Abstract
Research of antiviral textiles has received considerable attention owing to the continuous emergence of new infectious diseases. Antiviral textiles can effectively inhibit the spread of viruses and significantly reduce the risk of cross-infection and re-infection to protect people’s health and safety. In recent years, researchers studied various antiviral materials, which can prevent the spread and reproduction of viruses by killing and reducing their attachment. These materials can be applied to antiviral textiles through finishing and various spinning methods. This review organizes antiviral materials, analyzes their antiviral mechanisms and inhibition effects, and discusses the methods of combining antiviral materials with textiles, as well as their applications in healthcare and public transportation. In addition, prospects for antiviral textile research are proposed. This review provides references for the study of antiviral textiles and may stimulate the continuous research and development of antiviral textiles.
Graphical abstract
1 Introduction
Emerging infectious diseases (EIDs) refer to emerging or drug-resistant infectious diseases whose incidence in the population has increased over the past 20 years or signs indicate that their incidence may increase in the future. EIDs have various transmission routes and infection methods; however, humans generally lack sufficient immunity to EIDs, as well as treatment methods. Unfortunately, EIDs have been a consistent threat to people’s health and safety over the past 100 years, beginning with the Spanish influenza epidemic in 1918, Asian influenza in 1957, SARS in 2003, H1N1 influenza in 2009, Ebola hemorrhagic fever in West Africa in 2014, Zika virus disease in 2016, and more recently Coronavirus disease in 2019 (COVID-19). According to statistics, in the last 20 years, EIDs have reached as many as 30 kinds. Owing to their rapid spread and high fatality rate, EIDs are a serious health hazard and cause huge economic losses and social impacts [1]. For example, the SARS virus outbreak caused 8,422 confirmed cases and 919 deaths worldwide. In 2014, the West African EVD epidemic confirmed 28,646 cases and 11,323 deaths. To date, the total number of confirmed Coronavirus disease cases has exceeded 105 million, and the death toll has exceeded 2.3 million, causing negative growth in per capita income in more than 95% of countries [2,3].
In terms of emerging diseases control, biological protection textiles play an important role. Reports have shown that they can weaken the spread of viruses and reduce the sudden dangerous occurrence of the pandemic virus through the utilization of protective clothing, medical-surgical masks, and N95 masks. However, although protective clothing and masks can reduce the spread of the virus, the risk of infection cannot be eliminated [4]. According to studies, viruses can survive on textiles for 2–4 weeks at room temperature [5], which can easily cause re-infection and cross-infection [6,7]. In addition, in 2019, the global daily output of medical masks reached 40 million pieces, and the daily waste generated exceeded 15,000 tons. In 2020, the daily output of masks increased by 20.5 times compared with 2019. Many countries dispose of such waste through incineration, which creates great environmental concern [8]. Antiviral textiles can kill viruses on the surface of the fabric or inhibit the formation of biofilms, reducing the risk of infection/re-infection. Moreover, antiviral textiles can reduce environmental pollution due to their reusability. However, most textiles with antiviral properties have not been industrialized, and there is no systematic research on antiviral textiles. Therefore, the development of antiviral textiles is urgent.
This article summarizes the main antiviral mechanisms of antiviral materials, compares the current benefits and drawbacks of various materials, and analyzes the antiviral activities of various materials. We have comprehensively summarized the combination methods of antiviral materials and textiles, as well as the advantages and disadvantages of these methods, and the application in various fields is also discussed. Finally, we discussed specific existing problems of antiviral textiles and propose the direction of future development of antiviral textiles. This review provides a foundation for the research and development of antiviral textiles.
2 Classification and mechanism of antiviral materials
Viruses are mainly composed of genetic material and protein and are a form of material between life and nonlife. The genome is composed of one or more nucleic acid molecules (DNA or RNA), and there is a protective shell of protein or lipoprotein surrounding the genome (Figure 1a). Viruses can be classified into nonenveloped and enveloped viruses according to their external structure. The genetic material of nonenveloped viruses is surrounded by capsid proteins, which are resistant to acid and temperature, and are difficult to inactivate in vitro. Enveloped viruses not only have a protein shell but also have a lipid membrane, which provides adaptability to different physiological environments and has a longer survival period in the host. However, the lipid membrane cannot tolerate high temperature and acidic environments; hence, it is easier to inactivate in vitro. Viruses can survive in vitro for several hours and can spread through droplets and contact. Once they enter the host cell, they can reproduce rapidly. The proliferation cycle includes attachment and entry into the host cell (Figure 1b(1–3)), viral messenger RNA transcription, viral genome replication (Figure 1b(4–6)), protein synthesis, progeny virus particle assembly, and budding (Figure 1b(7 and 8)) [9]. Therefore, according to the aforementioned structural characteristics and replication process, the antiviral pathways mainly include: directly destroying the virus structure, interfering with virus adsorption, preventing viruses from penetrating cells, inhibiting virus biosynthesis, inhibiting virus release, or enhancing the host’s antiviral ability.
![Figure 1
(a) Coronavirus structure diagram. (b) Major steps in the generalized viral life cycle. Potential targets for inhibitors of entry, replication, assembly, and egress and cellular factors [10].](/document/doi/10.1515/ntrev-2021-0072/asset/graphic/j_ntrev-2021-0072_fig_001.jpg)
(a) Coronavirus structure diagram. (b) Major steps in the generalized viral life cycle. Potential targets for inhibitors of entry, replication, assembly, and egress and cellular factors [10].
In recent years, researchers have found that a variety of antiviral materials can inactivate viruses through different mechanisms. Currently, however, there is no standard classification of antiviral materials. This article divides commonly used antiviral materials into three categories according to their chemical structure: inorganic, organic, and natural (Figure 2). Inorganic antiviral material is a relatively new type of antiviral material and mainly refers to carbon-based material and those containing metal ions. Organic antiviral materials refer to polymer materials prepared via polymerization of compounds with antiviral properties such as quaternary ammonium salts, phenols, and benzimidazoles with other comonomers. Natural antiviral materials are currently mainly derived from plants and active substances with antiviral properties that are obtained through extraction, separation, and purification.

Classification of antiviral materials. TCM: traditional Chinese medicine; EPS: extracellular polysaccharides; QAC: quaternary ammonium compounds.
2.1 Inorganic antiviral materials
2.1.1 Metals and their compounds
Metal nanomaterials have characteristics such as large specific surface area, good stability, great biocompatibility, low toxicity, and easy functionalization, which provide broad application prospects in the field of biomedicine [11]. Metal materials, especially metal nanomaterials, have been proven to have a wide spectrum of antiviral properties. Metal nanomaterials can interact with virus surface glycoproteins to destroy its structure. Metal nanomaterials can also enter cells and affect virus replication by interacting with the viral genome (DNA or RNA) to exert antiviral activity. This antivirus mechanism is shown in Figure 3a. Currently, commonly used metal antiviral materials mainly include gold, silver, and copper.
![Figure 3
Antiviral mechanisms and antiviral activity of metals and their compounds. (a) Antiviral mechanism of metal nanoparticles [11]. (b) Effect of test compounds on the inactivation of HSV-1 [15]. (c) Potential antiviral principles of silver nanoparticles [27]. (d) The effect of AgNP on RVFV infection in Vero cells [25]. (e) Antimicrobial contact killing mechanisms for copper include membrane degradation, genotoxicity, and potentially ROS [39]. (f) Inhibition of the Ck/Yamaguchi/7/04 H5N1 virus NA after treatment with CuZeo-textile [38].](/document/doi/10.1515/ntrev-2021-0072/asset/graphic/j_ntrev-2021-0072_fig_003.jpg)
Antiviral mechanisms and antiviral activity of metals and their compounds. (a) Antiviral mechanism of metal nanoparticles [11]. (b) Effect of test compounds on the inactivation of HSV-1 [15]. (c) Potential antiviral principles of silver nanoparticles [27]. (d) The effect of AgNP on RVFV infection in Vero cells [25]. (e) Antimicrobial contact killing mechanisms for copper include membrane degradation, genotoxicity, and potentially ROS [39]. (f) Inhibition of the Ck/Yamaguchi/7/04 H5N1 virus NA after treatment with CuZeo-textile [38].
2.1.1.1 Gold nanoparticles (AuNPs)
AuNPs have attracted considerable attention in nanometer diagnosis and nanomedicine applications due to their small size effect, surface effect, and macroscopic quantum tunneling effect [12] and are often used as carriers for antiviral drugs. The antiviral mechanism of AuNPs has two main pathways: the first is that AuNPs can inhibit the binding of viruses to cell membranes, and the second is that AuNPs can inhibit the proliferation of viruses in cells by binding to the envelope glycoprotein gp120. Di Gianvincenzo et al. [13] described that AuNPs coated with sulfonic acid end ligands can bind to human immunodeficiency virus type 1 (HIV-1) envelope glycoprotein gp120 and inhibit HIV infection of T-cells in vitro at a nanomolar concentration. Fifty percent density of sulfonated ligand (other ligands are inert glucose derivatives) on 2 nm nanoparticles is sufficient to achieve the high anti-HIV activity. Papp et al. [14] tested 2 and 14 nm sialic acid-functionalized AuNPs against influenza viruses. AuNPs achieve antiviral effects through inhibition of the combination of viruses and cell membranes. In addition, they found that the activity of AuNPs depends on particle size and spatial distribution of ligand receptor molecules. Halder et al. [15] used gallic acid (GA) as a raw material to perform a one-pot reduction reaction to synthesize highly monodisperse gold nanoparticles (GAunps) with a diameter of 7.68 nm, which can effectively inhibit herpes simplex virus (HSV) infection. GAunp can interfere with virus attachment and inhibit the proliferation of HSV in Vero cells with low toxicity. Synthesized GAunp is less toxic and more efficient than GA and has been shown to exhibit a significant inhibitory effect on the HSV virus within 1 h (Figure 3b). AuNPs are used more in medicine, mainly as antiviral agents in the body.
2.1.1.2 Silver
Before the discovery of antibiotics, silver was commonly used in the treatment of wounds for antiseptic and antibacterial purposes. The development of nanotechnology has allowed the further development of silver in the field of antimicrobial. Silver nanoparticles (Ag NPs) have been widely used in medicine, textile, and other fields due to their broad spectrum, no drug-resistance mutations, safety, and relatively low price [16,17]. Reports have shown that Ag NPs can inactivate various types of viruses, such as monkeypox virus, hepatitis B, distemper, and arenaviruses [18,19,20,21]. In many studies, Ag NPs have been tested for the inhibition of viruses with different structures (enveloped or nonenveloped viruses) and different genetic information content (DNA or RNA, single or double stranded) [22,23,24]. The unique physical and chemical properties of Ag NPs have a strong adsorption capacity for viruses and interact to produce chemical reactions, thus exhibiting strong antibacterial and antiviral activities. Rai et al. [21] summarized the antiviral mechanism of Ag NPs as follows: Ag NPs (1) interact with viral envelope and viral surface proteins; (2) interact with cell membranes to prevent penetration of virus; (3) block the way for viruses to enter cells; (4) interact with the viral genome; (5) interact with viral factors required for virus replication; and (6) interact with cytokines to produce viral replication (Figure 3c). Vazquez-Muñoz et al. [25] found that Ag NPs reduce the infectivity of the Rift Valley fever virus (RVFV) through cell infection tests. Ag NPs at 12 μg/mL can decrease the infection rate by 98% (Figure 3d). Xiang et al. [26] reported the inhibitory effect of Ag NPs on H1N1 in vitro. Rai et al. [27] showed that the addition of Ag NPs as a coating has high inhibitory activity against HIV-1, HSV-1, and HSV-2. Castro-Mayorga et al. [28] produced a poly(3-114 hydroxybutyrate-co-18mol%-3-hydroxyvalerate)/Ag NPs nanofiber membrane by electrospinning as an antiviral coating on the surface and demonstrated the strongest antiviral activity at 37°C. This nanofiber membrane has good antiviral activity against nonenveloped feline calicivirus (FCV) and murine norovirus (MNV), and the titer is reduced by 1.42 and 0.14 log TCID50, respectively (log TCID50 is the half lethal dose after 10-fold dilution of the virus suspension and the quantitative unit of the virus infection value).
2.1.1.3 Copper and its compounds
Copper, a common antimicrobial material, is widely applied owing to its specific properties such as high efficiency, low toxicity, and broad spectrum. It exhibits a killing effect on infectious bronchitis virus, poliovirus, HIV-1, and so on [29]. In addition, copper can generate toxicity to microorganisms through several parallel nonspecific mechanisms, including damage to the microbial envelope, intracellular proteins, and nucleic acids [30]. Moreover, nanoparticles formed by copper compounds can combine with oxygen to generate and release reactive oxygen species (ROS), which oxidize the capsid protein of virus to effectively inactivate the virus. The antivirus mechanism is shown in Figure 3e. Numerous researches have shown that copper can reduce the infectivity of enveloped or nonenveloped, DNA or RNA viruses [29,31,32,33,34,35]. Noyce et al. [35] found that only 0.1% of influenza A (H1N1) can survive after being in contact with the copper surface for 6 h. In addition, copper ions (Cu2+) in CuCl2 and CuSO4 solutions at 2.5–250 μM concentration inactivate avian influenza virus (H9N2) subtype viruses within 3–6 h. Upon prolongation of contact time, the inactivation effect toward the virus increases [27]. Copper iodide (CuI) has also shown the inactivation effect toward H1N1 [36] and FCV [37]. Borkow et al. [33] dipped ordinary respiratory protective masks in copper oxide and performed antiviral tests on H1N1 and H9N2. Under the condition of simulated breathing, the virus titer reduces by >4.27 and >4.83 log TCID50, respectively, after 30 min. Imai et al. [38] discovered that highly pathogenic H5N1 and low pathogenic H5N3 viruses are inactivated on copper-treated fabric, in which the titer of A/chicken/Yamaguchi/7/04 (H5N1) in Madin-Darby Canine Kidney (MDCK) cells and eggs declines by >5.0 log 10 and 5.0 log 10 after 30 s, respectively. The titer of A/whooper swan/Hokkaido/1/08 (H5N1) in MDCK cells decreases by 2.3 and 3.5 within 1 and 5 min, respectively. Treatment of A/whistling swan/Shimane/499/83 (H5N3) on CuZeo-textile for 10 min reduces the titer by >5.0 log 10 in MDCK cells and by >3.5 log 10 in eggs. In contrast, such a reduction in titers is not observed on cotton fabrics treated with PBS buffer or containing only zeolite (Figure 3f).
2.1.2 Carbon-based nanomaterials
Carbon-based nanomaterials consist of carbon materials with at least one dimension less than 100 nm in the dispersed phase. Carbon-based nanomaterials have been found to exhibit good antiviral properties. In addition to their own nanostructures that may affect viral replication, they can also use their excellent electrochemical and electrothermal properties to inactivate viruses by heating to achieve antiviral properties.
2.1.2.1 Fullerene and its derivatives
Fullerene is the third allotrope of elemental carbon. Its antiviral mechanism mainly consists of two aspects: First, it inhibits the activity of internal enzymes of the virus. Friedman et al. [40] studied the ability of C60 (Bucky Ball) derivatives to interact with the active site of HIV-1 protease through modeling and physical and chemical analyses. They found that the main antiviral target of fullerene is HIV viral protease (HIVP), and fullerene inhibits its activity and terminates the life cycle of HIV. Second, fullerene also can be used as a biological structural scaffold for multivalent ligands to simulate virus morphology and inhibit virus reproduction. Muñoz et al. [41] reported a water-soluble 13-carbon fullerene modified by 120 peripheral carbohydrate subunits (Figure 4a). The prepared fullerene mimicked the shape of the virus, allowing binding to cell receptors at the early stage of infection, thus inhibiting the entry of the pathogen into the host cell. This has an excellent inhibitory effect on the Ebola virus.
![Figure 4
Antiviral mechanism of carbon-based materials. (a) Structure of the tridecafullerenes 17a–17c, the central C60 core is covalently connected to 12 hexakis adducts of C60. Each peripheral fullerene is endowed with ten monosaccharides and so a total of 120 carbohydrates decorate the periphery of each molecule [41]. (b) Schematic of bacteria capture, along with subsequent sterilization and depyrogenation by Joule-heating. (i) Schematic of the Joule-heating setup in which a potential is applied across the filter for Joule heating. (ii) Schematic of filtration followed by sterilization and depyrogenation through Joule heating [42]. (c) Possible mechanisms of the antiviral activity of GO. (i) Normal viruses are absorbed into cells by interacting with cell receptors to initiate infection. (ii) Negatively charged GO has more chances to interact with the positively charged viruses, resulting in virus damage and infection suppression. (iii) Infection was blocked by GO conjugated with nonionic PVP. (iv) Conjugation of GO with cationic PDDA cannot prevent the virus from infecting cells [47]. (d) Method for the elimination of a virus by using CNH complexes and NIR laser irradiation [48].](/document/doi/10.1515/ntrev-2021-0072/asset/graphic/j_ntrev-2021-0072_fig_004.jpg)
Antiviral mechanism of carbon-based materials. (a) Structure of the tridecafullerenes 17a–17c, the central C60 core is covalently connected to 12 hexakis adducts of C60. Each peripheral fullerene is endowed with ten monosaccharides and so a total of 120 carbohydrates decorate the periphery of each molecule [41]. (b) Schematic of bacteria capture, along with subsequent sterilization and depyrogenation by Joule-heating. (i) Schematic of the Joule-heating setup in which a potential is applied across the filter for Joule heating. (ii) Schematic of filtration followed by sterilization and depyrogenation through Joule heating [42]. (c) Possible mechanisms of the antiviral activity of GO. (i) Normal viruses are absorbed into cells by interacting with cell receptors to initiate infection. (ii) Negatively charged GO has more chances to interact with the positively charged viruses, resulting in virus damage and infection suppression. (iii) Infection was blocked by GO conjugated with nonionic PVP. (iv) Conjugation of GO with cationic PDDA cannot prevent the virus from infecting cells [47]. (d) Method for the elimination of a virus by using CNH complexes and NIR laser irradiation [48].
2.1.2.2 Graphene and its derivatives
Graphene, a carbon dioxide nanomaterial, is composed of carbon atoms sp2 hybrid orbitals honeycomb hexagonal lattice and has excellent optical, electrical, and mechanical properties. Graphene can be used to inactivate viruses by photoheating or electric heating due to the virus’s inability to withstand high temperatures. Stanford et al. [42] showed the ability of laser-induced porous conductive graphene foam to capture bacteria, viruses, and pollutants, which can reduce infection and inactivate viruses by electric heating (Figure 4b). Deokar et al. [43] designed and synthesized sulfonated magnetic nanoparticles, which were anchored on graphene sheets. The prepared graphene composite material captured viruses, followed by polymerization through external magnets to promote effective photothermal treatment. Type 1 HSV can be captured, heated, and destroyed. After 7 min of infrared radiation, 99% of the virus is inactivated. In fact, many reports have described that adsorption on graphene and gentle heat treatment at 56℃ for 30 min inactivate viruses [44,45].
Graphene oxide (GO) is formed via oxidation of graphene, which creates more active properties than graphene owing to increased oxygen-containing functional groups. In addition, currently, it is the most studied class of antibacterial and antiviral graphene materials [46]. GO is under a unique two-dimensional surface chemical structure and sharp physical edge structure, which can adsorb and block viruses. Moreover, the structure of GO itself has an inhibitory effect on viral infection. Ye et al. [47] found that GO can significantly inhibit the infection of the pseudorabies virus and porcine epidemic diarrhea virus. Both viruses can be inactivated through the destruction of their structure before the virus enters the cell, and the virus titer is reduced by 2 log TCID50. The potent antiviral activity of GO is attributed to its unique monolayer structure and negative charge. GO shows strong antiviral activity when combined with polyvinyl pyrrolidone (PVP, nonionic polymer), but not when combined with poly dimethyl diallyl ammonium chloride (PDDA, cationic polymer). In addition, the antiviral activity of GO’s precursor graphite and GO is much weaker than that of single-layer GO and reduced GO, indicating that the structure of nanoflakes is important to promote antiviral performance (Figure 4c).
The continuous development of biosensors has allowed researchers to create various types that detect and accurately locate viruses to prevent the virus from spreading further. Graphene has excellent electrochemical properties and can be used as a biosensor for virus monitoring. Kim et al. [49] prepared liquid coplanar gate graphene field-effect transistors on a flexible polyethylene terephthalate substrate for the detection of two typical viruses (HIV-1 and murine leukemia virus). In this case, the antibodies are used as probe molecules and modified on the graphene surface with 1-pyrene butyrate succinimidyl ester (PASE). When the solution containing the virus is dripped on the sensor, due to the electrostatic gating effect of the graphene in the antigen (namely, virus)–antibody complex, the Dirac point voltage moves downward (Figure 5a). Yang et al. [50] developed a new bandage-type wearable flexible microfluidics polymerase amplification sensor for visual detection of nucleic acids. The sensor is triggered by body temperature (30–37℃) and can be used for rapid, sensitive, intuitive, and highly portable nucleic acid detection. Kinnamon et al. [51] cross-linked influenza A-specific antibodies on the surface of GO to prepare a biosensor for virus monitoring. Wearable devices by high-risk groups allow virus detection before symptoms appear, as shown in Figure 5b.
![Figure 5
Fabrication and application of graphene sensors. (a) (i) Binding schematic of the antibody to PASE/graphene by condensation covalent bonding. (ii) Detection schematic of virus sensors using the interaction between antibody/PASE/graphene and target HIV-1 virus. (iii) A camera image of a virus sensor [49]. (b) Schematic of envisioned application space for the GO screen printed flexible impedance biosensors. Sensors arrays mounted on textiles and interfaced with flexible electronics can report the location of positive cases and identify sources of the outbreak [51].](/document/doi/10.1515/ntrev-2021-0072/asset/graphic/j_ntrev-2021-0072_fig_005.jpg)
Fabrication and application of graphene sensors. (a) (i) Binding schematic of the antibody to PASE/graphene by condensation covalent bonding. (ii) Detection schematic of virus sensors using the interaction between antibody/PASE/graphene and target HIV-1 virus. (iii) A camera image of a virus sensor [49]. (b) Schematic of envisioned application space for the GO screen printed flexible impedance biosensors. Sensors arrays mounted on textiles and interfaced with flexible electronics can report the location of positive cases and identify sources of the outbreak [51].
2.1.2.3 Carbon nanohorn (CNH)
CNH is a kind of “ox horn” carbon nanomaterial. Due to its unique structure, CNH has the characteristics of large specific surface area, strong thermal stability, and high porosity, making it ideal for the field of biological medicine. Miyako et al. [48] reported an antiviral approach to carbon nanotubes driven by a near-infrared laser, successfully synthesizing complex T7Tag antibody-CNH, which selectively binds to T7 phages and effectively inactivates under near-infrared laser irradiation. Therefore, it is speculated that it can have a photothermal killing effect on harmful viruses (such as HIV, SARS, and avian influenza viruses), and this mechanism is shown in Figure 4d.
2.1.3 Photocatalytic materials
Under photo-irradiation conditions, photocatalytic antiviral materials can absorb light and generate singlet oxygen using surrounding oxygen, which can oxidize and destroy the protein membrane, thereby destroying the viral envelope and promoting deactivation (Figure 6a). The process of photocatalytic antiviral material’s inactivation of microorganisms is shown in Figure 6b. This kind of material has the advantages of being green, pollution free, and high durability.
![Figure 6
(a) Principle of photocatalysis. (b) Microbial inactivation process [52].](/document/doi/10.1515/ntrev-2021-0072/asset/graphic/j_ntrev-2021-0072_fig_006.jpg)
(a) Principle of photocatalysis. (b) Microbial inactivation process [52].
Titanium dioxide (TiO2), a widely used photocatalytic material, can achieve antiviral effect through photocatalysis. Zan et al. [53] found that TiO2 suspension and TiO2-coated ceramic plates destroy most hepatitis B surface antigens and affect the adhesion of hepatitis B virus on cells under weak ultraviolet light, weak sunlight, or indoor sunlight irradiation, which inhibit the viral activity. After 4 h of exposure to 0.6 mW/cm2 mercury lamp, 97% of HBs can be destroyed in TiO2 suspension. Mazurkova et al. [54] tested the inactivation effect of TiO2 nanoparticles and TiO2 powder on influenza virus, in which TiO2 nanoparticles inactivate influenza viruses in the dark, while nonnanoparticles have no antiviral activity in the sun. Therefore, it is speculated that the main mechanism is that nanoparticles destroy the viral envelope, not just through photocatalysis. The degree of influenza virus inactivation depends on the concentration of nanoparticles and contact time. Upon incubation of influenza virus with TiO2 nanoparticles, it is killed after 30 min, and the optimal concentration is 2 mg/mL. Hajkova et al. [55] prepared TiO2 films using titanium isopropanol as precursors by plasma chemical vapor deposition, which inactivate herpes simplex virus (HSV-1) and exhibit 100% antiviral effect after 6 h of light exposure.
After nanocrystallization, zinc oxide (ZnO) has the advantages of low toxicity, safety, odorless, and high efficiency. Under light, it can form ROS, which can destroy the binding of virus and host cells, as well as the surface proteins of the virus; hence, it displays good antiviral properties. The effect of ZnO on biological function depends on its morphological structure, particle size, exposure time, concentration, pH value, and biocompatibility [56]. López de Dicastillo et al. [57] prepared ZnO nanotubes with enhanced antimicrobial activity compared to ZnO nanoparticles (ZnO NPs). Ghaffari et al. [58] used A/H1N1 strain A/Puerto Rico/8/34 (PR8) as A(H1N1) virus model and evaluated ZnO NPs, as well as the addition of polyethylene glycol (PEG) (PEG-ZnO NPs) antiviral characteristics of H1N1 virus. Compared with the controls, the highest nontoxic concentrations of PEG-ZnO NPs and pure ZnO NPs (200 and 75 μg/mL, respectively) after contact with the virus, the virus drops degree can be reduced by 2.8 and 1.2 log TCID50, respectively. Li et al. produced ZIF-8 (zinc-imidazolate MOF) nanocrystalline film with good photocatalytic and antibacterial properties, which far exceeds the performance of traditional semiconductors such as ZnO and TiO2 [9]. According to the indicated mechanism, the photoelectron captured by Zn+ center in ZIF-8 through ligand-metal charge transfer is the reason for the production of ROS related to oxygen reduction, which is the main disinfection mechanism. The inactivity of ZIF-8 against poliovirus and coronavirus is 99.99 and 99%, respectively.
2.2 Organic antiviral materials
Antiviral organic compounds have the characteristics of fast sterilization, easy synthesis, and high efficiency. They are often used to synthesize antiviral drugs and used in textile finishing to prepare antiviral textiles. Organic compounds can be polymerized and copolymerized with other comonomers to produce antiviral coatings or directly to produce antiviral fibers. Such compounds include quaternary ammonium salts, organic heterocyclic compounds, phenolic compounds, and organic metals.
2.2.1 Quaternary ammonium salt polymer
Quaternary ammonium salts are the most commonly used quaternary ammonium compounds. Their structure, especially the length of the alkyl chain, plays an important role in its antimicrobial activity. The double-chain quaternary ammonium salt has an inactivating effect on viruses. Their antiviral performance is also closely related to its concentration [59]. Hsu et al. [60] found that the dodecyl methyl polyethylene imine coating applied to the solid surface has a disinfecting effect on the influenza virus in the aqueous solution. Experiments based on scanning electron microscopy reveal that once in contact the influenza virus irreversibly adheres with the hydrophobic polycation coating, causing structural damage and inactivation, followed by the release of viral ribonucleic acid. The mechanism of its inactivation of influenza virus is shown in Figure 7a. Furthermore, monomer structure analogs of N,N-dodecyl, methyl-polyethyleneimine were analyzed, showing that the antiviral activity is inherent to the quaternary ammonium salt monomer unit and even remains in the form of polymer and surface fixation.
![Figure 7
(a) Mechanism of influenza virus inactivation by N,N-dodecyl, methyl-PEI coatings [60]. (b) PQAA antiviral mechanism: (i) binding of influenza virus to the N-acetylsialic acid receptor and engulfment by the cellular membrane, (ii) penetration of PQAA into the viral envelope followed by lipid destruction, (iii) inhibition of PQAA on ADV entry via blocking the association between CAR receptor and fiber protein, and (iv) binding of ADV to the CAR receptor mediated by the fiber protein and formation of Clarin pit. (c) Antiviral activity of PQAA against influenza virus (i) and adenovirus (ii) [61].](/document/doi/10.1515/ntrev-2021-0072/asset/graphic/j_ntrev-2021-0072_fig_007.jpg)
(a) Mechanism of influenza virus inactivation by N,N-dodecyl, methyl-PEI coatings [60]. (b) PQAA antiviral mechanism: (i) binding of influenza virus to the N-acetylsialic acid receptor and engulfment by the cellular membrane, (ii) penetration of PQAA into the viral envelope followed by lipid destruction, (iii) inhibition of PQAA on ADV entry via blocking the association between CAR receptor and fiber protein, and (iv) binding of ADV to the CAR receptor mediated by the fiber protein and formation of Clarin pit. (c) Antiviral activity of PQAA against influenza virus (i) and adenovirus (ii) [61].
The structure of quaternary phosphonium salt (QPS) is similar to that of quaternary ammonium salt; however, its polarization is stronger, making it is easier to adsorb bacteria and viruses and enhance antiviral performance. Xue and Xiao [61] found that trimer poly(QPM-r-AM-r-ATC) (PQAA), which can be prepared from quaternary phosphate salts, has antiviral properties and can effectively inactivate enveloped viruses (influenza viruses) and nonenveloped viruses (adenoviruses). Different antiviral mechanisms lead to different antiviral effects of PQAA on enveloped and nonenveloped viruses. The lipids of enveloped viruses are susceptible to high-density positive charges [62,63], and PQAA can bind and destroy influenza virus lipid envelopes through strong charge interactions. Furthermore, the receptor binding site, the envelope glycoprotein hemagglutinin, is also destroyed. The destruction of the viral envelope and loss of the binding site contribute to the inactivation of influenza virus, as shown in Figure 7b(i) and (ii). The negatively charged fiber knob acts as a receptor-binding site for cell entry. For adenovirus, a nonenveloped virus, the polymer QPS can block the adenovirus fiber knob–receptor interaction, prevent the adenovirus from entering the cell, followed by its inactivation, as shown in Figure 7b(iii) and (iv). Plaque reduction experiments for influenza and adenovirus show that the highest virus-killing efficiency of PQAA is 97.9% at a polymer concentration of 100 ppm for influenza viruses (Figure 7c(i)). For adenovirus, the maximum virus-killing efficiency is 66.4% at 10 ppm (Figure 7c(ii)).
2.2.2 Organic heterocyclic compounds
Organic heterocyclic compounds, especially nitrogen-containing heterocyclic compounds, such as pyrazoles, pyrimidines, and pyrrole heterocyclic compounds, have been broadly used as antibacterial agents [64]. El-Sabbagh et al. [65] synthesized novel antiviral N-acetyl 4,5-dihydropyrazole 7, which inactivates vaccinia virus at subtoxic concentrations. Kim et al. [66] used the redox properties of polyaniline-copyrrole polymerized nanotubes to synthesize polymerized nanotubes that regulate ROS levels. The activation of MEK (mitogen-activated protein kinase)/ERK (extracellular signal-regulated kinase) can be induced through the generation of ROS, thereby promoting cell survival and proliferation. They also confirmed that the three subtypes of influenza A virus (H1N1, H3N2, and H9N2) inhibit the spread of MDCK cells.
2.2.3 Others
In addition to the aforementioned two categories, organic antiviral materials also include biguanides, organotin, and so on. Park et al. [67] synthesized novel N,N-dodecyl methyl polyurethane (Quat-12-PU), which is easily processed into solutions, nanoparticles, and nanofibers. The surface of such materials has antiviral properties and inhibits influenza virus inactivation. Whether it is solution coating or nanocoating experiment, the material exhibits a strong inactivation effect on human influenza virus. It is speculated that hydrophobic Quat-12-PU coating destroys the lipid envelope of the virus, thereby promoting inactivation. However, Quat-12-PU coating cannot inactivate nonenveloped poliovirus. Rzaev and Sadykh-Zade [68] developed specific organotin polymers derived from trimethyltin and triethyltin ester, which show extraordinary antiviral activity due to the partial hydrolysis of organotin. Roner et al. [69] also verified that organotin polymers can inhibit virus replication.
Wang et al. [70] investigated the antiviral properties of other polymers by evaluating the virulent properties of poly(phenylacetylene)-based cationic conjugated polyelectrolytes (CPE) and oligophenylacetylene (OPE), which are commonly used as antimicrobial agents. CPE and OPE are also excellent candidates for antiviral applications and display the same virus-killing mechanisms as antimicrobials. Under UV-visible light irradiation, chlorinated paraffins and oxidized paraffins can produce singlet oxygen due to the π bond system in the main chain of the compound and then form more corrosive reactive oxygen intermediates, showing significant virus-killing properties. CPEs and OPEs exhibit intermediate antiviral activity through partial decomposition of phage particle structures in the dark and by photochemical damage of phage capsid proteins under UV/visible light irradiation. Their structures and sizes can be chemically modified for specific antiviral needs.
2.3 Natural antiviral materials
Due to environmental protection and health and safety awareness in recent years, natural antiviral substances have been paid more attention. Natural antiviral materials have a series of advantages, such as wide availability, safety, and low toxicity. Commonly used natural antiviral materials can be divided into plants, animals, and microbial extracts.
2.3.1 Traditional Chinese medicine
Traditional Chinese medicine has a long history in antiviral therapeutics, in which its ingredients can be widely sourced and exhibit low toxicity. Certain Chinese herbal medicines are also effective against drug-resistant virus strains. Traditional Chinese medicines such as Huoxiang Zhengqi, Lianhua Qingwen, Shufeng Jiedu, and Xuebijing have been verified to have significant effects on new coronary pneumonia [71]. The key components of Rhizobacteria sinensis, such as kaolin, quercetin, auricin, glycyrrhetinic acid, stigmastol, and indigo, have a good binding ability with 3CL(Mpro) and ACE2 enzyme proteins of SARS-CoV-2 and can act on COVID-19 through multiple components, multiple targets, and multiple pathways. The main ingredients of Shufeng detoxification are Polygonum cuspidatum, Forsythia suspensa, Isatis indigotica, Bupleurum, Corydalis, Verbena, Reed root, and Licorice, which can inhibit virus proliferation and anti-inflammatory effects, as well as possessing certain immunomodulatory effects [71]. More and more Chinese herbal medicines with antiviral activity have obtained experimental or clinical evidence of efficacy. Table 1 lists some antiviral traditional Chinese medicines and the corresponding viruses they can be effective against.
Partial list of traditional Chinese medicine names approved by the SFDA for the treatment of viral diseases
| Traditional Chinese medicine name | Species of virus | Epidemic disease | References |
|---|---|---|---|
| Radix bupleuri | Influenza A (H1N1) virus | Influenza | [72,73] |
| Human coronavirus 229E | Upper respiratory infection | ||
| Measles virus | |||
| Herpes simplex virus | |||
| 14 Varicella zoster viruses | |||
| Fructus forsythia | Influenza A (H1N1) virus | Acute bronchitis, pneumonia influenza | [74,75] |
| Respiratory syncytial virus | |||
| Infectious bronchitis virus | |||
| Honeysuckle | Influenza A (H1N1) virus | Influenza | [76,77] |
| Scutellaria root | Herpes simplex virus | Tonsillitis | |
| Adenovirus | Pharyngitis | ||
| Respiratory syncytial virus | Upper respiratory infection | ||
| Parainfluenza virus (PIV) | |||
| Indigowoad root | Influenza A (H1N1) virus | Influenza | [78,79] |
| Herpes simplex virus | Acute tonsillitis | ||
| Hepatitis B virus | Mumps |
2.3.2 Propolis
As a natural medicine, propolis has a wide range of biological and pharmacological activities. Modern science has confirmed the antibacterial and antiviral effects of propolis [80]. The mechanism of antiviral properties of propolis is related to viral inhibition to a certain extent. It can promote reverse transcriptase and virus proliferation inhibition. Tait et al. [81] reported that natural and synthetic flavonoids may interfere with the replication of picornaviruses, prevent the shedding of viral particles and release intracellular RNA, or prevent the synthesis of viral RNA. Gekker et al. [82] confirmed that propolis has strong virus-inactivation activity against HIV-1 virus cultured in CD4 T lymphocytes and microglia cells, and propolis has a concentrate-dependent inhibitory effect on virus expression. A total of 66.6 μg/mL propolis can inhibit HIV-1 virus cultured in CD4 T lymphocytes and microglia cells by 85 and 98%, respectively. According to the research of Huleihel and Isanu [83], the antiviral activity of propolis may be related to the effect of flavonoids, which play a critical role in the antiviral process. Propolis has 50% inhibitory effect on HSV infection. Indirect results indicate that propolis has a strong interaction with the surface of Vero cells, but has no interaction with HSV particles, thereby inhibiting the cell–virus binding process.
2.3.3 Exopolysaccharides (EPSs)
EPSs are synthesized by eukaryotes and prokaryotes. Compared to other natural materials, the production time of EPSs is usually much shorter. Moreover, the extract method of EPSs is very simple, and it has been extensively used in textile finishing [84]. Effective inactivation of the virus is known to depend on the ability of the infected host to generate pro-inflammatory immune response and Th1 immunity, which limits viral replication [85]. In addition, reports have shown that probiotics and their metabolites can fight viral infection through enhancement of innate and adaptive antiviral immunity, thereby reducing the duration and frequency of disease, shedding the virus, normalizing intestinal permeability, and increasing the production of virus-specific antibodies [86]. In vitro studies have shown that EPSs (especially sulfated polysaccharides such as dextran) have antiviral effects against enveloped viruses [87], where they interfere with viral absorption and penetration into host cells, as well as inhibit the reverse transcriptase activity of various retroviruses. Arena et al. [85] studied the antiviral and immunomodulatory effects of EPS-1, a novel extracellular polysaccharide produced by heat-resistant Bacillus teriformis strain, against HSV-2, and found that EPS-1 inhibits HSV-2 replication in human peripheral blood mononuclear cells (PBMCs) by inducing a large number of Th1 cytokines (IFN-A, IFN-C, TNF-A, IL-12, and IL-18). The antiviral activity of 300 µg/mL EPS-1 reached 85%. Gugliandolo et al. [88] showed that a new extracellular polysaccharide (EPS1-T14) from a strain of Bacillus licheniformis T14 has an antiviral effect on HSV-2, with the inhibition rate reaching 77%. Kanmani et al. [89] showed that the extracellular polysaccharide of Lactobacillus delbrueckii OLL1073R-1 has the potential antiviral activity against enteroviruses such as rotavirus.
2.4 Composite antiviral material
In recent years, to improve the antiviral performance of various materials, many researchers have used the aforementioned three materials to create composite antiviral materials through various methods. These composite materials can often produce synergistic effects and greatly improve the antiviral efficiency. Currently, Ag is the most commonly used antiviral material in combination with other materials for antiviral synergy.
Chen et al. [90] prepared GO–Ag nanocomposites by growing silver on GO and identified the antiviral activity of GO and GO–Ag against coated feline coronavirus (FCoV) and uncoated infectious bursa virus (IBDV) using virus inhibition assay. They found that GO–Ag inhibits 25% of FCoV infection and 23% of IBDV infection at noncytotoxic concentrations, while GO inhibits only 16% of FCoV infection and exhibited no antiviral activity against IBDV infection. The unique structure of GO tablets benefits the inhibition of FCOV infection of lipid envelope and can enhance the antiviral effect of silver particles by supporting the uniform dispersion of silver particles and formation of nonaggregated spherical particles. The fixation of Ag NPs on GO tablets reduces cytotoxicity. Iyigundogdu et al. [91] applied sodium pentaborate and triclosan to fabrics to obtain antiviral properties. The cotton fabric finished with 3% sodium pentaborate, 0.03% triclosan, and 7% glucagon has a good inactivation effect toward adenovirus type 5 and poliovirus type 1. Textile materials can reduce the titer of both viruses by 60%. Mori et al. [92] prepared Ag NPs/chitosan flocculent powder composite material with antiviral activity. Ag NPs of varying diameters were embedded in the chitosan matrix, where the antiviral activity increases with increasing Ag NPs concentration. The smaller Ag NPs in the composite material projected stronger antiviral activity. Chitosan itself has no antiviral activity, but it is conducive to the combination of virus and nano-silver, thereby improving the antiviral activity. Li et al. [93] studied the antibacterial synergy of TiO2 and Ag NPs. Ag NPs preferably break down the cell wall of the microorganism and attack the respiratory chain and cell division and ultimately lead to cell death. In addition, under light, TiO2 makes the surface of the fabric produce more active materials to ionize Ag. The ionized Ag is more active, can destroy the cell wall structure, and induces bacterial cell lysis. The NPs release Ag ions in the bacteria cell, which enhances the antibacterial activity. The synergistic antiviral mechanism of nanoparticles and photocatalysis is being further explored.
The aforementioned antiviral materials have been confirmed to have antiviral activity at a certain concentration, but these materials have their own advantages and disadvantages, as presented in Table 2. We can refer to these properties to prepare antiviral textiles for different purposes.
Advantages and disadvantages of various antiviral materials
| Antiviral material | Advantage | Disadvantage |
|---|---|---|
| Inorganic antiviral material | ||
| Metals and their compounds | Wide spectrum | High cost |
| Long effective antiviral period | Delayed effect | |
| Low toxicity | Ease of oxidation | |
| Resistance to drug resistance | ||
| Carbon-based nanomaterials | Low cytotoxicity | High cost |
| Specific antiviral activity | Limited range of use | |
| Photocatalytic material | Low cytotoxicity | High cost |
| Good antiviral performance | Poor antifungal effect | |
| Environmentally friendly | ||
| Organic antiviral material | Low cost | Easy to produce drug resistance |
| Fast germicidal speed | Toxic | |
| Easy to process | Poor heat resistance | |
| Good color stability | Pollution in the production process | |
| Natural antiviral material | Safe and nontoxic | Difficulty in processing |
| Environmentally friendly | Poor durability | |
| Poor heat resistance | ||
| Short service life | ||
| Narrow application range | ||
| Composite antiviral material | Prolonged durability | The coordination mechanism is not clear |
| Broad spectrum | ||
| Safe | ||
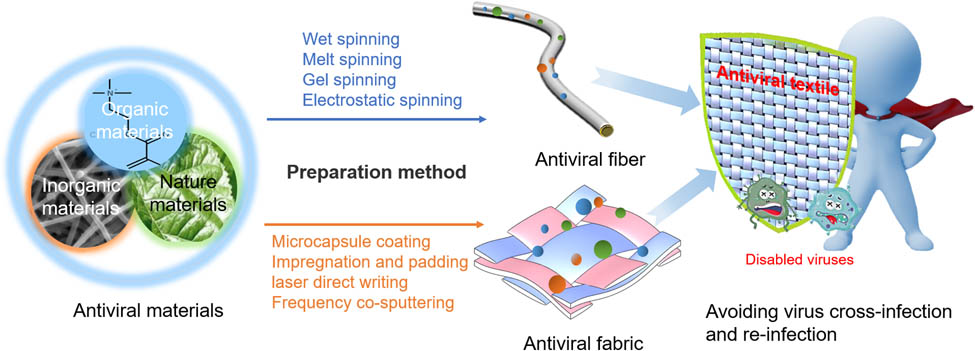
3 Preparation and detection method of antiviral textiles
To date, there are two main methods for the preparation of textiles that acquire antiviral functions. The first method is to mix the antiviral material with the polymer spinning solution and spin it. The second is through the finishing method, in which the antiviral material is added to the fabric finishing agent via impregnation or padding method to produce the fabric antiviral function. Table 3 summarizes the preparation methods of some antiviral textiles.
Preparation schemes and advantages and disadvantages of some antiviral textiles
| Method | Suitable material | Advantage | Disadvantage | References |
|---|---|---|---|---|
| Fabric finishing | ||||
| Microcapsule | Natural antiviral materials (especially Chinese herbal medicine) | Strong washing, resistance, slow-release function | Fabric feel and breathability deteriorate | [94,95] |
| Impregnation and padding | Inorganic organic natural | Simple operation, low cost, universal use | Reduce fabric softness and breathability | [91,96,97,98,99,100] |
| Spinning method | ||||
| Wet spinning | Inorganic and organic materials | Uniform structure | Low addition amount of antiviral materials, low efficiency, and high cost | [101] |
| Good quality | ||||
| Melt spinning | Inorganic organic materials with high heat resistance | Long-lasting antiviral effect | Agglomeration is easy to occur during the spinning process, and antiviral substances are not easily dispersed evenly | [102] |
| Good flexibility | ||||
| Gel spinning | Inorganic organic | Long-lasting antiviral effect, slow-release effect | Low output, low production efficiency | [103] |
| Electrostatic spinning | Inorganic organic | High specific surface area, high porosity, high antiviral activity | Low fiber strength and low production efficiency | [52,104] |
| Others | ||||
| Laser induction, radio frequency co-sputtering | Graphene | Simple operation, large-scale production | Cause damage to the structure of textiles, resulting in a decrease in mechanical properties | [105] |
The most commonly used method to combine organic natural antiviral materials and certain metal nano antiviral materials with textiles involves impregnation and padding, as shown in Figure 8a. Nanoparticles can be integrated on various textile fibers through finishing methods [106]. Joshi and Roy [97] mixed AgNO3 solution with various reducing agents, followed by immersion of fabric in the mixed solution to generate nanoparticles in situ, which was then dried to produce an antiviral fabric. Gabbay et al. [96] found that textiles impregnated with copper oxide solution have a strong inactivation effect on bacteria, fungi, and HIV virus. Borkow et al. [33] impregnated copper oxide solution on polypropylene fiber, which can effectively the inactivate influenza virus. The antiviral material prepared by attaching silver, copper, zinc, and other metals or their ions to zeolite through physical adsorption and ion exchange can also prepare antiviral textiles by finishing. Imai et al. [38] used the structural components of zeolite as raw materials to synthesize (Na12((AlO2)12(SiO2)12)·27H2O) zeolite on cotton fabrics to produce CuZeo textile, which can be utilized in the medical and health fields. MOFs, as a good carrier of functional nanoparticles, can be combined with zinc, silver, and other antiviral materials, followed by impregnation or grafting adhesive finishing on the textile. Li et al. [9] reported that ZIF-8 MOF finishing on textiles has a strong adsorption ability to viruses and bacteria and inactivation ability to bacteria under the light. The finishing processing method is simple to operate, low in cost, and suitable for inorganic, organic, and natural antiviral materials. However, this method has a mass release in the initial stage, with low durability, which affects the comfort of the fabric. As a result, a series of spinning methods, such as wet spinning and melt spinning, have emerged. The spinning method can generate antiviral substances evenly dispersed in the fiber, hence, the antiviral effect of the fabric, high washable resistance and durability, and greatly improve the feel of the fabric.
![Figure 8
Methods of processing antiviral textiles. (a) Antivirus textile soaking and rolling process. (b) Preparation of antiviral textiles by wet spinning. (c) Antiviral textiles prepared by melt spinning. (d) Basic equipment for electrostatic spinning. (e) With CW-LIFT transfer of graphene onto a second polyimide and then pulse-LIFT transfer of graphene onto a temperature-sensitive mask [105].](/document/doi/10.1515/ntrev-2021-0072/asset/graphic/j_ntrev-2021-0072_fig_008.jpg)
Methods of processing antiviral textiles. (a) Antivirus textile soaking and rolling process. (b) Preparation of antiviral textiles by wet spinning. (c) Antiviral textiles prepared by melt spinning. (d) Basic equipment for electrostatic spinning. (e) With CW-LIFT transfer of graphene onto a second polyimide and then pulse-LIFT transfer of graphene onto a temperature-sensitive mask [105].
Wet spinning is a chemical fiber spinning method in which the polymer is dissolved in the solvent. A thin stream is ejected through a nozzle hole to enter a coagulation bath to form fibers. This method can produce fibers with a skin-core structure (Figure 8b). The fiber-forming polymer suitable for wet spinning can be dissolved in a suitable solvent and cannot decompose in the coagulation bath. Kwak et al. [101] used UV-visible light method to prepare Ag NPs and prepared antibacterial Ag NPs doped gelatin fibers by the wet spinning method. Water and fructose can be employed as green solvents and cross-linking agents, allowing different amounts of Ag NPs to be embedded in gelatin fibers, and showed durable and effective antibacterial properties.
The melt spinning method involves the mixing of antiviral materials with various additives, which are uniformly combined with the fiber matrix resin. Finally, spinning under high-temperature conditions. Therefore, the material needs to be resistant to high temperature and has small particle size, good dispersibility, and compatibility. Compared with wet spinning, melt spinning draws more and produces finer fibers. In addition, it can be used to make antiviral yarns from most inorganic materials and some high temperature-resistant organic materials. The spinning device is shown in Figure 8c. Damerchely et al. [107] prepared 20 denier and 70 denier nylon 6/nanometer silver composite filaments via the melt spinning technique. The amount of nanometer silver added can reach 0.5–1 wt%. The yarn has good antibacterial properties and maintains excellent mechanical properties. Many studies have prepared antibacterial Lycell fibers via the addition of a variety of bioactive additives, including silver and chitosan derivatives, to N-methylmorpholine-N-oxide spinning solvent by physical blending. Silver zeolite and MOFs can also be blended and spun to produce antiviral fibers [108].
Gel spinning involves the extrusion of polymer solution or plasticized gel with a high concentration from the fine hole of the spinneret head into a gas medium. The thin stream is cooled, followed by solvent evaporation and polymer solidification to obtain the desired fiber. The equipment is similar to melt spinning. This spinning method can produce yarn with high strength and high modulus. Similar to melt spinning, the antiviral materials used in the method are subjected to high temperatures. Ma et al. [103] proposed a new high-throughput method for mechanically exfoliating graphene (MEG) in natural media. MEG can be well dispersed in the polyvinyl alcohol (PVA) matrix, where a certain amount of PVA and MEG are slowly added to mixed dimethyl sulfoxide (DMSO) solution in a specific ratio, and dispersed uniformly, and then passed through gel spinning and stretched 20 times to obtain PVA/MEG nanocomposite fiber. This nanocomposite fiber has high-strength characteristics and great antimicrobial, low cytotoxicity, and other properties.
However, there are some problems with these three approaches. The antiviral materials for the preparation of the antiviral fiber core layer cannot reach the fiber layer, which reduces the antiviral efficiency compared to the fabric finishing method. Therefore, electrospinning technology, which can prepare nanoscale and highly effective antiviral fibers, has received more attention. The nanofibers prepared by electrospinning also have a slow-release effect, which further enhances the safety and durability of the antiviral fibers. Electrospinning is a special fiber manufacturing process in which a polymer solution or melt is sprayed into a strong electric field to produce polymer filaments with nanoscale diameters (Figure 8d) [109]. Most organic polymers can be mixed with antiviral materials to produce antiviral electrospinning nanofibers through the electrospinning process. López de Dicastillo et al. [57] obtained ZnO nanotubes by atomic layer deposition on electrospinning polyvinyl alcohol nanofibers and then removed the polymer by calcination to obtain antimicrobial nanostructures with high specific surface area. Wang et al. [110] modified polyacrylonitrile (PAN) by nitrile modification reaction and synthesized PAN–Ag+ nanofiber membrane by the electrospinning method, which has high antibacterial activity and stability. Si et al. [52] used PVA-co-PE as a polymer precursor to fabricate a nanofiber web by electrospinning and finally dipped it in a photosensitizer to produce a daylight-driven antiviral nanofiber membrane. Many studies have improved the preparation method of electrospinning technology. Han et al. [111] used coaxial electrospinning technology to prepare nanofibers with high efficiency and a good slow-release effect. Dou et al. [112] introduced a high-speed thermal flow into the electrospinning, which can entangle the fibers and produce nanometer yarns with the tight arrangement. Some studies have also combined electrospinning and electrostatic spraying to spin nanofiber membranes with multilayer structures and functions [113]. Yu et al. [114] used a multi-needle conjugated electrospinning device to blend GO-polyethylenimine-silver composites with a PAN spinning solution to produce nanofiber yarn, which has high efficiency and durability, and enhanced strength compared to traditional electrospinning. Currently, the realization of large-scale production of electrospinning has also been further explored [115].
Laser-induced graphene is a new method for the preparation of graphene, using commercially available precursors (such as polyimide, SPEEK, and Bakelite) to produce functional graphene at a low cost. Zhong et al. [105] designed a double-membrane laser-induced forward transfer method as shown in Figure 8e, which can deposit multiple layers of graphene on a nonwoven mask with a low melting point. The surface of the fabric is superhydrophobic to prevent the infection of saliva soaking carrying the virus. Under the action of light, the temperature of the fabric can reach above 80℃, which can inactivate most viruses. Balagna et al. [116] used radio frequency (RF) co-sputtering technique to deposit silver nanoclusters/silica composite coating on the surface of cotton fabrics. The results demonstrated that the silver nanocluster/silica composite coating is characterized by strong virucidal activity against RSV and influenza virus, and the nanostructured coating is able to reduce influenza virus of about two orders of magnitude, compared to the uncoated filters or the control.
4 Application of antiviral textiles
4.1 Applications in healthcare settings
Healthcare facilities are crowded with patients exhibiting a variety of diseases, in which hazardous bacteria are more common compared to elsewhere. In addition, healthcare workers are at high risk of contracting virus. For example, during Ebola outbreak, a total of 852 healthcare workers were diagnosed, of whom 492 died. Healthcare workers are at 100 times more higher risk than the general population [117]. Therefore, the use of antiviral textiles in healthcare settings is vital. Lazary et al. [118] compared the incidence of healthcare-associated infections in two similar patients in the head injury care ward during two parallel periods A and B. Phase A served as a control group without treatment. In the case of Phase B, all conventional nonantimicrobial linens and employee uniforms were replaced with antimicrobial products impregnated with copper oxide, as shown in Figure 9a. The results showed that compared with Phase A, the patient’s infection index in Phase B decreases, the nosocomial infection index per 1,000 days of hospitalization reduces by 24%, the number of days of fever (>38.5℃) of patients reduces by 47%, and the total number of days of antibiotic administration reduces by 32.8%. Therefore, during Phase B, antibiotics, hospital infection-related treatments, X-rays, disposable supplies, labor, and laundry costs decrease by about 27% (Figure 9b). The filament prepared by the melt spinning of the nano additive and the spinning solution is prepared into anti-SARS masks for use by medical personnel [119].
![Figure 9
(a) Scanning electron microscope images of two representative pillowcases are shown. The white dots are copper oxide particles embedded in polyester fibers. (b) Bacterial loads were found in conventional and copper oxide impregnated sheets. Forty conventional sheets and Forty copper oxide impregnated sheets were swabbed immediately after being used by patients for 6–7 h. Swabs were taken from the area in contact with the patient’s upper back [118].](/document/doi/10.1515/ntrev-2021-0072/asset/graphic/j_ntrev-2021-0072_fig_009.jpg)
(a) Scanning electron microscope images of two representative pillowcases are shown. The white dots are copper oxide particles embedded in polyester fibers. (b) Bacterial loads were found in conventional and copper oxide impregnated sheets. Forty conventional sheets and Forty copper oxide impregnated sheets were swabbed immediately after being used by patients for 6–7 h. Swabs were taken from the area in contact with the patient’s upper back [118].
4.2 Antiviral protective textile
COVID-19 epidemic is affecting more than 210 countries and regions, mainly through respiratory droplets. In high-risk areas, disposable surgical masks are commonly used by patients, doctors, and even the public. Masks can directly block the spread of droplets, and the protection of masks plays an important role in the health of people. In recent years, many researchers have developed antivirus masks. Borkow et al. [33] made an antiviral mask with copper intermediate layer, consisting of the following four layers (Figure 10a): The outer layer A consisted of spunbonded polyamide fabric containing 2.2% weight (w/w) copper particles; the inner layer B consisted of fused polypropylene fabric containing 2% (w/w) copper oxide particles and forms the barrier layer that provides the physical filtration performance of the mask; the inner layer C consisted of ordinary polyester and used to shape the mask; and the outer layer D was the same as layer A, but closer to the wearer’s face when used. Respiratory masks were impregnated in copper oxide, giving them strong anti-influenza biocidal properties without changing their physical barrier properties. Zhong et al. [105] produced a layer of graphene on the surface of a common surgical mask by laser induction that kills virus with electrothermal properties (Figure 10b). Li et al. [9] used MOFilter as an intermediate layer, sandwiched between two layers of nonwoven fabric as a biological sterilization layer. Compared with the control group (N95 masks), it has a strong sterilization performance and did not affect the adsorption (Figure 10c). Tang et al. [120] exploited the strong electrostatic interaction between cationic cotton fibers and anionic photosensitizers to develop durable and reusable sun-induced antibacterial/antiviral cotton fabrics that can be used in face masks and protective clothing (Figure 10d). Virus substitutes (T7 phages) possess a microbiome reduction rate of about 5–6 logs for up to 60 min of sun exposure. Antiviral materials can be added to the adsorption layer in the middle of the mask. The upper and lower layers are hydrophobic layer and hydrophilic layer, respectively. Materials with special wettability can make the mask have better moisture permeability and surface moisture resistance, so as to prepare a mask with both disease resistance toxicity and comfort [121].
![Figure 10
Design of antiviral mask. (a) Copper oxide impregnated test mask composition [33]. (b) Illustration of the compatibility of the dual-mode LIFT for roll-to-roll production of a grapheme-coated mask. The direction of rolling movement is a black arrow, and the direction of the laser movement is a white arrow [105]. (c) Antiviral mask made by adding MOFilter layer to two layers of nonwoven fabric [9]. (d) Schematic illustration of the fabrication of daylight-induced antibacterial and antiviral textiles [120].](/document/doi/10.1515/ntrev-2021-0072/asset/graphic/j_ntrev-2021-0072_fig_010.jpg)
Design of antiviral mask. (a) Copper oxide impregnated test mask composition [33]. (b) Illustration of the compatibility of the dual-mode LIFT for roll-to-roll production of a grapheme-coated mask. The direction of rolling movement is a black arrow, and the direction of the laser movement is a white arrow [105]. (c) Antiviral mask made by adding MOFilter layer to two layers of nonwoven fabric [9]. (d) Schematic illustration of the fabrication of daylight-induced antibacterial and antiviral textiles [120].
In addition to the aforementioned two main applications, there is also a certain risk of infection in places such as public transportation, the catering industry, and public office learning. If seat fabrics, seat belts, interior textiles, tablecloths, carpets, towels, and other textiles have antiviral properties, the risk of cross-infection can be greatly reduced.
5 Prospects for antiviral textiles
So far, most antiviral researches focus on medicinal applications. However, relatively few studies researched antiviral materials, especially textiles. The materials for the preparation of antiviral textiles are mainly inorganic metals such as silver, copper, and their compounds, most of which are combined with textiles through finishing methods. However, the combination of copper and silver in fabrics can easily change the material’s color. Hence, the cost is high, and manufacturing is complex. After finishing the method, antiviral properties only exist on the fiber surface; it is not resistant to washing, initial dissolution is large, and there are safety problems in wearing the material. Hence, it is essential to develop green, safe and durable antivirus textiles.
In terms of materials, polypropylene fiber is the main component of mask melt-blown cloth. Its unique capillary structure provides a high surface area and electrostatic solid adsorption capacity. Therefore, viruses can be effectively intercepted and adsorbed by polypropylene fiber. Graphene has higher surface energy, which makes it stronger than polypropylene melt-blown fabric for electrostatic adsorption. When air passes through, almost all dust and toxic droplets can be electrostatically adsorbed and added to the melt-blown cloth of the mask. Therefore, graphene and other antiviral materials can be mixed through electrospinning to create masks with an excellent blocking effect and excellent antiviral performance. In recent years, Chinese medicine has been used more frequently, especially when it has played a vital role in the new crown pneumonia epidemic. The application of nanotechnology in the research and development of Chinese medicine is one of the essential directions for the development of Chinese medicine. The application of nanotechnology can improve the water solubility of the effective ingredients of traditional Chinese medicine and increase the utilization rate of the medicine. Packaging the practical components of traditional Chinese medicine into nano-carriers can prevent the damage of pH and enzymes and maintain its stability. There are many ways to combine Chinese medicine with textiles, such as Chinese medicine extract, padding, sol, and microcapsule. Nano-processing the active ingredients of traditional Chinese medicine, adding the spinning solution to blending or adding adhesive on the fabric’s surface, can effectively improve the antiviral performance of the fabric. Composite antiviral materials have extensive application prospects. The combination of multiple antiviral materials can enhance spectral properties and antiviral activity. However, currently, only a few studies examine composite antiviral materials, and the research on its mechanism and ratio is still lacking. Therefore, researchers should explore antiviral textiles made of multiple materials.
In terms of technology, spinning to produce antiviral fibers has slow release and durability, which is an important trend in the development of antiviral textiles in the future. The antiviral material can be made into antiviral yarn or fiber through electrostatic spinning, blending spinning, and wet spinning methods, which can significantly improve antiviral fabrics’ safety and washing durability. However, due to the limited number of antiviral substances added in the spinning process, researchers can further explore such materials and determine optimal concentration.
Bio-flexible sensors also play an important role in the epidemic outbreak. However, few such studies focus on sensors, which are mainly used for virus detection and the location of virus-infected persons. Researchers can use textiles as a substrate to create biosensors, combined with 5G wireless communication technology and equipment to control the spread of the epidemic.
Currently, there is an urgent need to develop low-cost, green, safe, high-efficiency, and industrially produced antiviral textiles. It is very necessary to cooperate in textile materials science, microbiology, and pharmacy to learn and develop together, realize industrialized production, and make antiviral textiles play an important role in the fields of medical protection and daily human protection.
-
Funding information: The authors acknowledge the financial support from National Natural Science Foundation of China (No. 52073224), National Key Research and Development Program of China (No. 2019YFA0706801), Innovation Capacity Support Plan of Shaanxi, China (No. 2020PT-043), Scientific and Technology Project for Overseas Students of Shaanxi, China (No. 12), Scientific Research Program Funded by Shaanxi Provincial Education Department, China (No. 20JK0651), and Thousand Talents Program of Shaanxi, China.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
References
[1] Barac A, Poljak M, Ong DSY. Editorial: innovative approaches in diagnosis of emerging/re-emerging infectious diseases. Front Microbiol. 2020;11:619498.10.3389/fmicb.2020.619498Search in Google Scholar PubMed PubMed Central
[2] Olival KJ, Hosseini PR, Zambrana-Torrelio C, Ross N, Bogich TL, Daszak P. Host and viral traits predict zoonotic spillover from mammals. Nature. 2017;546(7660):646–50.10.1038/nature22975Search in Google Scholar PubMed PubMed Central
[3] Metcalf CJE, Lessler J. Opportunities and challenges in modeling emerging infectious diseases. Science. 2017;357(6347):149–52.10.1126/science.aam8335Search in Google Scholar PubMed PubMed Central
[4] Bibby K, Casson LW, Stachler E, Haas CN. Ebola virus persistence in the environment: state of the knowledge and research needs. Env Sci Tech Let. 2014;2(1):2–6.10.1021/ez5003715Search in Google Scholar
[5] Kampf G. How long can nosocomial pathogens survive on textiles? a systematic review. GMS Hyg Infect Contr. 2020;15:Doc10.Search in Google Scholar
[6] Beam EL, Schwedhelm S, Boulter K, Kratochvil C, Lowe J, Hewlett A, et al. Personal protective equipment processes and rationale for the Nebraska biocontainment unit during the 2014 activations for Ebola virus disease. AM J Infect Control. 2016;44(3):340–2.10.1016/j.ajic.2015.09.031Search in Google Scholar PubMed
[7] Vázquez-Muñoz R, Borrego B, Juárez-Moreno KO, García-García M, Mota-Morales J, Bogdanchikova N, et al. Toxicity of silver nanoparticles in biological systems: does the complexity of biological systems matter? Toxicol Lett. 2016;259:S190–1.10.1016/j.toxlet.2016.07.455Search in Google Scholar
[8] Alam Q, Schollbach K, Rijnders M, van Hoek C, van der Laan S, Brouwers HJH. The immobilization of potentially toxic elements due to incineration and weathering of bottom ash fines. J Hazard Mater. 2019;379:120798.10.1016/j.jhazmat.2019.120798Search in Google Scholar PubMed
[9] Li P, Li J, Feng X, Li J, Hao Y, Zhang J, et al. Metal-organic frameworks with photocatalytic bactericidal activity for integrated air cleaning. Nat Commun. 2019;10(1):2177.10.1038/s41467-019-10218-9Search in Google Scholar PubMed PubMed Central
[10] Li T, Peng T. Traditional Chinese herbal medicine as a source of molecules with antiviral activity. Antivir Res. 2013;97(1):1–9.10.1016/j.antiviral.2012.10.006Search in Google Scholar PubMed PubMed Central
[11] Galdiero S, Falanga A, Vitiello M, Cantisani M, Marra V, Galdiero M. Silver nanoparticles as potential antiviral agents. Molecules. 2011;16(10):8894–918.10.3390/molecules16108894Search in Google Scholar PubMed PubMed Central
[12] Chen L, Liang J. An overview of functional nanoparticles as novel emerging antiviral therapeutic agents. Mater Sci Eng C. 2020;112:110924.10.1016/j.msec.2020.110924Search in Google Scholar PubMed PubMed Central
[13] Di Gianvincenzo P, Marradi M, Martínez-Ávila OM, Bedoya LM, Alcamí J, Penadés S. Gold nanoparticles capped with sulfate-ended ligands as anti-HIV agents. Bioorg Med Chem Lett. 2010;20(9):2718–21.10.1016/j.bmcl.2010.03.079Search in Google Scholar PubMed
[14] Papp I, Sieben C, Ludwig K, Roskamp M, Böttcher C, Schlecht S, et al. Inhibition of influenza virus infection by multivalent sialic-acid-functionalized gold nanoparticles. Small. 2010;6(24):2900–6.10.1002/smll.201001349Search in Google Scholar PubMed
[15] Halder A, Das S, Ojha D, Chattopadhyay D, Mukherjee A. Highly monodispersed gold nanoparticles synthesis and inhibition of herpes simplex virus infections. Mater Sci Eng C. 2018;89:413–21.10.1016/j.msec.2018.04.005Search in Google Scholar PubMed
[16] Dizaj SM, Lotfipour F, Barzegar-Jalali M, Zarrintan MH, Adibkia K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater Sci Eng C. 2014;44:278–84.10.1016/j.msec.2014.08.031Search in Google Scholar PubMed
[17] Lara HH, Garza-Treviño EN, Ixtepan-Turrent L, Singh DK. Silver nanoparticles are broad-spectrum bactericidal and virucidal compounds. J Nanobiotechnol. 2011;9(1):30.10.1186/1477-3155-9-30Search in Google Scholar PubMed PubMed Central
[18] Rogers JV, Parkinson CV, Choi YW, Speshock JL, Hussain SM. A preliminary assessment of silver nanoparticle inhibition of monkeypox virus plaque formation. Nanoscale Res Lett. 2008;3(4):129–33.10.1007/s11671-008-9128-2Search in Google Scholar
[19] Bogdanchikova N, Muñoz RV, Saquero AH, Jasso AP, Uzcanga GA, Díaz PLP, et al. Silver nanoparticles composition for treatment of distemper in dogs. Int J Nanotechnol. 2016;13(1–3):227.10.1504/IJNT.2016.074536Search in Google Scholar
[20] Lu L, Sun RW, Chen R, Hui CK, Ho CM, Luk JM, et al. Silver nanoparticles inhibit hepatitis B virus replication. Antivir Ther. 2008;13(2):253–62.10.1177/135965350801300210Search in Google Scholar
[21] Rai M, Deshmukh SD, Ingle AP, Gupta IR, Galdiero M, Galdiero S. Metal nanoparticles: the protective nanoshield against virus infection. Crit Rev Microbiol. 2016;42(1):46–56.10.3109/1040841X.2013.879849Search in Google Scholar PubMed
[22] Elechiguerra JL, Burt JL, Morones JR, Camacho-Bragado A, Gao X, Lara HH, et al. Interaction of silver nanoparticles with HIV-1. J Nanobiotechnology. 2005;3(1):6.10.1186/1477-3155-3-6Search in Google Scholar PubMed PubMed Central
[23] Trefry JC, Wooley DP. Rapid assessment of antiviral activity and cytotoxicity of silver nanoparticles using a novel application of the tetrazolium-based colorimetric assay. J Virol Methods. 2012;183(1):19–24.10.1016/j.jviromet.2012.03.014Search in Google Scholar PubMed
[24] You J, Zhang Y, Hu Z. Bacteria and bacteriophage inactivation by silver and zinc oxide nanoparticles. Colloid Surf B. 2011;85(2):161–7.10.1016/j.colsurfb.2011.02.023Search in Google Scholar PubMed
[25] Vazquez-Muñoz R, Borrego B, Juárez-Moreno K, García-García M, Mota Morales JD, Bogdanchikova N, et al. Toxicity of silver nanoparticles in biological systems: does the complexity of biological systems matter? Toxicol Lett. 2017;276:11–20.10.1016/j.toxlet.2017.05.007Search in Google Scholar PubMed
[26] Xiang DX, Chen Q, Pang L, Zheng CL. Inhibitory effects of silver nanoparticles on H1N1 influenza A virus in vitro. J Virol Methods. 2011;178(1–2):137–42.10.1016/j.jviromet.2011.09.003Search in Google Scholar PubMed
[27] Rai M, Birla S, Ingle AP, Gupta I, Gade A, Abd-Elsalam K, et al. Nanosilver: an inorganic nanoparticle with myriad potential applications. Nanotechnol Rev. 2014;3(3):281–309.10.1515/ntrev-2014-0001Search in Google Scholar
[28] Castro-Mayorga JL, Randazzo W, Fabra MJ, Lagaron JM, Aznar R, Sánchez G. Antiviral properties of silver nanoparticles against norovirus surrogates and their efficacy in coated polyhydroxyalkanoates systems. LWT-Food Sci Technol. 2017;79:503–10.10.1016/j.lwt.2017.01.065Search in Google Scholar
[29] Borkow G, Sidwell RW, Smee DF, Barnard DL, Morrey JD, Lara-Villegas HH, et al. Neutralizing viruses in suspensions by copper oxide-based filters. Antimicrob Agents Chemother. 2007;51(7):2605–7.10.1128/AAC.00125-07Search in Google Scholar PubMed PubMed Central
[30] Borkow G. Using copper to fight microorganisms. Curr Chem Biol. 2012;6(2):93–103.10.2174/187231312801254723Search in Google Scholar
[31] Borkow G, Gabbay J. Putting copper into action: copper-impregnated products with potent biocidal activities. Faseb J. 2004;18(14):1728–30.10.1096/fj.04-2029fjeSearch in Google Scholar PubMed
[32] Borkow G, Lara HH, Covington CY, Nyamathi A, Gabbay J. Deactivation of human immunodeficiency virus type 1 in medium by copper oxide-containing filters. Antimicrob Agents Chemother. 2008;52(2):518–25.10.1128/AAC.00899-07Search in Google Scholar PubMed PubMed Central
[33] Borkow G, Zhou SS, Page T, Gabbay J. A novel anti-influenza copper oxide containing respiratory face mask. PLoS One. 2010;5(6):e11295.10.1371/journal.pone.0011295Search in Google Scholar PubMed PubMed Central
[34] Horie M, Ogawa H, Yoshida Y, Yamada K, Hara A, Ozawa K, et al. Inactivation and morphological changes of avian influenza virus by copper ions. Arch Virol. 2008;153(8):1467–72.10.1007/s00705-008-0154-2Search in Google Scholar PubMed
[35] Noyce JO, Michels H, Keevil CW. Inactivation of influenza A virus on copper versus stainless steel surfaces. Appl Env Microb. 2007;73(8):2748–50.10.1128/AEM.01139-06Search in Google Scholar PubMed PubMed Central
[36] Fujimori Y, Sato T, Hayata T, Nagao T, Nakayama M, Nakayama T, et al. Novel antiviral characteristics of nanosized copper(i) iodide particles showing inactivation activity against 2009 pandemic H1N1 influenza virus. Appl Env Microb. 2012;78(4):951–5.10.1128/AEM.06284-11Search in Google Scholar PubMed PubMed Central
[37] Shionoiri N, Sato T, Fujimori Y, Nakayama T, Nemoto M, Matsunaga T, et al. Investigation of the antiviral properties of copper iodide nanoparticles against feline calicivirus. J Biosci Bioeng. 2012;113(5):580–6.10.1016/j.jbiosc.2011.12.006Search in Google Scholar PubMed
[38] Imai K, Ogawa H, Bui VN, Inoue H, Fukuda J, Ohba M, et al. Inactivation of high and low pathogenic avian influenza virus H5 subtypes by copper ions incorporated in zeolite-textile materials. Antivir Res. 2012;93(2):225–33.10.1016/j.antiviral.2011.11.017Search in Google Scholar PubMed
[39] Vincent M, Duval RE, Hartemann P, Engels-Deutsch M. Contact killing and antimicrobial properties of copper. J Appl Microbiol. 2018;124(5):1032–46.10.1111/jam.13681Search in Google Scholar PubMed
[40] Friedman SH, DeCamp DL, Sijbesma RP, Srdanov G, Wudl F, Kenyon GL. Inhibition of the HIV-1 protease by fullerene derivatives: model building studies and experimental verification. J Am Chem Soc. 1993;115(15):6506–9.10.1021/ja00068a005Search in Google Scholar
[41] Muñoz A, Sigwalt D, Illescas BM, Luczkowiak J, Rodríguez-Pérez L, Nierengarten I, et al. Synthesis of giant globular multivalent glycofullerenes as potent inhibitors in a model of Ebola virus infection. Nat Chem. 2015;8(1):50–7.10.1038/nchem.2387Search in Google Scholar PubMed
[42] Stanford MG, Li JT, Chen Y, McHugh EA, Liopo A, Xiao H, et al. Self-sterilizing laser-induced graphene bacterial air filter. ACS Nano. 2019;13(10):11912–20.10.1021/acsnano.9b05983Search in Google Scholar PubMed
[43] Deokar AR, Nagvenkar AP, Kalt I, Shani L, Yeshurun Y, Gedanken A, et al. Graphene-based “hot plate” for the capture and destruction of the herpes simplex virus type 1. Bioconjugate Chem. 2017;28(4):1115–22.10.1021/acs.bioconjchem.7b00030Search in Google Scholar PubMed
[44] Darnell MER, Subbarao K, Feinstone SM, Taylor DR. Inactivation of the coronavirus that induces severe acute respiratory syndrome, SARS-CoV. J Virol Methods. 2004;121(1):85–91.10.1016/j.jviromet.2004.06.006Search in Google Scholar PubMed PubMed Central
[45] Song Z, Wang X, Zhu G, Nian Q, Zhou H, Yang D, et al. Virus capture and destruction by label-free graphene oxide for detection and disinfection applications. Small. 2015;11(9–10):1171–6.10.1002/smll.201401706Search in Google Scholar PubMed
[46] Karahan HE, Wang Y, Li W, Liu F, Wang L, Sui X, et al. Antimicrobial graphene materials: the interplay of complex materials characteristics and competing mechanisms. Biomater Sci. 2018;6(4):766–73.10.1039/C7BM00987ASearch in Google Scholar
[47] Ye S, Shao K, Li Z, Guo N, Zuo Y, Li Q, et al. Antiviral activity of graphene oxide: how sharp edged structure and charge matter. ACS Appl Mater Interfaces. 2015;7(38):21571–9.10.1021/acsami.5b06876Search in Google Scholar PubMed
[48] Miyako E, Nagata H, Hirano K, Sakamoto K, Makita Y, Nakayama K, et al. Photoinduced antiviral carbon nanohorns. Nanotechnology. 2008;19(7):075106.10.1088/0957-4484/19/7/075106Search in Google Scholar PubMed
[49] Kim JW, Kim S, Jang Y-H, Lim K-I, Lee WH. Attomolar detection of virus by liquid coplanar-gate graphene transistor on plastic. Nanotechnology. 2019;30(34):345502.10.1088/1361-6528/ab0f52Search in Google Scholar PubMed
[50] Yang B, Kong J, Fang X. Bandage-like wearable flexible microfluidic recombinase polymerase amplification sensor for the rapid visual detection of nucleic acids. Talanta. 2019;204:685–92.10.1016/j.talanta.2019.06.031Search in Google Scholar PubMed
[51] Kinnamon DS, Krishnan S, Brosler S, Sun E, Prasad S. Screen printed graphene oxide textile biosensor for applications in inexpensive and wearable point-of-exposure detection of influenza for at-risk populations. J Electrochem Soc. 2018;165(8):B3084–90.10.1149/2.0131808jesSearch in Google Scholar
[52] Si Y, Zhang Z, Wu W, Fu Q, Huang K, Nitin N, et al. Daylight-driven rechargeable antibacterial and antiviral nanofibrous membranes for bioprotective applications. Sci Adv. 2018;4(3):eaar5931.10.1126/sciadv.aar5931Search in Google Scholar PubMed PubMed Central
[53] Zan L, Fa W, Peng T, Gong Z-K. Photocatalysis effect of nanometer TiO2 and TiO2-coated ceramic plate on Hepatitis B virus. J Photochem Photobiol B. 2007;86(2):165–9.10.1016/j.jphotobiol.2006.09.002Search in Google Scholar PubMed
[54] Mazurkova NA, Spitsyna YE, Shikina NV, Ismagilov ZR, Zagrebel’nyi SN, Ryabchikova EI. Interaction of titanium dioxide nanoparticles with influenza virus. Nanotechnol Russ. 2010;5(5–6):417–20.10.1134/S1995078010050174Search in Google Scholar
[55] Hajkova P, Spatenka P, Horsky J, Horska I, Kolouch A. Photocatalytic effect of TiO2 films on viruses and bacteria. Plasma Process Polym. 2007;4(S1):S397–401.10.1002/ppap.200731007Search in Google Scholar
[56] Siddiqi KS, ur Rahman A, Tajuddin, Husen A. Properties of zinc oxide nanoparticles and their activity against microbes. Nanoscale Res Lett. 2018;13(1):141.10.1186/s11671-018-2532-3Search in Google Scholar PubMed PubMed Central
[57] López de Dicastillo C, Patiño Vidal C, Falcó I, Sánchez G, Márquez P, Escrig J. Antimicrobial bilayer nanocomposites based on the incorporation of as-synthetized hollow zinc oxide nanotubes. Nanomaterials. 2020;10(3):503.10.3390/nano10030503Search in Google Scholar PubMed PubMed Central
[58] Ghaffari H, Tavakoli A, Moradi A, Tabarraei A, Bokharaei-Salim F, Zahmatkeshan M, et al. Inhibition of H1N1 influenza virus infection by zinc oxide nanoparticles: another emerging application of nanomedicine. J Biomed Sci. 2019;26(1):70.10.1186/s12929-019-0563-4Search in Google Scholar PubMed PubMed Central
[59] Kataev VE, Strobykina IY, Zakharova LY. Quaternary ammonium derivatives of natural terpenoids. Synth Prop Russ Chem Bull. 2015;63(9):1884–900.10.1007/s11172-014-0680-xSearch in Google Scholar
[60] Hsu BB, Yinn Wong S, Hammond PT, Chen J, Klibanov AM. Mechanism of inactivation of influenza viruses by immobilized hydrophobic polycations. Proc Natl Acad Sci U S A. 2011;108(1):61–6.10.1073/pnas.1017012108Search in Google Scholar PubMed PubMed Central
[61] Xue Y, Xiao H. Characterization and antipathogenic evaluation of a novel quaternary phosphonium tripolyacrylamide and elucidation of the inactivation mechanisms. J Biomed Mater Res A. 2016;104(3):747–57.10.1002/jbm.a.35613Search in Google Scholar PubMed
[62] Smith AE. How viruses enter animal cells. Science. 2004;304(5668):237–42.10.1126/science.1094823Search in Google Scholar PubMed
[63] Ashkenazi A, Shai Y. Insights into the mechanism of HIV-1 envelope induced membrane fusion as revealed by its inhibitory peptides. Eur Biophys J. 2011;40(4):349–57.10.1007/s00249-010-0666-zSearch in Google Scholar PubMed
[64] Nada A, Al-Moghazy M, Soliman AAF, Rashwan GMT, Eldawy THA, Hassan AAE, et al. Pyrazole-based compounds in chitosan liposomal emulsion for antimicrobial cotton fabrics. Int J Biol Macromal. 2018;107:585–94.10.1016/j.ijbiomac.2017.09.031Search in Google Scholar PubMed
[65] El-Sabbagh OI, Baraka MM, Ibrahim SM, Pannecouque C, Andrei G, Snoeck R, et al. Synthesis and antiviral activity of new pyrazole and thiazole derivatives. Eur J Med Chem. 2009;44(9):3746–53.10.1016/j.ejmech.2009.03.038Search in Google Scholar PubMed
[66] Kim HO, Yeom M, Kim J, Kukreja A, Na W, Choi J, et al. Reactive oxygen species-regulating polymersome as an antiviral agent against influenza virus. Small. 2017;13(32):1700818.10.1002/smll.201700818Search in Google Scholar PubMed
[67] Park D, Larson AM, Klibanov AM, Wang Y. Antiviral and antibacterial polyurethanes of various modalities. Appl Biochem Biotechnol. 2013;169(4):1134–46.10.1007/s12010-012-9999-7Search in Google Scholar PubMed
[68] Rzaev ZM, Sadykh-Zade SI. Radical copolymerization of maleic anhydride with organotin acrylates. J Polym Sci Part C: Polym Symp. 2007;42(2):541–52.10.1002/polc.5070420205Search in Google Scholar
[69] Roner MR, Carraher Jr CE, Shahi K, Barot G. Antiviral activity of metal-containing polymers – organotin and cisplatin-like polymers. Materials. 2011;4(6):991–1012.10.3390/ma4060991Search in Google Scholar PubMed PubMed Central
[70] Wang Y, Canady TD, Zhou Z, Tang Y, Price DN, Bear DG, et al. Cationic phenylene ethynylene polymers and oligomers exhibit efficient antiviral activity. ACS Appl Mater Interfaces. 2011;3(7):2209–14.10.1021/am200575ySearch in Google Scholar PubMed
[71] Tong T, Wu YQ, Ni WJ, Shen AZ, Liu S. The potential insights of Traditional Chinese Medicine on treatment of COVID-19. Chin Med-UK. 2020;15:51.10.1186/s13020-020-00326-wSearch in Google Scholar PubMed PubMed Central
[72] Yuan B, Yang R, Ma Y, Zhou S, Zhang X, Liu Y. A systematic review of the active saikosaponins and extracts isolated from Radix Bupleuri and their applications. Pharm Biol. 2016;55(1):620–35.10.1080/13880209.2016.1262433Search in Google Scholar
[73] Cheng PW, Ng LT, Chiang LC, Lin CC. Antiviral effects of Saikosaponins on human coronavirus 229e in vitro. Clin Clin Exp Pharmacol P. 2006;33(7):612–6.10.1111/j.1440-1681.2006.04415.xSearch in Google Scholar
[74] Dong Z, Lu X, Tong X, Dong Y, Tang L, Liu M. Forsythiae fructus: a review on its phytochemistry, quality control, pharmacology and pharmacokinetics. Molecules. 2017;22(9):1466.10.3390/molecules22091466Search in Google Scholar
[75] Zhou W, Yin A, Shan J, Wang S, Cai B, Di L. Study on the rationality for antiviral activity of flos lonicerae japonicae-fructus forsythiae herb couple preparations improved by chito-oligosaccharide via integral pharmacokinetics. Molecules. 2017;22(4):654.10.3390/molecules22040654Search in Google Scholar
[76] Ma SC, Du J, But PP, Deng XL, Zhang YW, Ooi VE, et al. Antiviral Chinese medicinal herbs against respiratory syncytial virus. J Ethnopharmacol. 2002;79(2):205–11.10.1016/S0378-8741(01)00389-0Search in Google Scholar
[77] Shang X, He X, He X, Li M, Zhang R, Fan P, et al. The genus Scutellaria an ethnopharmacological and phytochemical review. J Ethnopharmacol. 2010;128(2):279–313.10.1016/j.jep.2010.01.006Search in Google Scholar PubMed
[78] Nie LX, Wu YL, Dai Z, Ma SC. Antiviral activity of Isatidis Radix derived glucosinolate isomers and their breakdown products against influenza A in vitro/ovo and mechanism of action. J Ethnopharmacol. 2020;251:112550.10.1016/j.jep.2020.112550Search in Google Scholar PubMed PubMed Central
[79] Wang T, Wang X, Zhuo Y, Si C, Yang L, Meng L, et al. Antiviral activity of a polysaccharide from Radix Isatidis (Isatis indigotica Fortune) against hepatitis B virus (HBV) in vitro via activation of JAK/STAT signal pathway. J Ethnopharmacol. 2020;257:112782.10.1016/j.jep.2020.112782Search in Google Scholar PubMed
[80] Sforcin JM, Bankova V. Propolis: is there a potential for the development of new drugs? J Ethnopharmacol. 2011;133(2):253–60.10.1016/j.jep.2010.10.032Search in Google Scholar PubMed
[81] Tait S, Salvati AL, Desideri N, Fiore L. Antiviral activity of substituted homoisoflavonoids on enteroviruses. Antivir Res. 2006;72(3):252–5.10.1016/j.antiviral.2006.07.003Search in Google Scholar PubMed
[82] Gekker G, Hu S, Spivak M, Lokensgard JR, Peterson PK. Anti-HIV-1 activity of propolis in CD4+ lymphocyte and microglial cell cultures. J Ethnopharmacol. 2005;102(2):158–63.10.1016/j.jep.2005.05.045Search in Google Scholar PubMed
[83] Huleihel M, Ishano V. Effect of propolis extract on malignant cell transformation by moloney murine sarcoma virus. Arch Virol. 2001;146(8):1517–26.10.1007/s007050170075Search in Google Scholar PubMed
[84] Yildiz H, Karatas N. Microbial exopolysaccharides: resources and bioactive properties. Process Biochem. 2018;72:41–6.10.1016/j.procbio.2018.06.009Search in Google Scholar
[85] Arena A, Maugeri TL, Pavone B, Iannello D, Gugliandolo C, Bisignano G. Antiviral and immunoregulatory effect of a novel exopolysaccharide from a marine thermotolerant Bacillus licheniformis. Int Immunopharmacol. 2006;6(1):8–13.10.1016/j.intimp.2005.07.004Search in Google Scholar PubMed
[86] Villena J, Vizoso-Pinto MG, Kitazawa H. Intestinal innate antiviral immunity and immunobiotics: beneficial effects against rotavirus infection. Front Immunol. 2016;7:563.10.3389/fimmu.2016.00563Search in Google Scholar PubMed PubMed Central
[87] Baba M, Snoeck R, Pauwels R, de Clercq E. Sulfated polysaccharides are potent and selective inhibitors of various enveloped viruses, including herpes simplex virus, cytomegalovirus, vesicular stomatitis virus, and human immunodeficiency virus. Antimicrob Agents Ch. 1988;32(11):1742–5.10.1128/AAC.32.11.1742Search in Google Scholar PubMed PubMed Central
[88] Gugliandolo C, Spanò A, Lentini V, Arena A, Maugeri TL. Antiviral and immunomodulatory effects of a novel bacterial exopolysaccharide of shallow marine vent origin. J Appl Microbiol. 2014;116(4):1028–34.10.1111/jam.12422Search in Google Scholar PubMed
[89] Kanmani P, Albarracin L, Kobayashi H, Iida H, Komatsu R, Humayun Kober AKM, et al. Exopolysaccharides from Lactobacillus delbrueckii OLL1073R-1 modulate innate antiviral immune response in porcine intestinal epithelial cells. Mol Immunol. 2018;93:253–65.10.1016/j.molimm.2017.07.009Search in Google Scholar PubMed
[90] Chen YN, Hsueh YH, Hsieh CT, Tzou DY, Chang PL. Antiviral activity of graphene-silver nanocomposites against non-enveloped and enveloped viruses. Int J Env Res Public Health. 2016;13(4):430.10.3390/ijerph13040430Search in Google Scholar PubMed PubMed Central
[91] Iyigundogdu ZU, Demir O, Asutay AB, Sahin F. Developing novel antimicrobial and antiviral textile products. Appl Biochem Biotechnol. 2017;181(3):1155–66.10.1007/s12010-016-2275-5Search in Google Scholar PubMed PubMed Central
[92] Mori Y, Ono T, Miyahira Y, Nguyen VQ, Matsui T, Ishihara M. Antiviral activity of silver nanoparticle/chitosan composites against H1N1 influenza A virus. Nanoscale Res Lett. 2013;8(1):93.10.1186/1556-276X-8-93Search in Google Scholar PubMed PubMed Central
[93] Li S, Zhu T, Huang J, Guo Q, Chen G, Lai Y. Durable antibacterial and UV-protective Ag/TiO2@ fabrics for sustainable biomedical application. Int J Nanomed. 2017;12:2593–606.10.2147/IJN.S132035Search in Google Scholar PubMed PubMed Central
[94] Hui PC, Wang WY, Kan CW, Ng FS, Wat E, Zhang VX, et al. Microencapsulation of traditional Chinese herbs-pentaherbs extracts and potential application in healthcare textiles. Colloids Surf B. 2013;111:156–61.10.1016/j.colsurfb.2013.05.036Search in Google Scholar PubMed
[95] Wang R, Li M, Liu X, Sun Y. Preparation of composite fabric loaded with mi-croencapsulated plant extracts and its inhibitory effect on lipase. Pigm Resin Technol. 2019;48(3):202–9.10.1108/PRT-12-2017-0104Search in Google Scholar
[96] Gabbay J, Borkow G, Mishal J, Magen E, Zatcoff R, Shemer-Avni Y. Copper oxide impregnated textiles with potent biocidal activities. J Ind Text. 2016;35(4):323–35.10.1177/1528083706060785Search in Google Scholar
[97] Joshi M, Roy A. Antimicrobial textiles based on metal and metal oxide nano-particles. Nanomaterials in the Wet Processing of Textiles. John Wiley & Sons Ltd; 2018. p. 71–111.10.1002/9781119459804.ch2Search in Google Scholar
[98] Kiwi J, Pulgarin C. Self-cleaning textiles modified by Tio2 and bactericide textiles modified by Ag and Cu. Self-Cleaning Materials and Surfaces. John Wiley & Sons Ltd; 2013. p. 203–27.10.1002/9781118652336.ch7Search in Google Scholar
[99] Balagna C, Irfan M, Perero S, Miola M, Maina G, Santella D, et al. Characterization of antibacterial silver nanocluster/silica composite coating on high performance Kevlar® textile. Surf Coat Technol. 2017;321:438–47.10.1016/j.surfcoat.2017.05.009Search in Google Scholar
[100] Foksowicz-Flaczyk J, Walentowska J, Przybylak M, Maciejewski H. Multifunctional durable properties of textile materials modified by biocidal agents in the sol-gel process. Surf Coat Technol. 2016;304:160–6.10.1016/j.surfcoat.2016.06.062Search in Google Scholar
[101] Kwak HW, Kim JE, Lee KH. Green fabrication of antibacterial gelatin fiber for biomedical application. React Funct Polym. 2019;136:86–94.10.1016/j.reactfunctpolym.2018.12.020Search in Google Scholar
[102] Saini S, Belgacem N, Mendes J, Elegir G, Bras J. Contact antimicrobial surface obtained by chemical grafting of microfibrillated cellulose in aqueous solution limiting antibiotic release. ACS Appl Mater Interfaces. 2015;7(32):18076–85.10.1021/acsami.5b04938Search in Google Scholar PubMed
[103] Ma Y, Bai D, Hu X, Ren N, Gao W, Chen S, et al. Robust and antibacterial polymer/mechanically exfoliated graphene nanocomposite fibers for biomedical applications. ACS Appl Mater Interfaces. 2018;10(3):3002–10.10.1021/acsami.7b17835Search in Google Scholar PubMed
[104] Ashkarran AA, Aghigh SM, Kavianipour M, Farahani NJ. Visible light photo-and bioactivity of Ag/TiO2 nanocomposite with various silver contents. Curr Appl Phys. 2011;11(4):1048–55.10.1016/j.cap.2011.01.042Search in Google Scholar
[105] Zhong H, Zhu Z, Lin J, Cheung CF, Lu VL, Yan F, et al. Reusable and recyclable graphene masks with outstanding superhydrophobic and photothermal performances. ACS Nano. 2020;14(5):6213–21.10.1021/acsnano.0c02250Search in Google Scholar PubMed
[106] Sawhney PS, Singh K, Condon B, Sachinvala N, Hui D. Scope of nanotechnology in modern textiles. World J Eng. 2010;7(1):1–4.Search in Google Scholar
[107] Damerchely R, Mohammad Esmail Y, Rashidi A-S, Khajavi R. Morphology and mechanical properties of antibacterial nylon 6/nano-silver nano-composite multifilament yarns. Text Res J. 2011;81(16):1694–701.10.1177/0040517511410104Search in Google Scholar
[108] Kalaj M, Denny MS Jr, Bentz KC, Palomba JM, Cohen SM. Nylon-MOF composites through postsynthetic polymerization. Angew Chem Int Ed Engl. 2019;58(8):2336–40.10.1002/anie.201812655Search in Google Scholar PubMed
[109] Liu J, Lai H, Xiong Z, Chen B, Chen T. Review: applications, effects and the prospects for electrospun nanofibrous mats in membrane separation. J Mater Sci. 2019;55(3):893–924.10.1007/s10853-019-04012-7Search in Google Scholar
[110] Wang WX, Liu Y, Wang YX, Chen H, Bai LJ. A novel and convenient preparation of antibacterial polyacrylonitrile nanofibers via post-modification using nitrile click chemistry and electrospinning. Chem Pap. 2017;72(1):191–200.10.1007/s11696-017-0270-0Search in Google Scholar
[111] Han L, Ma Y, Dou H, Fan W. Dual-functional SFP/PAN based nano drug release system for treatment and nutrients. Text Res J Forthcom. 2021;8:579236.10.1177/0040517520988094Search in Google Scholar
[112] Dou H, Yao S-W, Meng X, Fan W, Li Y-Y, Liu H-Y. Effect of air-flow parameters on the morphology of nanofibrous yarns by blown bubble-spinning. Therm Sci. 2020;24(4):2637–43.10.2298/TSCI2004637DSearch in Google Scholar
[113] Shi J, Wu T, Teng K, Wang W, Shan M, Xu Z, et al. Simultaneous electrospinning and spraying toward branch-like nanofibrous membranes functionalised with carboxylated MWCNTs for dye removal. Mater Lett. 2016;166:26–9.10.1016/j.matlet.2015.12.024Search in Google Scholar
[114] Yu W, Li X, He J, Chen Y, Qi L, Yuan P, et al. Graphene oxide-silver nanocomposites embedded nanofiber core-spun yarns for durable antibacterial textiles. J Colloid Interface Sci. 2021;584:164–73.10.1016/j.jcis.2020.09.092Search in Google Scholar PubMed
[115] Wei L, Liu C, Mao X, Dong J, Fan W, Zhi C, et al. Multiple-jet needleless electrospinning approach via a linear flume spinneret. Polym (Basel). 2019;11(12):2052.10.3390/polym11122052Search in Google Scholar PubMed PubMed Central
[116] Balagna C, Francese R, Perero S, Lembo D, Ferraris M. Nanostructured composite coating endowed with antiviral activity against human respiratory viruses deposited on fibre-based air filters. Surf Coat Technol. 2021;409:126873.10.1016/j.surfcoat.2021.126873Search in Google Scholar PubMed PubMed Central
[117] Olu O, Kargbo B, Kamara S, Wurie AH, Amone J, Ganda L, et al. Epidemiology of Ebola virus disease transmission among health care workers in Sierra Leone, May to December 2014: a retrospective descriptive study. BMC Infect Dis. 2015;15(1):416.10.1186/s12879-015-1166-7Search in Google Scholar PubMed PubMed Central
[118] Lazary A, Weinberg I, Vatine JJ, Jefidoff A, Bardenstein R, Borkow G, et al. Reduction of healthcare-associated infections in a long-term care brain injury ward by replacing regular linens with biocidal copper oxide impregnated linens. Int J Infect Dis. 2014;24:23–9.10.1016/j.ijid.2014.01.022Search in Google Scholar PubMed
[119] Sawhney APS, Condon B, Singh KV, Pang SS, Li G, Hui D. Modern applications of nanotechnology in textiles. Text Res J. 2008;78(8):731–9.10.1177/0040517508091066Search in Google Scholar
[120] Tang P, Zhang Z, El-Moghazy AY, Wisuthiphaet N, Nitin N, Sun G. Daylight-induced antibacterial and antiviral cotton cloth for offensive personal protection. ACS Appl Mater Interfaces. 2020;12(44):49442–51.10.1021/acsami.0c15540Search in Google Scholar PubMed
[121] Li S, Huang J, Chen Z, Chen G, Lai Y. A review on special wettability textiles: theoretical models, fabrication technologies and multifunctional applications. J Mater Chem A. 2017;5(1):31–55.10.1039/C6TA07984ASearch in Google Scholar
© 2021 Yao Zhang et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Improved impedance matching by multi-componential metal-hybridized rGO toward high performance of microwave absorption
- Pure-silk fibroin hydrogel with stable aligned micropattern toward peripheral nerve regeneration
- Effective ion pathways and 3D conductive carbon networks in bentonite host enable stable and high-rate lithium–sulfur batteries
- Fabrication and characterization of 3D-printed gellan gum/starch composite scaffold for Schwann cells growth
- Synergistic strengthening mechanism of copper matrix composite reinforced with nano-Al2O3 particles and micro-SiC whiskers
- Deformation mechanisms and plasticity of ultrafine-grained Al under complex stress state revealed by digital image correlation technique
- On the deformation-induced grain rotations in gradient nano-grained copper based on molecular dynamics simulations
- Removal of sulfate from aqueous solution using Mg–Al nano-layered double hydroxides synthesized under different dual solvent systems
- Microwave-assisted sol–gel synthesis of TiO2-mixed metal oxide nanocatalyst for degradation of organic pollutant
- Electrophoretic deposition of graphene on basalt fiber for composite applications
- Polyphenylene sulfide-coated wrench composites by nanopinning effect
- Thermal conductivity and thermoelectric properties in 3D macroscopic pure carbon nanotube materials
- An effective thermal conductivity and thermomechanical homogenization scheme for a multiscale Nb3Sn filaments
- Friction stir spot welding of AA5052 with additional carbon fiber-reinforced polymer composite interlayer
- Improvement of long-term cycling performance of high-nickel cathode materials by ZnO coating
- Quantum effects of gas flow in nanochannels
- An approach to effectively improve the interfacial bonding of nano-perfused composites by in situ growth of CNTs
- Effects of nano-modified polymer cement-based materials on the bending behavior of repaired concrete beams
- Effects of the combined usage of nanomaterials and steel fibres on the workability, compressive strength, and microstructure of ultra-high performance concrete
- One-pot solvothermal synthesis and characterization of highly stable nickel nanoparticles
- Comparative study on mechanisms for improving mechanical properties and microstructure of cement paste modified by different types of nanomaterials
- Effect of in situ graphene-doped nano-CeO2 on microstructure and electrical contact properties of Cu30Cr10W contacts
- The experimental study of CFRP interlayer of dissimilar joint AA7075-T651/Ti-6Al-4V alloys by friction stir spot welding on mechanical and microstructural properties
- Vibration analysis of a sandwich cylindrical shell in hygrothermal environment
- Water barrier and mechanical properties of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch (TPS)/poly(lactic acid) (PLA) blend bionanocomposites
- Strong quadratic acousto-optic coupling in 1D multilayer phoxonic crystal cavity
- Three-dimensional shape analysis of peripapillary retinal pigment epithelium-basement membrane layer based on OCT radial images
- Solvent regulation synthesis of single-component white emission carbon quantum dots for white light-emitting diodes
- Xanthate-modified nanoTiO2 as a novel vulcanization accelerator enhancing mechanical and antibacterial properties of natural rubber
- Effect of steel fiber on impact resistance and durability of concrete containing nano-SiO2
- Ultrasound-enhanced biosynthesis of uniform ZnO nanorice using Swietenia macrophylla seed extract and its in vitro anticancer activity
- Temperature dependence of hardness prediction for high-temperature structural ceramics and their composites
- Study on the frequency of acoustic emission signal during crystal growth of salicylic acid
- Controllable modification of helical carbon nanotubes for high-performance microwave absorption
- Role of dry ozonization of basalt fibers on interfacial properties and fracture toughness of epoxy matrix composites
- Nanosystem’s density functional theory study of the chlorine adsorption on the Fe(100) surface
- A rapid nanobiosensing platform based on herceptin-conjugated graphene for ultrasensitive detection of circulating tumor cells in early breast cancer
- Improving flexural strength of UHPC with sustainably synthesized graphene oxide
- The role of graphene/graphene oxide in cement hydration
- Structural characterization of microcrystalline and nanocrystalline cellulose from Ananas comosus L. leaves: Cytocompatibility and molecular docking studies
- Evaluation of the nanostructure of calcium silicate hydrate based on atomic force microscopy-infrared spectroscopy experiments
- Combined effects of nano-silica and silica fume on the mechanical behavior of recycled aggregate concrete
- Safety study of malapposition of the bio-corrodible nitrided iron stent in vivo
- Triethanolamine interface modification of crystallized ZnO nanospheres enabling fast photocatalytic hazard-free treatment of Cr(vi) ions
- Novel electrodes for precise and accurate droplet dispensing and splitting in digital microfluidics
- Construction of Chi(Zn/BMP2)/HA composite coating on AZ31B magnesium alloy surface to improve the corrosion resistance and biocompatibility
- Experimental and multiscale numerical investigations on low-velocity impact responses of syntactic foam composites reinforced with modified MWCNTs
- Comprehensive performance analysis and optimal design of smart light pole for cooperative vehicle infrastructure system
- Room temperature growth of ZnO with highly active exposed facets for photocatalytic application
- Influences of poling temperature and elongation ratio on PVDF-HFP piezoelectric films
- Large strain hardening of magnesium containing in situ nanoparticles
- Super stable water-based magnetic fluid as a dual-mode contrast agent
- Photocatalytic activity of biogenic zinc oxide nanoparticles: In vitro antimicrobial, biocompatibility, and molecular docking studies
- Hygrothermal environment effect on the critical buckling load of FGP microbeams with initial curvature integrated by CNT-reinforced skins considering the influence of thickness stretching
- Thermal aging behavior characteristics of asphalt binder modified by nano-stabilizer based on DSR and AFM
- Building effective core/shell polymer nanoparticles for epoxy composite toughening based on Hansen solubility parameters
- Structural characterization and nanoscale strain field analysis of α/β interface layer of a near α titanium alloy
- Optimization of thermal and hydrophobic properties of GO-doped epoxy nanocomposite coatings
- The properties of nano-CaCO3/nano-ZnO/SBR composite-modified asphalt
- Three-dimensional metallic carbon allotropes with superhardness
- Physical stability and rheological behavior of Pickering emulsions stabilized by protein–polysaccharide hybrid nanoconjugates
- Optimization of volume fraction and microstructure evolution during thermal deformation of nano-SiCp/Al–7Si composites
- Phase analysis and corrosion behavior of brazing Cu/Al dissimilar metal joint with BAl88Si filler metal
- High-efficiency nano polishing of steel materials
- On the rheological properties of multi-walled carbon nano-polyvinylpyrrolidone/silicon-based shear thickening fluid
- Fabrication of Ag/ZnO hollow nanospheres and cubic TiO2/ZnO heterojunction photocatalysts for RhB degradation
- Fabrication and properties of PLA/nano-HA composite scaffolds with balanced mechanical properties and biological functions for bone tissue engineering application
- Investigation of the early-age performance and microstructure of nano-C–S–H blended cement-based materials
- Reduced graphene oxide coating on basalt fabric using electrophoretic deposition and its role in the mechanical and tribological performance of epoxy/basalt fiber composites
- Effect of nano-silica as cementitious materials-reducing admixtures on the workability, mechanical properties and durability of concrete
- Machine-learning-assisted microstructure–property linkages of carbon nanotube-reinforced aluminum matrix nanocomposites produced by laser powder bed fusion
- Physical, thermal, and mechanical properties of highly porous polylactic acid/cellulose nanofibre scaffolds prepared by salt leaching technique
- A comparative study on characterizations and synthesis of pure lead sulfide (PbS) and Ag-doped PbS for photovoltaic applications
- Clean preparation of washable antibacterial polyester fibers by high temperature and high pressure hydrothermal self-assembly
- Al 5251-based hybrid nanocomposite by FSP reinforced with graphene nanoplates and boron nitride nanoparticles: Microstructure, wear, and mechanical characterization
- Interlaminar fracture toughness properties of hybrid glass fiber-reinforced composite interlayered with carbon nanotube using electrospray deposition
- Microstructure and life prediction model of steel slag concrete under freezing-thawing environment
- Synthesis of biogenic silver nanoparticles from the seed coat waste of pistachio (Pistacia vera) and their effect on the growth of eggplant
- Study on adaptability of rheological index of nano-PUA-modified asphalt based on geometric parameters of parallel plate
- Preparation and adsorption properties of nano-graphene oxide/tourmaline composites
- A study on interfacial behaviors of epoxy/graphene oxide derived from pitch-based graphite fibers
- Multiresponsive carboxylated graphene oxide-grafted aptamer as a multifunctional nanocarrier for targeted delivery of chemotherapeutics and bioactive compounds in cancer therapy
- Piezoresistive/piezoelectric intrinsic sensing properties of carbon nanotube cement-based smart composite and its electromechanical sensing mechanisms: A review
- Smart stimuli-responsive biofunctionalized niosomal nanocarriers for programmed release of bioactive compounds into cancer cells in vitro and in vivo
- Photoremediation of methylene blue by biosynthesized ZnO/Fe3O4 nanocomposites using Callistemon viminalis leaves aqueous extract: A comparative study
- Study of gold nanoparticles’ preparation through ultrasonic spray pyrolysis and lyophilisation for possible use as markers in LFIA tests
- Review Articles
- Advance on the dispersion treatment of graphene oxide and the graphene oxide modified cement-based materials
- Development of ionic liquid-based electroactive polymer composites using nanotechnology
- Nanostructured multifunctional electrocatalysts for efficient energy conversion systems: Recent perspectives
- Recent advances on the fabrication methods of nanocomposite yarn-based strain sensor
- Review on nanocomposites based on aerospace applications
- Overview of nanocellulose as additives in paper processing and paper products
- The frontiers of functionalized graphene-based nanocomposites as chemical sensors
- Material advancement in tissue-engineered nerve conduit
- Carbon nanostructure-based superhydrophobic surfaces and coatings
- Functionalized graphene-based nanocomposites for smart optoelectronic applications
- Interfacial technology for enhancement in steel fiber reinforced cementitious composite from nano to macroscale
- Metal nanoparticles and biomaterials: The multipronged approach for potential diabetic wound therapy
- Review on resistive switching mechanisms of bio-organic thin film for non-volatile memory application
- Nanotechnology-enabled biomedical engineering: Current trends, future scopes, and perspectives
- Research progress on key problems of nanomaterials-modified geopolymer concrete
- Smart stimuli-responsive nanocarriers for the cancer therapy – nanomedicine
- An overview of methods for production and detection of silver nanoparticles, with emphasis on their fate and toxicological effects on human, soil, and aquatic environment
- Effects of chemical modification and nanotechnology on wood properties
- Mechanisms, influencing factors, and applications of electrohydrodynamic jet printing
- Application of antiviral materials in textiles: A review
- Phase transformation and strengthening mechanisms of nanostructured high-entropy alloys
- Research progress on individual effect of graphene oxide in cement-based materials and its synergistic effect with other nanomaterials
- Catalytic defense against fungal pathogens using nanozymes
- A mini-review of three-dimensional network topological structure nanocomposites: Preparation and mechanical properties
- Mechanical properties and structural health monitoring performance of carbon nanotube-modified FRP composites: A review
- Nano-scale delivery: A comprehensive review of nano-structured devices, preparative techniques, site-specificity designs, biomedical applications, commercial products, and references to safety, cellular uptake, and organ toxicity
- Effects of alloying, heat treatment and nanoreinforcement on mechanical properties and damping performances of Cu–Al-based alloys: A review
- Recent progress in the synthesis and applications of vertically aligned carbon nanotube materials
- Thermal conductivity and dynamic viscosity of mono and hybrid organic- and synthetic-based nanofluids: A critical review
- Recent advances in waste-recycled nanomaterials for biomedical applications: Waste-to-wealth
- Layup sequence and interfacial bonding of additively manufactured polymeric composite: A brief review
- Quantum dots synthetization and future prospect applications
- Approved and marketed nanoparticles for disease targeting and applications in COVID-19
- Strategies for improving rechargeable lithium-ion batteries: From active materials to CO2 emissions
Articles in the same Issue
- Research Articles
- Improved impedance matching by multi-componential metal-hybridized rGO toward high performance of microwave absorption
- Pure-silk fibroin hydrogel with stable aligned micropattern toward peripheral nerve regeneration
- Effective ion pathways and 3D conductive carbon networks in bentonite host enable stable and high-rate lithium–sulfur batteries
- Fabrication and characterization of 3D-printed gellan gum/starch composite scaffold for Schwann cells growth
- Synergistic strengthening mechanism of copper matrix composite reinforced with nano-Al2O3 particles and micro-SiC whiskers
- Deformation mechanisms and plasticity of ultrafine-grained Al under complex stress state revealed by digital image correlation technique
- On the deformation-induced grain rotations in gradient nano-grained copper based on molecular dynamics simulations
- Removal of sulfate from aqueous solution using Mg–Al nano-layered double hydroxides synthesized under different dual solvent systems
- Microwave-assisted sol–gel synthesis of TiO2-mixed metal oxide nanocatalyst for degradation of organic pollutant
- Electrophoretic deposition of graphene on basalt fiber for composite applications
- Polyphenylene sulfide-coated wrench composites by nanopinning effect
- Thermal conductivity and thermoelectric properties in 3D macroscopic pure carbon nanotube materials
- An effective thermal conductivity and thermomechanical homogenization scheme for a multiscale Nb3Sn filaments
- Friction stir spot welding of AA5052 with additional carbon fiber-reinforced polymer composite interlayer
- Improvement of long-term cycling performance of high-nickel cathode materials by ZnO coating
- Quantum effects of gas flow in nanochannels
- An approach to effectively improve the interfacial bonding of nano-perfused composites by in situ growth of CNTs
- Effects of nano-modified polymer cement-based materials on the bending behavior of repaired concrete beams
- Effects of the combined usage of nanomaterials and steel fibres on the workability, compressive strength, and microstructure of ultra-high performance concrete
- One-pot solvothermal synthesis and characterization of highly stable nickel nanoparticles
- Comparative study on mechanisms for improving mechanical properties and microstructure of cement paste modified by different types of nanomaterials
- Effect of in situ graphene-doped nano-CeO2 on microstructure and electrical contact properties of Cu30Cr10W contacts
- The experimental study of CFRP interlayer of dissimilar joint AA7075-T651/Ti-6Al-4V alloys by friction stir spot welding on mechanical and microstructural properties
- Vibration analysis of a sandwich cylindrical shell in hygrothermal environment
- Water barrier and mechanical properties of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch (TPS)/poly(lactic acid) (PLA) blend bionanocomposites
- Strong quadratic acousto-optic coupling in 1D multilayer phoxonic crystal cavity
- Three-dimensional shape analysis of peripapillary retinal pigment epithelium-basement membrane layer based on OCT radial images
- Solvent regulation synthesis of single-component white emission carbon quantum dots for white light-emitting diodes
- Xanthate-modified nanoTiO2 as a novel vulcanization accelerator enhancing mechanical and antibacterial properties of natural rubber
- Effect of steel fiber on impact resistance and durability of concrete containing nano-SiO2
- Ultrasound-enhanced biosynthesis of uniform ZnO nanorice using Swietenia macrophylla seed extract and its in vitro anticancer activity
- Temperature dependence of hardness prediction for high-temperature structural ceramics and their composites
- Study on the frequency of acoustic emission signal during crystal growth of salicylic acid
- Controllable modification of helical carbon nanotubes for high-performance microwave absorption
- Role of dry ozonization of basalt fibers on interfacial properties and fracture toughness of epoxy matrix composites
- Nanosystem’s density functional theory study of the chlorine adsorption on the Fe(100) surface
- A rapid nanobiosensing platform based on herceptin-conjugated graphene for ultrasensitive detection of circulating tumor cells in early breast cancer
- Improving flexural strength of UHPC with sustainably synthesized graphene oxide
- The role of graphene/graphene oxide in cement hydration
- Structural characterization of microcrystalline and nanocrystalline cellulose from Ananas comosus L. leaves: Cytocompatibility and molecular docking studies
- Evaluation of the nanostructure of calcium silicate hydrate based on atomic force microscopy-infrared spectroscopy experiments
- Combined effects of nano-silica and silica fume on the mechanical behavior of recycled aggregate concrete
- Safety study of malapposition of the bio-corrodible nitrided iron stent in vivo
- Triethanolamine interface modification of crystallized ZnO nanospheres enabling fast photocatalytic hazard-free treatment of Cr(vi) ions
- Novel electrodes for precise and accurate droplet dispensing and splitting in digital microfluidics
- Construction of Chi(Zn/BMP2)/HA composite coating on AZ31B magnesium alloy surface to improve the corrosion resistance and biocompatibility
- Experimental and multiscale numerical investigations on low-velocity impact responses of syntactic foam composites reinforced with modified MWCNTs
- Comprehensive performance analysis and optimal design of smart light pole for cooperative vehicle infrastructure system
- Room temperature growth of ZnO with highly active exposed facets for photocatalytic application
- Influences of poling temperature and elongation ratio on PVDF-HFP piezoelectric films
- Large strain hardening of magnesium containing in situ nanoparticles
- Super stable water-based magnetic fluid as a dual-mode contrast agent
- Photocatalytic activity of biogenic zinc oxide nanoparticles: In vitro antimicrobial, biocompatibility, and molecular docking studies
- Hygrothermal environment effect on the critical buckling load of FGP microbeams with initial curvature integrated by CNT-reinforced skins considering the influence of thickness stretching
- Thermal aging behavior characteristics of asphalt binder modified by nano-stabilizer based on DSR and AFM
- Building effective core/shell polymer nanoparticles for epoxy composite toughening based on Hansen solubility parameters
- Structural characterization and nanoscale strain field analysis of α/β interface layer of a near α titanium alloy
- Optimization of thermal and hydrophobic properties of GO-doped epoxy nanocomposite coatings
- The properties of nano-CaCO3/nano-ZnO/SBR composite-modified asphalt
- Three-dimensional metallic carbon allotropes with superhardness
- Physical stability and rheological behavior of Pickering emulsions stabilized by protein–polysaccharide hybrid nanoconjugates
- Optimization of volume fraction and microstructure evolution during thermal deformation of nano-SiCp/Al–7Si composites
- Phase analysis and corrosion behavior of brazing Cu/Al dissimilar metal joint with BAl88Si filler metal
- High-efficiency nano polishing of steel materials
- On the rheological properties of multi-walled carbon nano-polyvinylpyrrolidone/silicon-based shear thickening fluid
- Fabrication of Ag/ZnO hollow nanospheres and cubic TiO2/ZnO heterojunction photocatalysts for RhB degradation
- Fabrication and properties of PLA/nano-HA composite scaffolds with balanced mechanical properties and biological functions for bone tissue engineering application
- Investigation of the early-age performance and microstructure of nano-C–S–H blended cement-based materials
- Reduced graphene oxide coating on basalt fabric using electrophoretic deposition and its role in the mechanical and tribological performance of epoxy/basalt fiber composites
- Effect of nano-silica as cementitious materials-reducing admixtures on the workability, mechanical properties and durability of concrete
- Machine-learning-assisted microstructure–property linkages of carbon nanotube-reinforced aluminum matrix nanocomposites produced by laser powder bed fusion
- Physical, thermal, and mechanical properties of highly porous polylactic acid/cellulose nanofibre scaffolds prepared by salt leaching technique
- A comparative study on characterizations and synthesis of pure lead sulfide (PbS) and Ag-doped PbS for photovoltaic applications
- Clean preparation of washable antibacterial polyester fibers by high temperature and high pressure hydrothermal self-assembly
- Al 5251-based hybrid nanocomposite by FSP reinforced with graphene nanoplates and boron nitride nanoparticles: Microstructure, wear, and mechanical characterization
- Interlaminar fracture toughness properties of hybrid glass fiber-reinforced composite interlayered with carbon nanotube using electrospray deposition
- Microstructure and life prediction model of steel slag concrete under freezing-thawing environment
- Synthesis of biogenic silver nanoparticles from the seed coat waste of pistachio (Pistacia vera) and their effect on the growth of eggplant
- Study on adaptability of rheological index of nano-PUA-modified asphalt based on geometric parameters of parallel plate
- Preparation and adsorption properties of nano-graphene oxide/tourmaline composites
- A study on interfacial behaviors of epoxy/graphene oxide derived from pitch-based graphite fibers
- Multiresponsive carboxylated graphene oxide-grafted aptamer as a multifunctional nanocarrier for targeted delivery of chemotherapeutics and bioactive compounds in cancer therapy
- Piezoresistive/piezoelectric intrinsic sensing properties of carbon nanotube cement-based smart composite and its electromechanical sensing mechanisms: A review
- Smart stimuli-responsive biofunctionalized niosomal nanocarriers for programmed release of bioactive compounds into cancer cells in vitro and in vivo
- Photoremediation of methylene blue by biosynthesized ZnO/Fe3O4 nanocomposites using Callistemon viminalis leaves aqueous extract: A comparative study
- Study of gold nanoparticles’ preparation through ultrasonic spray pyrolysis and lyophilisation for possible use as markers in LFIA tests
- Review Articles
- Advance on the dispersion treatment of graphene oxide and the graphene oxide modified cement-based materials
- Development of ionic liquid-based electroactive polymer composites using nanotechnology
- Nanostructured multifunctional electrocatalysts for efficient energy conversion systems: Recent perspectives
- Recent advances on the fabrication methods of nanocomposite yarn-based strain sensor
- Review on nanocomposites based on aerospace applications
- Overview of nanocellulose as additives in paper processing and paper products
- The frontiers of functionalized graphene-based nanocomposites as chemical sensors
- Material advancement in tissue-engineered nerve conduit
- Carbon nanostructure-based superhydrophobic surfaces and coatings
- Functionalized graphene-based nanocomposites for smart optoelectronic applications
- Interfacial technology for enhancement in steel fiber reinforced cementitious composite from nano to macroscale
- Metal nanoparticles and biomaterials: The multipronged approach for potential diabetic wound therapy
- Review on resistive switching mechanisms of bio-organic thin film for non-volatile memory application
- Nanotechnology-enabled biomedical engineering: Current trends, future scopes, and perspectives
- Research progress on key problems of nanomaterials-modified geopolymer concrete
- Smart stimuli-responsive nanocarriers for the cancer therapy – nanomedicine
- An overview of methods for production and detection of silver nanoparticles, with emphasis on their fate and toxicological effects on human, soil, and aquatic environment
- Effects of chemical modification and nanotechnology on wood properties
- Mechanisms, influencing factors, and applications of electrohydrodynamic jet printing
- Application of antiviral materials in textiles: A review
- Phase transformation and strengthening mechanisms of nanostructured high-entropy alloys
- Research progress on individual effect of graphene oxide in cement-based materials and its synergistic effect with other nanomaterials
- Catalytic defense against fungal pathogens using nanozymes
- A mini-review of three-dimensional network topological structure nanocomposites: Preparation and mechanical properties
- Mechanical properties and structural health monitoring performance of carbon nanotube-modified FRP composites: A review
- Nano-scale delivery: A comprehensive review of nano-structured devices, preparative techniques, site-specificity designs, biomedical applications, commercial products, and references to safety, cellular uptake, and organ toxicity
- Effects of alloying, heat treatment and nanoreinforcement on mechanical properties and damping performances of Cu–Al-based alloys: A review
- Recent progress in the synthesis and applications of vertically aligned carbon nanotube materials
- Thermal conductivity and dynamic viscosity of mono and hybrid organic- and synthetic-based nanofluids: A critical review
- Recent advances in waste-recycled nanomaterials for biomedical applications: Waste-to-wealth
- Layup sequence and interfacial bonding of additively manufactured polymeric composite: A brief review
- Quantum dots synthetization and future prospect applications
- Approved and marketed nanoparticles for disease targeting and applications in COVID-19
- Strategies for improving rechargeable lithium-ion batteries: From active materials to CO2 emissions

