield in semi-arid environments, at Birfarm, Upper Blue Nile, Ethiopia
- Chemometrics for mapping the spatial nitrate distribution on the leaf lamina of fenugreek grown under varying nitrogenous fertilizer doses
- Pomegranate peel ethanolic extract: A promising natural antioxidant, antimicrobial agent, and novel approach to mitigate rancidity in used edible oils
- Transformative learning and engagement with organic farming: Lessons learned from Indonesia
- Tourism in rural areas as a broader concept: Some insights from the Portuguese reality
- Assessment enhancing drought tolerance in henna (Lawsonia inermis L.) ecotypes through sodium nitroprusside foliar application
- Edible insects: A survey about perceptions regarding possible beneficial health effects and safety concerns among adult citizens from Portugal and Romania
- Phenological stages analysis in peach trees using electronic nose
- Harvest date and salicylic acid impact on peanut (Arachis hypogaea L.) properties under different humidity conditions
- Hibiscus sabdariffa L. petal biomass: A green source of nanoparticles of multifarious potential
- Use of different vegetation indices for the evaluation of the kinetics of the cherry tomato (Solanum lycopersicum var. cerasiforme) growth based on multispectral images by UAV
- First evidence of microplastic pollution in mangrove sediments and its ingestion by coral reef fish: Case study in Biawak Island, Indonesia
- Physical and textural properties and sensory acceptability of wheat bread partially incorporated with unripe non-commercial banana cultivars
- Cereibacter sphaeroides ST16 and ST26 were used to solubilize insoluble P forms to improve P uptake, growth, and yield of rice in acidic and extreme saline soil
- Avocado peel by-product in cattle diets and supplementation with oregano oil and effects on production, carcass, and meat quality
- Optimizing inorganic blended fertilizer application for the maximum grain yield and profitability of bread wheat and food barley in Dawuro Zone, Southwest Ethiopia
- The acceptance of social media as a channel of communication and livestock information for sheep farmers
- Adaptation of rice farmers to aging in Thailand
- Combined use of improved maize hybrids and nitrogen application increases grain yield of maize, under natural Striga hermonthica infestation
- From aquatic to terrestrial: An examination of plant diversity and ecological shifts
- Statistical modelling of a tractor tractive performance during ploughing operation on a tropical Alfisol
- Participation in artisanal diamond mining and food security: A case study of Kasai Oriental in DR Congo
- Assessment and multi-scenario simulation of ecosystem service values in Southwest China’s mountainous and hilly region
- Analysis of agricultural emissions and economic growth in Europe in search of ecological balance
- Bacillus thuringiensis strains with high insecticidal activity against insect larvae of the orders Coleoptera and Lepidoptera
- Technical efficiency of sugarcane farming in East Java, Indonesia: A bootstrap data envelopment analysis
- Comparison between mycobiota diversity and fungi and mycotoxin contamination of maize and wheat
- Evaluation of cultivation technology package and corn variety based on agronomy characters and leaf green indices
- Exploring the association between the consumption of beverages, fast foods, sweets, fats, and oils and the risk of gastric and pancreatic cancers: Findings from case–control study
- Phytochemical composition and insecticidal activity of Acokanthera oblongifolia (Hochst.) Benth & Hook.f. ex B.D.Jacks. extract on life span and biological aspects of Spodoptera littoralis (Biosd.)
- Land use management solutions in response to climate change: Case study in the central coastal areas of Vietnam
- Evaluation of coffee pulp as a feed ingredient for ruminants: A meta-analysis
- Interannual variations of normalized difference vegetation index and potential evapotranspiration and their relationship in the Baghdad area
- Harnessing synthetic microbial communities with nitrogen-fixing activity to promote rice growth
- Agronomic and economic benefits of rice–sweetpotato rotation in lowland rice cropping systems in Uganda
- Response of potato tuber as an effect of the N-fertilizer and paclobutrazol application in medium altitude
- Bridging the gap: The role of geographic proximity in enhancing seed sustainability in Bandung District
- Evaluation of Abrams curve in agricultural sector using the NARDL approach
- Challenges and opportunities for young farmers in the implementation of the Rural Development Program 2014–2020 of the Republic of Croatia
- Yield stability of ten common bean (Phaseolus vulgaris L.) genotypes at different sowing dates in Lubumbashi, South-East of DR Congo
- Effects of encapsulation and combining probiotics with different nitrate forms on methane emission and in vitro rumen fermentation characteristics
- Phytochemical analysis of Bienertia sinuspersici extract and its antioxidant and antimicrobial activities
- Evaluation of relative drought tolerance of grapevines by leaf fluorescence parameters
- Yield assessment of new streak-resistant topcross maize hybrids in Benin
- Improvement of cocoa powder properties through ultrasonic- and microwave-assisted alkalization
- Potential of ecoenzymes made from nutmeg (Myristica fragrans) leaf and pulp waste as bioinsecticides for Periplaneta americana
- Analysis of farm performance to realize the sustainability of organic cabbage vegetable farming in Getasan Semarang, Indonesia
- Revealing the influences of organic amendment-derived dissolved organic matter on growth and nutrient accumulation in lettuce seedlings (Lactuca sativa L.)
- Identification of viruses infecting sweetpotato (Ipomoea batatas Lam.) in Benin
- Assessing the soil physical and chemical properties of long-term pomelo orchard based on tree growth
- Investigating access and use of digital tools for agriculture among rural farmers: A case study of Nkomazi Municipality, South Africa
- Does sex influence the impact of dietary vitD3 and UVB light on performance parameters and welfare indicators of broilers?
- Design of intelligent sprayer control for an autonomous farming drone using a multiclass support vector machine
- Deciphering salt-responsive NB-ARC genes in rice transcriptomic data: A bioinformatics approach with gene expression validation
- Review Articles
- Impact of nematode infestation in livestock production and the role of natural feed additives – A review
- Role of dietary fats in reproductive, health, and nutritional benefits in farm animals: A review
- Climate change and adaptive strategies on viticulture (Vitis spp.)
- The false tiger of almond, Monosteira unicostata (Hemiptera: Tingidae): Biology, ecology, and control methods
- A systematic review on potential analogy of phytobiomass and soil carbon evaluation methods: Ethiopia insights
- A review of storage temperature and relative humidity effects on shelf life and quality of mango (Mangifera indica L.) fruit and implications for nutrition insecurity in Ethiopia
- Green extraction of nutmeg (Myristica fragrans) phytochemicals: Prospective strategies and roadblocks
- Potential influence of nitrogen fertilizer rates on yield and yield components of carrot (Dacus carota L.) in Ethiopia: Systematic review
- Corn silk: A promising source of antimicrobial compounds for health and wellness
- State and contours of research on roselle (Hibiscus sabdariffa L.) in Africa
- The potential of phosphorus-solubilizing purple nonsulfur bacteria in agriculture: Present and future perspectives
- Minor millets: Processing techniques and their nutritional and health benefits
- Meta-analysis of reproductive performance of improved dairy cattle under Ethiopian environmental conditions
- Review on enhancing the efficiency of fertilizer utilization: Strategies for optimal nutrient management
- The nutritional, phytochemical composition, and utilisation of different parts of maize: A comparative analysis
- Motivations for farmers’ participation in agri-environmental scheme in the EU, literature review
- Evolution of climate-smart agriculture research: A science mapping exploration and network analysis
- Short Communications
- Music enrichment improves the behavior and leukocyte profile of dairy cattle
- Effect of pruning height and organic fertilization on the morphological and productive characteristics of Moringa oleifera Lam. in the Peruvian dry tropics
- Corrigendum
- Corrigendum to “Bioinformatics investigation of the effect of volatile and non-volatile compounds of rhizobacteria in inhibiting late embryogenesis abundant protein that induces drought tolerance”
- Corrigendum to “Composition and quality of winter annual agrestal and ruderal herbages of two different land-use types”
- Special issue: Smart Agriculture System for Sustainable Development: Methods and Practices
- Construction of a sustainable model to predict the moisture content of porang powder (Amorphophallus oncophyllus) based on pointed-scan visible near-infrared spectroscopy
- FruitVision: A deep learning based automatic fruit grading system
- Energy harvesting and ANFIS modeling of a PVDF/GO-ZNO piezoelectric nanogenerator on a UAV
- Effects of stress hormones on digestibility and performance in cattle: A review
- Special Issue of The 4th International Conference on Food Science and Engineering (ICFSE) 2022 - Part II
- Assessment of omega-3 and omega-6 fatty acid profiles and ratio of omega-6/omega-3 of white eggs produced by laying hens fed diets enriched with omega-3 rich vegetable oil
- Special Issue on FCEM - International Web Conference on Food Choice & Eating Motivation - Part II
- Special Issue on FCEM – International Web Conference on Food Choice & Eating Motivation: Message from the editor
- Fruit and vegetable consumption: Study involving Portuguese and French consumers
- Knowledge about consumption of milk: Study involving consumers from two European Countries – France and Portugal
Articles in the same Issue
- Regular Articles
- Supplementation of P-solubilizing purple nonsulfur bacteria, Rhodopseudomonas palustris improved soil fertility, P nutrient, growth, and yield of Cucumis melo L.
- Yield gap variation in rice cultivation in Indonesia
- Effects of co-inoculation of indole-3-acetic acid- and ammonia-producing bacteria on plant growth and nutrition, soil elements, and the relationships of soil microbiomes with soil physicochemical parameters
- Impact of mulching and planting time on spring-wheat (Triticum aestivum) growth: A combined field experiment and empirical modeling approach
- Morphological diversity, correlation studies, and multiple-traits selection for yield and yield components of local cowpea varieties
- Participatory on-farm evaluation of new orange-fleshed sweetpotato varieties in Southern Ethiopia
- Yield performance and stability analysis of three cultivars of Gayo Arabica coffee across six different environments
- Biology of Spodoptera frugiperda (Lepidoptera: Noctuidae) on different types of plants feeds: Potency as a pest on various agricultural plants
- Antidiabetic activity of methanolic extract of Hibiscus sabdariffa Linn. fruit in alloxan-induced Swiss albino diabetic mice
- Bioinformatics investigation of the effect of volatile and non-volatile compounds of rhizobacteria in inhibiting late embryogenesis abundant protein that induces drought tolerance
- Nicotinamide as a biostimulant improves soybean growth and yield
- Farmer’s willingness to accept the sustainable zoning-based organic farming development plan: A lesson from Sleman District, Indonesia
- Uncovering hidden determinants of millennial farmers’ intentions in running conservation agriculture: An application of the Norm Activation Model
- Mediating role of leadership and group capital between human capital component and sustainability of horticultural agribusiness institutions in Indonesia
- Biochar technology to increase cassava crop productivity: A study of sustainable agriculture on degraded land
- Effect of struvite on the growth of green beans on Mars and Moon regolith simulants
- UrbanAgriKG: A knowledge graph on urban agriculture and its embeddings
- Provision of loans and credit by cocoa buyers under non-price competition: Cocoa beans market in Ghana
- Effectiveness of micro-dosing of lime on selected chemical properties of soil in Banja District, North West, Ethiopia
- Effect of weather, nitrogen fertilizer, and biostimulators on the root size and yield components of Hordeum vulgare
- Effects of selected biostimulants on qualitative and quantitative parameters of nine cultivars of the genus Capsicum spp.
- Growth, yield, and secondary metabolite responses of three shallot cultivars at different watering intervals
- Design of drainage channel for effective use of land on fully mechanized sugarcane plantations: A case study at Bone Sugarcane Plantation
- Technical feasibility and economic benefit of combined shallot seedlings techniques in Indonesia
- Control of Meloidogyne javanica in banana by endophytic bacteria
- Comparison of important quality components of red-flesh kiwifruit (Actinidia chinensis) in different locations
- Efficiency of rice farming in flood-prone areas of East Java, Indonesia
- Comparative analysis of alpine agritourism in Trentino, Tyrol, and South Tyrol: Regional variations and prospects
- Detection of Fusarium spp. infection in potato (Solanum tuberosum L.) during postharvest storage through visible–near-infrared and shortwave–near-infrared reflectance spectroscopy
- Forage yield, seed, and forage qualitative traits evaluation by determining the optimal forage harvesting stage in dual-purpose cultivation in safflower varieties (Carthamus tinctorius L.)
- The influence of tourism on the development of urban space: Comparison in Hanoi, Danang, and Ho Chi Minh City
- Optimum intra-row spacing and clove size for the economical production of garlic (Allium sativum L.) in Northwestern Highlands of Ethiopia
- The role of organic rice farm income on farmer household welfare: Evidence from Yogyakarta, Indonesia
- Exploring innovative food in a developing country: Edible insects as a sustainable option
- Genotype by environment interaction and performance stability of common bean (Phaseolus vulgaris L.) cultivars grown in Dawuro zone, Southwestern Ethiopia
- Factors influencing green, environmentally-friendly consumer behaviour
- Factors affecting coffee farmers’ access to financial institutions: The case of Bandung Regency, Indonesia
- Morphological and yield trait-based evaluation and selection of chili (Capsicum annuum L.) genotypes suitable for both summer and winter seasons
- Sustainability analysis and decision-making strategy for swamp buffalo (Bubalus bubalis carabauesis) conservation in Jambi Province, Indonesia
- Understanding factors affecting rice purchasing decisions in Indonesia: Does rice brand matter?
- An implementation of an extended theory of planned behavior to investigate consumer behavior on hygiene sanitation-certified livestock food products
- Information technology adoption in Indonesia’s small-scale dairy farms
- Draft genome of a biological control agent against Bipolaris sorokiniana, the causal phytopathogen of spot blotch in wheat (Triticum turgidum L. subsp. durum): Bacillus inaquosorum TSO22
- Assessment of the recurrent mutagenesis efficacy of sesame crosses followed by isolation and evaluation of promising genetic resources for use in future breeding programs
- Fostering cocoa industry resilience: A collaborative approach to managing farm gate price fluctuations in West Sulawesi, Indonesia
- Field investigation of component failures for selected farm machinery used in small rice farming operations
- Near-infrared technology in agriculture: Rapid, simultaneous, and non-destructive determination of inner quality parameters on intact coffee beans
- The synergistic application of sucrose and various LED light exposures to enhance the in vitro growth of Stevia rebaudiana (Bertoni)
- Weather index-based agricultural insurance for flower farmers: Willingness to pay, sales, and profitability perspectives
- Meta-analysis of dietary Bacillus spp. on serum biochemical and antioxidant status and egg quality of laying hens
- Biochemical characterization of trypsin from Indonesian skipjack tuna (Katsuwonus pelamis) viscera
- Determination of C-factor for conventional cultivation and soil conservation technique used in hop gardens
- Empowering farmers: Unveiling the economic impacts of contract farming on red chilli farmers’ income in Magelang District, Indonesia
- Evaluating salt tolerance in fodder crops: A field experiment in the dry land
- Labor productivity of lowland rice (Oryza sativa L.) farmers in Central Java Province, Indonesia
- Cropping systems and production assessment in southern Myanmar: Informing strategic interventions
- The effect of biostimulants and red mud on the growth and yield of shallots in post-unlicensed gold mining soil
- Effects of dietary Adansonia digitata L. (baobab) seed meal on growth performance and carcass characteristics of broiler chickens: A systematic review and meta-analysis
- Analysis and structural characterization of the vid-pisco market
- Pseudomonas fluorescens SP007s enhances defense responses against the soybean bacterial pustule caused by Xanthomonas axonopodis pv. glycines
- A brief investigation on the prospective of co-composted biochar as a fertilizer for Zucchini plants cultivated in arid sandy soil
- Supply chain efficiency of red chilies in the production center of Sleman Indonesia based on performance measurement system
- Investment development path for developed economies: Is agriculture different?
- Power relations among actors in laying hen business in Indonesia: A MACTOR analysis
- High-throughput digital imaging and detection of morpho-physiological traits in tomato plants under drought
- Converting compression ignition engine to dual-fuel (diesel + CNG) engine and experimentally investigating its performance and emissions
- Structuration, risk management, and institutional dynamics in resolving palm oil conflicts
- Spacing strategies for enhancing drought resilience and yield in maize agriculture
- Composition and quality of winter annual agrestal and ruderal herbages of two different land-use types
- Investigating Spodoptera spp. diversity, percentage of attack, and control strategies in the West Java, Indonesia, corn cultivation
- Yield stability of biofertilizer treatments to soybean in the rainy season based on the GGE biplot
- Evaluating agricultural yield and economic implications of varied irrigation depths on maize yield in semi-arid environments, at Birfarm, Upper Blue Nile, Ethiopia
- Chemometrics for mapping the spatial nitrate distribution on the leaf lamina of fenugreek grown under varying nitrogenous fertilizer doses
- Pomegranate peel ethanolic extract: A promising natural antioxidant, antimicrobial agent, and novel approach to mitigate rancidity in used edible oils
- Transformative learning and engagement with organic farming: Lessons learned from Indonesia
- Tourism in rural areas as a broader concept: Some insights from the Portuguese reality
- Assessment enhancing drought tolerance in henna (Lawsonia inermis L.) ecotypes through sodium nitroprusside foliar application
- Edible insects: A survey about perceptions regarding possible beneficial health effects and safety concerns among adult citizens from Portugal and Romania
- Phenological stages analysis in peach trees using electronic nose
- Harvest date and salicylic acid impact on peanut (Arachis hypogaea L.) properties under different humidity conditions
- Hibiscus sabdariffa L. petal biomass: A green source of nanoparticles of multifarious potential
- Use of different vegetation indices for the evaluation of the kinetics of the cherry tomato (Solanum lycopersicum var. cerasiforme) growth based on multispectral images by UAV
- First evidence of microplastic pollution in mangrove sediments and its ingestion by coral reef fish: Case study in Biawak Island, Indonesia
- Physical and textural properties and sensory acceptability of wheat bread partially incorporated with unripe non-commercial banana cultivars
- Cereibacter sphaeroides ST16 and ST26 were used to solubilize insoluble P forms to improve P uptake, growth, and yield of rice in acidic and extreme saline soil
- Avocado peel by-product in cattle diets and supplementation with oregano oil and effects on production, carcass, and meat quality
- Optimizing inorganic blended fertilizer application for the maximum grain yield and profitability of bread wheat and food barley in Dawuro Zone, Southwest Ethiopia
- The acceptance of social media as a channel of communication and livestock information for sheep farmers
- Adaptation of rice farmers to aging in Thailand
- Combined use of improved maize hybrids and nitrogen application increases grain yield of maize, under natural Striga hermonthica infestation
- From aquatic to terrestrial: An examination of plant diversity and ecological shifts
- Statistical modelling of a tractor tractive performance during ploughing operation on a tropical Alfisol
- Participation in artisanal diamond mining and food security: A case study of Kasai Oriental in DR Congo
- Assessment and multi-scenario simulation of ecosystem service values in Southwest China’s mountainous and hilly region
- Analysis of agricultural emissions and economic growth in Europe in search of ecological balance
- Bacillus thuringiensis strains with high insecticidal activity against insect larvae of the orders Coleoptera and Lepidoptera
- Technical efficiency of sugarcane farming in East Java, Indonesia: A bootstrap data envelopment analysis
- Comparison between mycobiota diversity and fungi and mycotoxin contamination of maize and wheat
- Evaluation of cultivation technology package and corn variety based on agronomy characters and leaf green indices
- Exploring the association between the consumption of beverages, fast foods, sweets, fats, and oils and the risk of gastric and pancreatic cancers: Findings from case–control study
- Phytochemical composition and insecticidal activity of Acokanthera oblongifolia (Hochst.) Benth & Hook.f. ex B.D.Jacks. extract on life span and biological aspects of Spodoptera littoralis (Biosd.)
- Land use management solutions in response to climate change: Case study in the central coastal areas of Vietnam
- Evaluation of coffee pulp as a feed ingredient for ruminants: A meta-analysis
- Interannual variations of normalized difference vegetation index and potential evapotranspiration and their relationship in the Baghdad area
- Harnessing synthetic microbial communities with nitrogen-fixing activity to promote rice growth
- Agronomic and economic benefits of rice–sweetpotato rotation in lowland rice cropping systems in Uganda
- Response of potato tuber as an effect of the N-fertilizer and paclobutrazol application in medium altitude
- Bridging the gap: The role of geographic proximity in enhancing seed sustainability in Bandung District
- Evaluation of Abrams curve in agricultural sector using the NARDL approach
- Challenges and opportunities for young farmers in the implementation of the Rural Development Program 2014–2020 of the Republic of Croatia
- Yield stability of ten common bean (Phaseolus vulgaris L.) genotypes at different sowing dates in Lubumbashi, South-East of DR Congo
- Effects of encapsulation and combining probiotics with different nitrate forms on methane emission and in vitro rumen fermentation characteristics
- Phytochemical analysis of Bienertia sinuspersici extract and its antioxidant and antimicrobial activities
- Evaluation of relative drought tolerance of grapevines by leaf fluorescence parameters
- Yield assessment of new streak-resistant topcross maize hybrids in Benin
- Improvement of cocoa powder properties through ultrasonic- and microwave-assisted alkalization
- Potential of ecoenzymes made from nutmeg (Myristica fragrans) leaf and pulp waste as bioinsecticides for Periplaneta americana
- Analysis of farm performance to realize the sustainability of organic cabbage vegetable farming in Getasan Semarang, Indonesia
- Revealing the influences of organic amendment-derived dissolved organic matter on growth and nutrient accumulation in lettuce seedlings (Lactuca sativa L.)
- Identification of viruses infecting sweetpotato (Ipomoea batatas Lam.) in Benin
- Assessing the soil physical and chemical properties of long-term pomelo orchard based on tree growth
- Investigating access and use of digital tools for agriculture among rural farmers: A case study of Nkomazi Municipality, South Africa
- Does sex influence the impact of dietary vitD3 and UVB light on performance parameters and welfare indicators of broilers?
- Design of intelligent sprayer control for an autonomous farming drone using a multiclass support vector machine
- Deciphering salt-responsive NB-ARC genes in rice transcriptomic data: A bioinformatics approach with gene expression validation
- Review Articles
- Impact of nematode infestation in livestock production and the role of natural feed additives – A review
- Role of dietary fats in reproductive, health, and nutritional benefits in farm animals: A review
- Climate change and adaptive strategies on viticulture (Vitis spp.)
- The false tiger of almond, Monosteira unicostata (Hemiptera: Tingidae): Biology, ecology, and control methods
- A systematic review on potential analogy of phytobiomass and soil carbon evaluation methods: Ethiopia insights
- A review of storage temperature and relative humidity effects on shelf life and quality of mango (Mangifera indica L.) fruit and implications for nutrition insecurity in Ethiopia
- Green extraction of nutmeg (Myristica fragrans) phytochemicals: Prospective strategies and roadblocks
- Potential influence of nitrogen fertilizer rates on yield and yield components of carrot (Dacus carota L.) in Ethiopia: Systematic review
- Corn silk: A promising source of antimicrobial compounds for health and wellness
- State and contours of research on roselle (Hibiscus sabdariffa L.) in Africa
- The potential of phosphorus-solubilizing purple nonsulfur bacteria in agriculture: Present and future perspectives
- Minor millets: Processing techniques and their nutritional and health benefits
- Meta-analysis of reproductive performance of improved dairy cattle under Ethiopian environmental conditions
- Review on enhancing the efficiency of fertilizer utilization: Strategies for optimal nutrient management
- The nutritional, phytochemical composition, and utilisation of different parts of maize: A comparative analysis
- Motivations for farmers’ participation in agri-environmental scheme in the EU, literature review
- Evolution of climate-smart agriculture research: A science mapping exploration and network analysis
- Short Communications
- Music enrichment improves the behavior and leukocyte profile of dairy cattle
- Effect of pruning height and organic fertilization on the morphological and productive characteristics of Moringa oleifera Lam. in the Peruvian dry tropics
- Corrigendum
- Corrigendum to “Bioinformatics investigation of the effect of volatile and non-volatile compounds of rhizobacteria in inhibiting late embryogenesis abundant protein that induces drought tolerance”
- Corrigendum to “Composition and quality of winter annual agrestal and ruderal herbages of two different land-use types”
- Special issue: Smart Agriculture System for Sustainable Development: Methods and Practices
- Construction of a sustainable model to predict the moisture content of porang powder (Amorphophallus oncophyllus) based on pointed-scan visible near-infrared spectroscopy
- FruitVision: A deep learning based automatic fruit grading system
- Energy harvesting and ANFIS modeling of a PVDF/GO-ZNO piezoelectric nanogenerator on a UAV
- Effects of stress hormones on digestibility and performance in cattle: A review
- Special Issue of The 4th International Conference on Food Science and Engineering (ICFSE) 2022 - Part II
- Assessment of omega-3 and omega-6 fatty acid profiles and ratio of omega-6/omega-3 of white eggs produced by laying hens fed diets enriched with omega-3 rich vegetable oil
- Special Issue on FCEM - International Web Conference on Food Choice & Eating Motivation - Part II
- Special Issue on FCEM – International Web Conference on Food Choice & Eating Motivation: Message from the editor
- Fruit and vegetable consumption: Study involving Portuguese and French consumers
- Knowledge about consumption of milk: Study involving consumers from two European Countries – France and Portugal

