Crystal structure of [(E)-6′-(diethylamino)-2-(2-(((E)-pyren-1-ylmethylene)amino)ethyl)-4′-(2-((E)-1,3,3-trimethylindolin-2-ylidene)ethylidene)-1′,2′,3′,4′-tetrahydrospiro[isoindoline-1,9′-xanthen]-3-one]-methanol, solvate C57H56N4O3
Abstract
C57H56N4O3, monoclinic, P21/c (no. 14), a = 10.1943(2) Å, b = 25.1257(4) Å, c = 18.6057(3) Å, β = 105.462(2)°, Z = 4, V = 4593.16(14) Å3 R gt (F) = 0.0603, wR ref (F2) = 0.1869, T = 173(10) K.
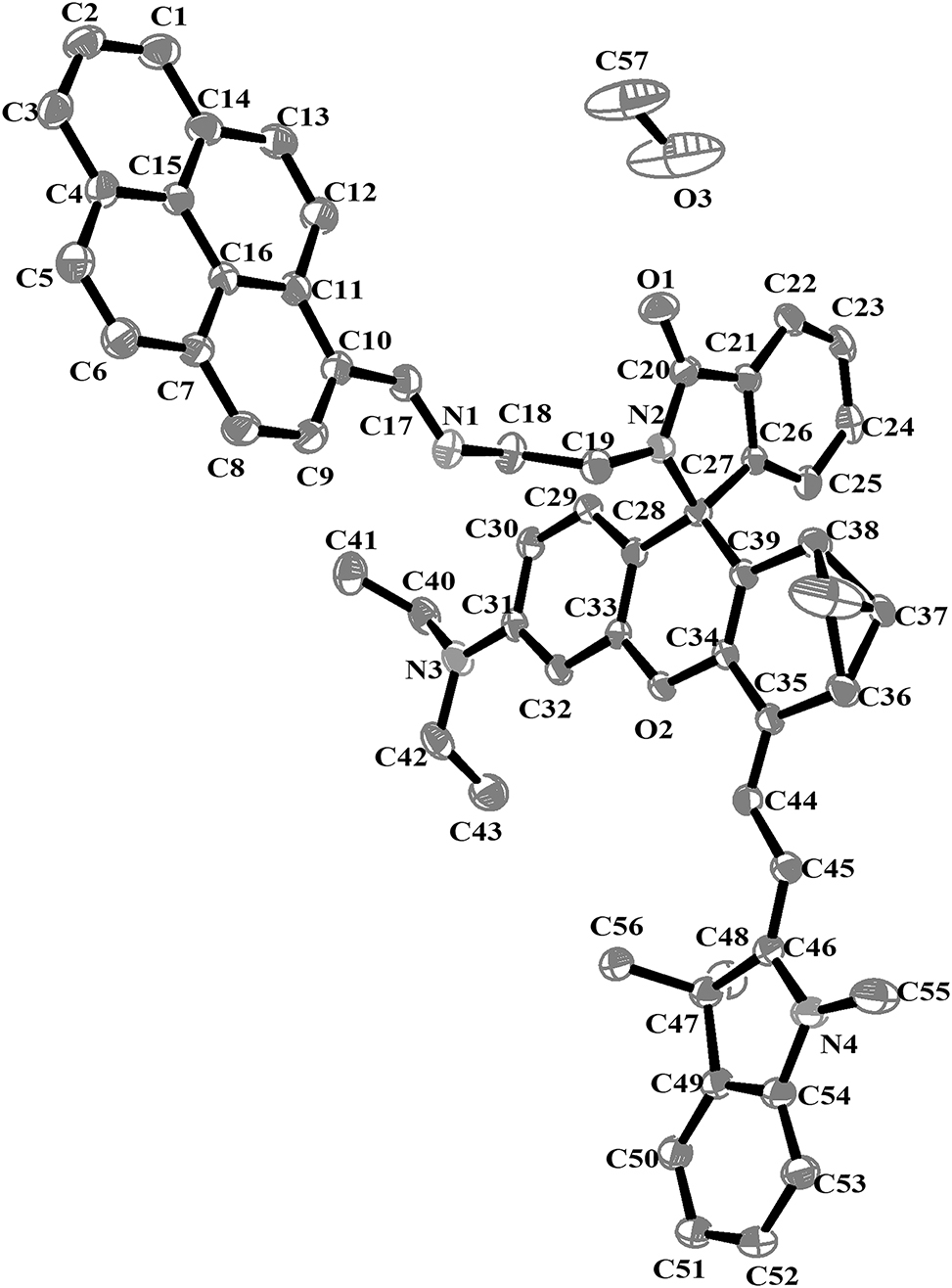
The molecular structure is shown in the figure. Table 1 contains the crystallographic data. The list of the atoms including atomic coordinates and displacement parameters can be found in the cif-file attached to this article.
Data collection and handling.
| Crystal: | Clear light yellow block |
| Size: | 0.20 × 0.15 × 0.10 mm |
| Wavelength: | Cu Kα radiation (1.54184 Å) |
| μ: | 0.59 mm−1 |
| Diffractometer, scan mode: | Rigaku SuperNova, ω scan |
| θmax, completeness: | 65.1°, 99 % |
| N(hkl)measured, N(hkl)unique, Rint: | 29090, 7712, 0.029 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 6,280 |
| N(param)refined: | 593 |
| Programs: | Rigaku, 1 Olex2, 2 SHELX 3 , 4 |
1 Source of materials
All reagents were used without further purification. The title compound was synthesized via a four-step process as described in the literature. 5 , 6 , 7
Synthesis of intermediate 1: Under ice-bath conditions, cyclohexanone (0.99 mL, 4.8 mmol) was carefully dripped into concentrated sulfuric acid (10.0 mL). Subsequently, 4-diethylaminolevulinic acid (1.50 g, 4.8 mmol) was slowly added under vigorous stirring. After addition, the mixture was heated to 363 K and maintained about 4 h to promote the reaction. Then, the reaction mixture was cooled and poured into crushed ice (100.0 g). When perchloric acid (1.5 mL, 70 %) was added, a red precipitate appeared immediately, which was filtered, washed with cold water for three times and air-dried to yield a red solid, yielding 80 %, named intermediate 1. Intermediate 1 was used without further purification.
Synthesis of intermediate 2: Equimolar amounts of intermediate 1 (1.25 g, 3.33 mmol) and 2-(1,3,3–trimethylindolin-2-ylidene)acetaldehyde (0.67 g, 3.33 mmol) were successively dissolved in acetic anhydride (20.0 mL) and stirred for 30 min at room temperature. The reaction was promptly quenched by cooling it to 273 K. Then, the crude product was filtered and purified by column chromatography, ethanol-dichloromethane (CH2Cl2/CH3CH2OH = 200:1∼20:1 v/v) as the gradient eluent. At last, a dark green solid was obtained, named intermediate 2, yielding 42 %.
Synthesis of intermediate 3: Intermediate 2 (1.2 g, 2.15 mmol), PyBOP (1.4 g, 2.68 mmol) and ethylenediamine (1.07 mL, 16 mmol) were successively dissolved in dichloromethane (25 mL). And then, the mixture was stirred 10 h at room temperature. Next, the mixture was distilled under reduced pressure to obtain the crude product. Purification was performed by column chromatography, using ethanol-dichloromethane (CH2Cl2/CH3CH2OH = 200:1∼50:1 v/v) as the gradient eluent. A yellow powder was collected, which was identified as intermediate 3, yielding 30 %.
Synthesis of the title compound: Intermediate 3 (0.42 g, 0.734 mmol) and 1-pyrenecarboxaldehyde (0.25 g, 1.1 mmol) were dissolved in ethanol (20 mL) and refluxed for 3 h, during which a yellow precipitate formed. Then, the precipitate was filtered and washed thoroughly with cold water for three times and purified through column chromatography using a mixture of ethanol and dichloromethane (CH2Cl2/CH3CH2OH = 200:1∼50:1 v/v) as the eluent. The yellow powder was collected and labeled as the title compound, yielding 32 %.
2 Experimental details
The H atoms were added using riding models. Their Uiso values were set to 1.2 Ueq of the parent atoms.
3 Comment
Pyrene-based fluorophores constitute structurally versatile systems endowed with inherent chemical stability, extended radiative lifetimes, and superior quantum efficiencies. While extensive investigations have established their prominence in visible-range sensing applications, systematic exploration of near-infrared (NIR) emissive pyrene derivatives remains limited. 8 , 9 , 10 Expanding the π-conjugated system drives NIR fluorophore design. Yuan et al. pioneered xanthene-hemicyanine hybrids inducing bathochromism via extended conjugation, advancing NIR dyes. 11 , 12 , 13 Building on this, we engineered pyrene-integrated hemicyanine-xanthene fluorophores with NIR emission. 14 However, The study found that this dye exhibits low sensitivity in metal ion binding. Prompting structural optimization through precise modulation of the xanthene-pyrene interchromophoric distance. This rational design approach yielded the title compound. Cu2+ homeostasis dysfunction, implicated in Alzheimer’s, Parkinson’s, and Wilson’s diseases, drives demand for specific probes. 15 Given the potential of the title compound to bind selectively to Cu2+, it may act as a valuable Cu2+ probe, facilitating the visualization of copper dynamics in vivo and offering critical insights for disease diagnosis and therapeutic intervention.
The title compound crystallizes within the monoclinic system, belonging to the space group P21/c. The asymmetric unit of the title compound comprises a title neutral molecule and a methanol molecule. The bond lengths and angles within the crystal structure are within normal ranges. The disordered treatment was applied to the C37, revealing a statistical coexistence of two conformational states with refined occupancies of 0.837(11) and 0.163(11), respectively. Notably, the dihedral angles between the xanthene and pyrene moieties in the current structure are measured at 83.0°, and the dihedral angle between the plane of the spirolactam and the pyrene plane is merely 22.3°. In contrast, angular parameters for the previously synthesized analogs were reported as 68.6° (xanthene-pyrene) and 26.3° (spirolactam-pyrene), 14 demonstrating substantial conformational modifications in the newly designed compound. This implies that the coordination of the N and O atoms on the title compound with copper ions may trigger the ring-opening of the spirolactam, thereby conferring the compound with the ability to release near-infrared fluorescence. 16 The C17=N1 bond distance, measured as 1.255(4) Å, aligns well with the C=N double bond length of Schiff bases as reported in the literature. 12 , 14
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Project No.22101076), the Natural Science Foundation of Henan, China (Project No.252300420129). The authors extend their gratitude to Mr. Tian gaofeng from Scientific Compass (www.shiyanjia.com) for providing invaluable assistance with the X-ray diffraction analysis.
References
1. CrysAlis Pro Software System, Version 1. 171. 38. 43f, Rigaku Oxford Diffraction. 2015.Search in Google Scholar
2. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinementand Analysis Program. J. Appl. Cryst. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
3. Sheldrick, G. M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Sheldrick, G. M. SHELXT-Integrated space-group and crystal-structure Determin Ation. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar PubMed PubMed Central
5. Xie, J. Y.; Li, C. Y.; Li, Y. F.; Fu, Y. J.; Nie, S. X.; Tan, H. Y. A near-infrared Chemosensor for Determination of Trivalent Aluminum Ions in Living Cells and Tissues. Dyes Pigm. 2017, 136, 817–824; https://doi.org/10.1016/j.dyepig.2016.09.046.Search in Google Scholar
6. Yao, S. K.; Qian, Y.; Qi, Z. Q. A Smart Two-photon Fluorescent Platform Based on Besulfurization-cyclization: A Phthalimide-Rhodamine Chemodosimeter for Hg2+ NIR Emission at 746 nm and Through-bond Energy Transfer. New J. Chem. 2017, 41, 13495–13503; https://doi.org/10.1039/C7NJ02814H.Search in Google Scholar
7. Wang, J. L.; Li, W. L.; Long, L. P. Development of a near-infrared Fluorescence turn-on Probe for Imaging Hg2+ in Living Cells and Animals. Sens. Actuator. B: Chem. 2017, 245, 462–469; https://doi.org/10.1016/j.snb.2017.01.002.Search in Google Scholar
8. Yang, H. H.; Xu, Q. Q.; Feng, H. D.; Yuan, J. Crystal Structure of (E)–N′-((1,8-dihydropyren-1-yl)-methylene)picolinohydrazide, C23H15N3O. Z. Kristallogr. N. Cryst. Struct. 2019, 234, 1103–1104; https://doi.org/10.1515/ncrs-2019-0297.Search in Google Scholar
9. Wang, L. L. Crystal Structure of (E)-N′-((1,6-dihydropyren-1-yl) methylene)isonicotinohydrazide-methanol (1/1), C24H19N3O2. Z. Kristallogr. N. Cryst. Struct. 2019, 234, 1249–1250; https://doi.org/10.1515/ncrs-2019-0370.Search in Google Scholar
10. Tang, J. W.; Wang, M. X.; Li, H. M.; Liang, Q.; Zhang, Z.; Liu, H.; Sun, J.; Ma, L. J. Simply Structural, Reversible and Chemical Stable Ratiometric pH-responsive Fluorescence Probe of Pyrene Derivatives and its Applications. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2025, 238, 126205; https://doi.org/10.1016/j.saa.2025.126205.Search in Google Scholar PubMed
11. Yuan, L.; Lin, W.; Xie, Y.; Chen, H. A Unique Class of near-infrared Functional Fluorescent Dyes with carboxylic-acid-modulated Fluorescence on/off Switching: Rational Design, Synthesis, Optical Properties, Theoretical Calculations, and Applications for Fluorescence Imaging in Living Animals. J. Am. Chem. Soc. 2012, 134, 1200–1211; https://doi.org/10.1021/ja209292b.Search in Google Scholar PubMed
12. Li, K. H.; Wu, J. P.; Lan, H. R.; Wang, J.; Yuan, J. Crystal structure of (4′E)-6′-(diethylamino)-2-[(E)-[(6-methylpyridin-2-yl)methylidene]amino]-4′-{2-[(2E)-1,3,3-trimethyl-2,3-dihydro-1H-indol-2-ylidene]ethylidene}-1′, 2,2′,3,3′,4′-hexahydrospiro[isoindole-1,9′-xanthene]-3-one, C44H45N5O2. Z. Kristallogr. N. Cryst. Struct. 2022, 237, 51–54; https://doi.org/10.1515/ncrs-2021-0363.Search in Google Scholar
13. Wu, J. P.; Xing, A. P.; Yuan, Y. Y.; Hao, Y. T.; Pan, P.; He, S. N.; Yuan, J.; Zeng, D. A near-infrared Hemicyanine-based Colorimetric and Fluorescent Chemosensor for Highly Sensitive Detection of Cu2+ and Imaging in Living Cells and in Vivo. Dyes Pigments 2024, 221, 111797; https://doi.org/10.1016/j.dyepig.2023.111797.Search in Google Scholar
14. Wu, J. P.; Li, K. H.; Lan, H. R.; Chu, Y. Crystal structure of (4′E)-6′-(diethylamino)-2-[(E)-[(pyren-1-yl)methylidene]amino]-4′-{2-[(2E)-1,3,3-trimethyl-2,3-dihydro-1H-indol-2-ylidene]ethylidene}-1′, 2,2′,3,3′,4′-hexahydrospiro[isoindole-1,9′-xanthene]-3-one, C54H48N4O2. Z. Kristallogr. N. Cryst. Struct. 2022, 237, 113–116; https://doi.org/10.1515/ncrs-2021-0383.Search in Google Scholar
15. Zhou, J.; Jangili, P.; Son, S.; Ji, M. S.; Won, M.; Kim, J. S. Fluorescent Diagnostic Probes in Neurodegenerative Diseases. Adv. Mater. 2020, 32, 2001945; https://doi.org/10.1002/adma.202001945.Search in Google Scholar PubMed
16. Li, Z.; Ho, J. T.; Wang, S. Recent Advances of Luminescent Sensors for Iron and Copper: Platforms, Mechanisms, and bio-applications. Coord. Chem. Rev. 2022, 469, 214695; https://doi.org/10.1016/j.ccr.2022.214695.Search in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of (S)-N-(10-((2,2-dimethoxyethyl)amino)-1,2,3-trimethoxy-9-oxo-5,6,7,9-tetrahydrobenzo[a]heptalen-7-yl)acetamide, C25H32N2O7
- The crystal structure of 6,6′-difluoro-3,3′-dimethyl-5,5′-di(10H-phenoxazin-10-yl)- [1,1′-biphenyl]-2,2′-dicarbonitrile, C40H24F2N4O2
- Crystal structure of poly[(di-ethylenediamine-κ2N,N′)cadmium(II) tetradedocyloxidohexavanadate] (V4+/V5+ = 2/1), C4H16CdN4O14V6
- The crystal structure of poly[bis(dimethylformamide-κ1N)-(μ4-2′,3,3″,5′-tetrakis(trifluoromethyl)-[1,1′:4′,1″-terphenyl]-4,4″-dicarboxylato-κ4 O,O′: O″,O‴)dicadmium(II)], C27H15CdF12NO5
- Crystal structure of bis(μ2-ferrocenylcarboxylato-O,O′)-(μ3-oxido-κ3O:O:O)-bis(μ2-salicyladoximato-κ2N,O,O′)-(μ2-isopropoxo)-tris(isopropoxy-κ1O trititanium(IV)), C48H55N2O13Fe2Ti3
- Crystal structure of 3-(diethylamino)-7,9,11-trimethyl-8-phenyl-6H,13H-12λ4,13λ4-chromeno[3′,4′:4,5]pyrrolo[1,2-c]pyrrolo[2,1-f][1,3,2]diazaborinin-6-one, C28H26BF2N3O2
- The crystal structure of catena-poly[aqua-μ2-2-nitro-benzene-1,3-dicarboxylato-κ2O,O′)-(1,10-phenanthroline-κ2N,N′)-zinc(II)], C20H13N3O7Zn
- Crystal structure of poly[diaqua-{μ3-1-(3-carboxylatophenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylato-κ4O,O′:O′′:O′′′′}manganese(II)] hydrate
- Crystal structure of N′-((1-hydroxycyclohexyl)(phenyl)methyl)-2-methoxybenzohydrazide methanol solvate, C22H28N2O4
- The cocrystal of caffeic acid — progesterone — water (1/2/1), C51H70O9
- Crystal structure of (((oxido(quinolin-6-yl)methoxy)triphenyl-λ5-stibanyl)oxy)(quinolin-7-yl)methanolate
- Crystal structure of [(E)-6′-(diethylamino)-2-(2-(((E)-pyren-1-ylmethylene)amino)ethyl)-4′-(2-((E)-1,3,3-trimethylindolin-2-ylidene)ethylidene)-1′,2′,3′,4′-tetrahydrospiro[isoindoline-1,9′-xanthen]-3-one]-methanol, solvate C57H56N4O3
- The crystal structure of 1-(acridin-9-yl)pyrrolidine-2,5-dione, C17H22N2O2
- Crystal structure of N-(4-acetylphenyl)-2-(6-methoxynaphthalen-2-yl)propanamide, C22H21NO3
- The crystal structure of 5,10,15,20-tetrakis(4-(1H-1,2,4-triazol-1-yl)phenyl)porphyrin, C52H34N16
- Crystal structure of hexacarbonyl-μ2-[phenylmethanedithiolato-κ4S:S,S′:S′]diiron (Fe–Fe) C13H6Fe2O6S2
- Crystal structure of diiodo-bis(1-((2-propyl-1H-benzo[d]imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κ1N)cadmium(II), C34H34CdI2N10
- Crystal structure of (E)-(3-(3-bromophenyl)acryloyl)ferrocene, C19H15BrFeO
- Crystal structure of catena-poly(μ2-6-chloropyridine-2-carboxylato-κ3N,O:O′)(6-chloropyridine-2-carboxylato-κ2O,N)copper(II), C12H6Cl2N2O4Cu
- Crystal structure of poly[diaqua-μ 3-(5-(3,5-dicarboxy-2,4,6-trimethylbenzyl)-2,4,6-trimethylisophthalato)-κ 6O,O′:O″,O‴:O‴′,O‴″) terbium(III)-monohydrate], C23H28TbO12
- Crystal structure of (E)-2-(((5-chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)methylene)amino)-3′,6′-dihydroxyspiro[isoindoline-1,9′-xanthen]-3-one – ethanol (1/2), C35H33ClN4O6
- The crystal structure of 3-(5-amino-3-phenylisoxazol-4-yl)-4-chloro-3-hydroxyindolin-2-one, C17H12ClN3O3
- The crystal structure of dimethylammonium 4-[2-(4-fluorophenyl)-4, 5-diphenyl-1H-imidazol-1-yl]benzenesulfonate, C29H26FN3O3S
- Crystal structure of (R)-2-ammonio-3-((5-carboxypentyl)thio)propanoate
- Crystal structure of 4-cyclohexyl-5-(thiophen-2-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione, C12H15N3S2
- The crystal structure of 4,6-bis(dimethylamino)-2-fluoroisophthalonitrile, C12H13FN4
- Hydrogen bonding in the crystal structure of nicotin-1,1′-dium tetrabromidomanganate(II)
- The crystal structure of bis(2-bromobenzyl)(2-((2-oxybenzylidene)amino)-4-methylpentanoato-κ3N, O,O′)tin(IV), C27H27Br2NO3Sn
- Crystal structure of (E)-(3-(p-tolyl)acryloyl)ferrocene, C20H18FeO
- Crystal structure of (E)-7-fluoro-2-((5-(4-methylpiperazin-1-yl)pyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C21H22FN3O
- Crystal structure of (E)-7-methoxy-2-((5-(4-methylpiperazin-1-yl)pyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C22H25N3O2
- The crystal structure of poly(bis(μ2-1,3,5-tri(1H-imidazol-1-yl)benzene-κ2N:N′)-(μ2-2,3,5,6-tetrafluoroterephthalato-κ2O:O′)-manganese(II), C38H24F4N12O4Mn
- Crystal structure of (3,4-dimethoxybenzyl)triphenylphosphonium bromide ethanol solvate, C29H32BrO3P
- Crystal structure of tetraethylammonium hydrogencarbonate – (diaminomethylene)thiourea – water (2/1/3)
- Crystal structure of N, N-Dimethyl-N′-tosylformimidamide, C10H14N2O2S
- The crystal structure of ethyl 2-methyl-5-oxo-4-(2-methoxyphenyl)-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C20H23N2O4
- Crystal structure of bis(μ2-1,5-bis[(E)-1-(2-hydroxyphenyl)ethylidene] thiocarbonohydrazide)-bis(dimethylformamide)-dizinc(II) dimethylformamide solvate, C40H46N10O6S2Zn2⋅C3H7NO
- Crystal structure of azido-κ1N{hydridotris(3-tert-butyl-5-methylpyrazol-1-yl)borato-κ3N,N′,N″}copper(II), C24H40BCuN9
- The crystal structure of fac-tricarbonyl(1,10-phenanthroline-κ2N,N′)-(azido-κ1N)rhenium(I), C15H8N5O3Re
- Crystal structure of 4-((triphenylphosphonio)methyl)pyridin-1-ium tetrachloridozincate(II), C24H22Cl4NPZn
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of (S)-N-(10-((2,2-dimethoxyethyl)amino)-1,2,3-trimethoxy-9-oxo-5,6,7,9-tetrahydrobenzo[a]heptalen-7-yl)acetamide, C25H32N2O7
- The crystal structure of 6,6′-difluoro-3,3′-dimethyl-5,5′-di(10H-phenoxazin-10-yl)- [1,1′-biphenyl]-2,2′-dicarbonitrile, C40H24F2N4O2
- Crystal structure of poly[(di-ethylenediamine-κ2N,N′)cadmium(II) tetradedocyloxidohexavanadate] (V4+/V5+ = 2/1), C4H16CdN4O14V6
- The crystal structure of poly[bis(dimethylformamide-κ1N)-(μ4-2′,3,3″,5′-tetrakis(trifluoromethyl)-[1,1′:4′,1″-terphenyl]-4,4″-dicarboxylato-κ4 O,O′: O″,O‴)dicadmium(II)], C27H15CdF12NO5
- Crystal structure of bis(μ2-ferrocenylcarboxylato-O,O′)-(μ3-oxido-κ3O:O:O)-bis(μ2-salicyladoximato-κ2N,O,O′)-(μ2-isopropoxo)-tris(isopropoxy-κ1O trititanium(IV)), C48H55N2O13Fe2Ti3
- Crystal structure of 3-(diethylamino)-7,9,11-trimethyl-8-phenyl-6H,13H-12λ4,13λ4-chromeno[3′,4′:4,5]pyrrolo[1,2-c]pyrrolo[2,1-f][1,3,2]diazaborinin-6-one, C28H26BF2N3O2
- The crystal structure of catena-poly[aqua-μ2-2-nitro-benzene-1,3-dicarboxylato-κ2O,O′)-(1,10-phenanthroline-κ2N,N′)-zinc(II)], C20H13N3O7Zn
- Crystal structure of poly[diaqua-{μ3-1-(3-carboxylatophenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylato-κ4O,O′:O′′:O′′′′}manganese(II)] hydrate
- Crystal structure of N′-((1-hydroxycyclohexyl)(phenyl)methyl)-2-methoxybenzohydrazide methanol solvate, C22H28N2O4
- The cocrystal of caffeic acid — progesterone — water (1/2/1), C51H70O9
- Crystal structure of (((oxido(quinolin-6-yl)methoxy)triphenyl-λ5-stibanyl)oxy)(quinolin-7-yl)methanolate
- Crystal structure of [(E)-6′-(diethylamino)-2-(2-(((E)-pyren-1-ylmethylene)amino)ethyl)-4′-(2-((E)-1,3,3-trimethylindolin-2-ylidene)ethylidene)-1′,2′,3′,4′-tetrahydrospiro[isoindoline-1,9′-xanthen]-3-one]-methanol, solvate C57H56N4O3
- The crystal structure of 1-(acridin-9-yl)pyrrolidine-2,5-dione, C17H22N2O2
- Crystal structure of N-(4-acetylphenyl)-2-(6-methoxynaphthalen-2-yl)propanamide, C22H21NO3
- The crystal structure of 5,10,15,20-tetrakis(4-(1H-1,2,4-triazol-1-yl)phenyl)porphyrin, C52H34N16
- Crystal structure of hexacarbonyl-μ2-[phenylmethanedithiolato-κ4S:S,S′:S′]diiron (Fe–Fe) C13H6Fe2O6S2
- Crystal structure of diiodo-bis(1-((2-propyl-1H-benzo[d]imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κ1N)cadmium(II), C34H34CdI2N10
- Crystal structure of (E)-(3-(3-bromophenyl)acryloyl)ferrocene, C19H15BrFeO
- Crystal structure of catena-poly(μ2-6-chloropyridine-2-carboxylato-κ3N,O:O′)(6-chloropyridine-2-carboxylato-κ2O,N)copper(II), C12H6Cl2N2O4Cu
- Crystal structure of poly[diaqua-μ 3-(5-(3,5-dicarboxy-2,4,6-trimethylbenzyl)-2,4,6-trimethylisophthalato)-κ 6O,O′:O″,O‴:O‴′,O‴″) terbium(III)-monohydrate], C23H28TbO12
- Crystal structure of (E)-2-(((5-chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)methylene)amino)-3′,6′-dihydroxyspiro[isoindoline-1,9′-xanthen]-3-one – ethanol (1/2), C35H33ClN4O6
- The crystal structure of 3-(5-amino-3-phenylisoxazol-4-yl)-4-chloro-3-hydroxyindolin-2-one, C17H12ClN3O3
- The crystal structure of dimethylammonium 4-[2-(4-fluorophenyl)-4, 5-diphenyl-1H-imidazol-1-yl]benzenesulfonate, C29H26FN3O3S
- Crystal structure of (R)-2-ammonio-3-((5-carboxypentyl)thio)propanoate
- Crystal structure of 4-cyclohexyl-5-(thiophen-2-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione, C12H15N3S2
- The crystal structure of 4,6-bis(dimethylamino)-2-fluoroisophthalonitrile, C12H13FN4
- Hydrogen bonding in the crystal structure of nicotin-1,1′-dium tetrabromidomanganate(II)
- The crystal structure of bis(2-bromobenzyl)(2-((2-oxybenzylidene)amino)-4-methylpentanoato-κ3N, O,O′)tin(IV), C27H27Br2NO3Sn
- Crystal structure of (E)-(3-(p-tolyl)acryloyl)ferrocene, C20H18FeO
- Crystal structure of (E)-7-fluoro-2-((5-(4-methylpiperazin-1-yl)pyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C21H22FN3O
- Crystal structure of (E)-7-methoxy-2-((5-(4-methylpiperazin-1-yl)pyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C22H25N3O2
- The crystal structure of poly(bis(μ2-1,3,5-tri(1H-imidazol-1-yl)benzene-κ2N:N′)-(μ2-2,3,5,6-tetrafluoroterephthalato-κ2O:O′)-manganese(II), C38H24F4N12O4Mn
- Crystal structure of (3,4-dimethoxybenzyl)triphenylphosphonium bromide ethanol solvate, C29H32BrO3P
- Crystal structure of tetraethylammonium hydrogencarbonate – (diaminomethylene)thiourea – water (2/1/3)
- Crystal structure of N, N-Dimethyl-N′-tosylformimidamide, C10H14N2O2S
- The crystal structure of ethyl 2-methyl-5-oxo-4-(2-methoxyphenyl)-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C20H23N2O4
- Crystal structure of bis(μ2-1,5-bis[(E)-1-(2-hydroxyphenyl)ethylidene] thiocarbonohydrazide)-bis(dimethylformamide)-dizinc(II) dimethylformamide solvate, C40H46N10O6S2Zn2⋅C3H7NO
- Crystal structure of azido-κ1N{hydridotris(3-tert-butyl-5-methylpyrazol-1-yl)borato-κ3N,N′,N″}copper(II), C24H40BCuN9
- The crystal structure of fac-tricarbonyl(1,10-phenanthroline-κ2N,N′)-(azido-κ1N)rhenium(I), C15H8N5O3Re
- Crystal structure of 4-((triphenylphosphonio)methyl)pyridin-1-ium tetrachloridozincate(II), C24H22Cl4NPZn

