Abstract
C28H26BF2N3O2, triclinic,
The molecular structure is shown in the Figure 1. Table 1 contains the crystallographic data and the list of the atoms including atomic coordinates and displacement parameters can be found in the cif-file attached to this article.
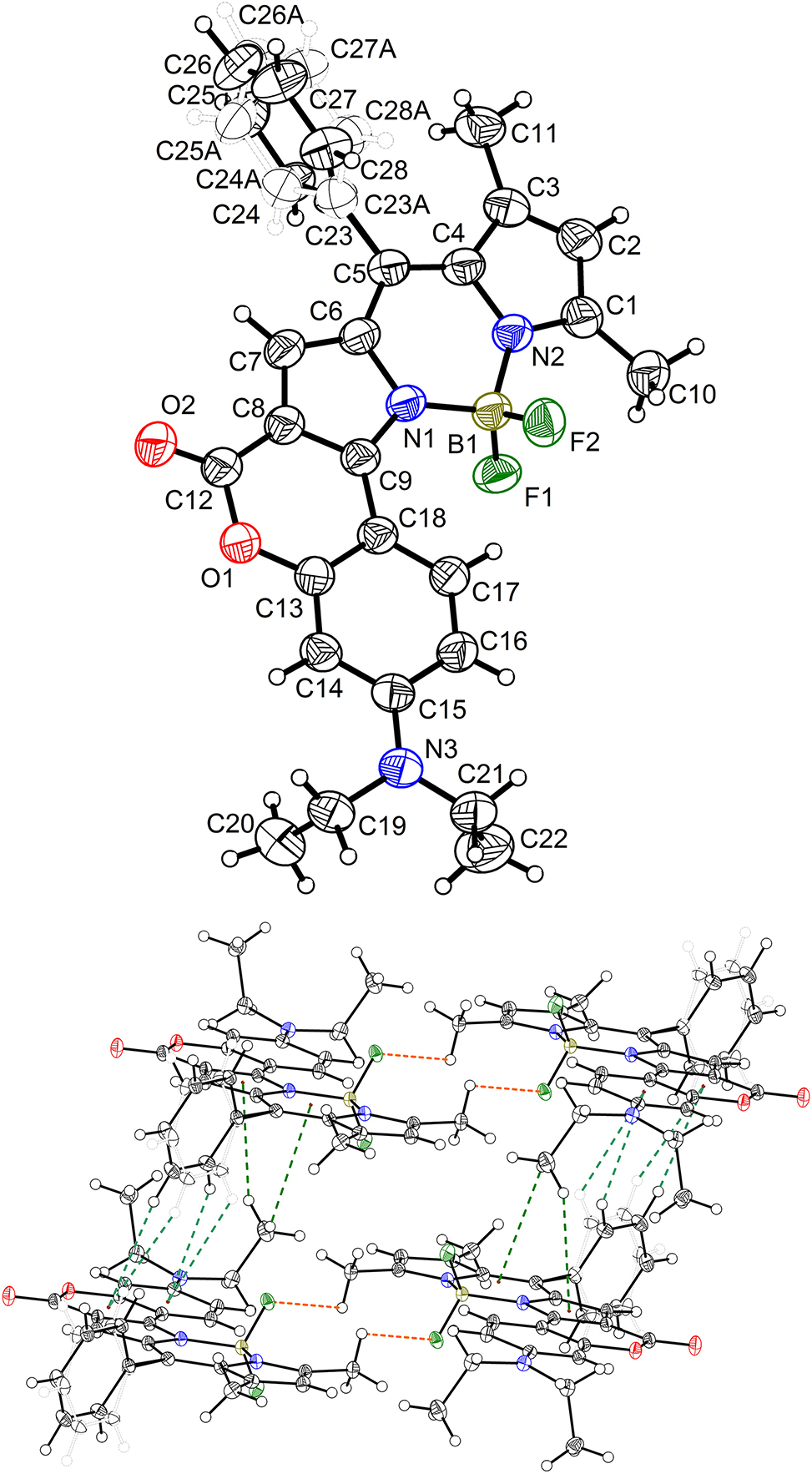
A view of the molecule. Displacement ellipsoids are drawn at the 50 % probability level and H atoms are shown as small spheres of arbitrary radii.
Data collection and handling.
| Crystal: | Clear reddish black block |
| Size: | 0.18 × 0.15 × 0.13 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.10 mm−1 |
| Diffractometer, scan mode: | Bruker SMART, ω scans |
| θmax, completeness: | 29.5°, 99 % |
| N(hkl)measured, N(hkl)unique, Rint: | 53008, 6517, 0.055 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 4,823 |
| N(param)refined: | 384 |
| Programs: | Bruker, 1 SHELX, 2 , 3 , 4 Olex2 5 |
1 Source of material
The title compound, 3-(diethylamino)-7,9,11-trimethyl-8-phenyl-6H,13H-12λ4,13λ4-chromeno[3′,4′:4,5]pyrrolo[1,2-c]pyrrolo[2,1-f][1,3,2]diazaborinin-6-one, was synthesized according to the literature method with a slight modification. 6 Under the argon atmosphere, 2-benzoylchromeno[4,3-b]pyrrol-4(1H)-one (650 mg, 1.8 mmol) was charged in a dry three-neck flask and mixed with dichloromethane (130 mL), 2,4-dimethylpyrrole (0.18 mL, 1.8 mmol), and phosphoryl chloride (0.17 mL, 1.8 mmol). The reaction mixture was stirred at room temperature for about 3 days. The reaction progress was monitored by thin-layer chromatography (TLC). When the TLC analysis indicated the complete consumption of 2-benzoylchromeno[4,3-b]pyrrol-4(1H)-one, triethylamine (2.5 mL) was added and stirring the mixture continuously for 15 min. Then, boron trifluoride etherate (BF3·Et2O, 2.5 mL) was added and the reaction mixture was stirred furtherly for more than 8 h at room temperature. The solution color turned to red. The mixture was quenched with distilled water (50 mL) and then it was extracted with dichloromethane (50 mL × 3), washed with distilled water (50 mL × 3). The collected organic phase was dried over MgSO4. The volatile component was evaporated under reduced pressure. The residue was purified by column chromatography on silica gel (200–300 mesh), using dichloromethane as the eluent. The crystal growth was carried in saturated dichloromethane/methanol (10:1) and the solution sealed in a 10 mL vial with parafilm. The number of holes on the parafilm was used to control the solvent evaporation speed. Finally, regular shaped crystals can be obtained and mounted for X-ray diffraction.
2 Experimental details
The single-crystal X-ray diffraction measurement of the title compound, 3-(diethylamino)-7,9,11-trimethyl-8-phenyl-6H,13H-12λ4,13λ4-chromeno[3′,4′:4,5]pyrrolo[1,2-c]pyrrolo[2,1-f][1,3,2]diazaborinin-6-one, was carried out on a Bruker D8 Venture. Data reduction was carried out using Appex5 and empirical absorption corrections were made using SADABS-2016/2. The structure was solved by using the programs of SHELX and refined by ShelXl through the Olex2 interface. 1 , 2 , 3 , 4 Hydrogen atoms attached to C atoms were placed geometrically and refined using a riding model approximation, assigned isotropic thermal parameters with d(C–H) = 0.93, 0.96 or 0.97 Å (–CH, –CH3, and –CH2). Uiso(H) = 1.2Ueq(C) for CH and Uiso(H) = 1.5Ueq(C) for CH3 and CH2 groups. 2 The benzene ring (C23/C24/C25/C26/C27/C28) attached at the meso-position of BODIPY was disordered due to the rotation around the single bond (C5–C23). Therefore, it was split to two parts with 54 % and 46 %, respectively. SAME and SIMU command were used to restrict the two disordered benzene rings to maintain regular geometry configuration. No other constraints or restraints were used in further data refining. The molecular graphics were drawn using DIAMOND software with 50 % probability ellipsoids in the figure (top).
3 Comment
The structure of the title compound incorporates the typical fluorescent coumarin and BODIPY (4,4-difluoro-4-bora-3a,4a-diaza-s-indacene) both. It combines the photo-character of two fluorophores and broadens the application in fluorescent sensors, fluorescent labeling of biomolecules and cells, photodynamic therapy, and light-emitting materials, etc. 7 , 8 , 9 , 10 , 11 The crystal structure demonstrates that the coumarin moiety fused with that of the BODIPY part and a significant red-shift in fluorescent emission maximum. The conjugate electron cloud could be delocalized to both coumarin and BODIPY part, which endows its unique character in fluorescent emission. 6
In the title crystal structure, the asymmetric unit contains only one molecules. The BODIPY part is coplanar with the RMSD in distance estimated to be 0.032 Å. However, the BODIPY and coumarin fused together through C8–C9 (1.408 Å), which is slightly longer than that of the classical carbon–carbon double bond (1.339 Å) and has the partial character of double bond. Therefore, the whole part of coumarin-BODIPY part fused by the partially π character of C8–C9 also becomes planarization with the RMSD 0.052 Å. This large conjugate system leads to a strong absorption shift toward the longer wavelength (around 600 nm). In addition, the bond length of N3–C15 is 1.365 Å, which is shorter than that of N3–C19 (1.463 Å) and N3–C21 (1.455 Å). It demonstrates that the electron rich N3 conjugated with the coumarin ring, which can effectively donate the electron toward the coumarin and BODIPY moiety. The electron donor (NEt2) and carbonyl group (C12/O2) configures a strong intramolecular electron “push–pull” effect, which determines the emission performance of coumarin-/BODIPY-containing dyes. 12 , 13 , 14 , 15 Additionally, the configuration of amino (N3/C15/C19/C21) does not construct the ideal trigonal pyramid geometry but coplanar with the RMSD 0.042 Å. It indicates that the lone pair electron of N3 partial involved in the bond formation of N3–C15 and leads to its partial π character, showing the electron donation of N3 to coumarin ring. The adjacent parallel molecules are fixed by the C–H⋯π interactions (figure bottom). The hydrogen atoms (H27/H27A/H28/H28A/H22A/H22C) locate directly above the carbon ring. The H⋯centroid distance ranged from 2.95 to 3.45 Å, which is well inside the interval 2.65 to 4.0 Å. 16 The C–H bonds point to the ring carbon rings center and corresponds to the type III geometry. In addition, typical C–H⋯O interactions are also found in the crystal. The C⋯O distances are 3.455 and 3.834 Å (C14⋯O2 and C19⋯O2). The hydrogen bonds configuration in the crystal is significant to uncover the principle of the solid emission behavior of the fluorescent dyes. 17 , 18 , 19 , 20 Therefore, reasonable introduction of inter- and/or intra-molecular hydrogen bonds into the crystal lattice is an effective methodology to inhibit the ration of non-radiative channels. 21 , 22 The existence of heteroatom O/N/S is key to configure intermolecular hydrogen bonds. 23 , 24 , 25 When there exist highly stronger intermolecular interactions, a significant emission quench will be unavoided. 26 , 27 , 28
The related crystal structures are mainly stabilized by the hydrogen bonds and C–H⋯π interactions. The hydrogen bonds join the adjacent molecules side by side. The hydrogen bond join the paralleled molecules to form the network. 29 , 30 , 31 Generally, the stronger hydrogen bonds are the determining factor to the optical properties of the compound in crystal state. 32 , 33 Therefore, it is important that the heteroatoms involved hydrogen bonds are key to the optical properties. 34 , 35 , 36 Both the bond lengths and the angles are in the expected ranges.
Acknowledgments
The authors appreciates the finical supporting by the Department of Science and Technology of Henan province (252102230148), the Young Backbone Teacher Training Objects of Colleges and Universities in Henan Province (2024GGJS154), the Henan Science and Technology Program (252102311246), the Young Core Instructor Training Program of Xinyang Agriculture and Forestry University (2023-9), Young Teachers' Research Fund Project of Xinyang Agriculture and Forestry University (QN20230024). The authors also gratefully thanks Prof. Xiaochuan Li (Henan Normal University) for his assistance in chemical purification, single crystal X-ray diffraction and his expertise on crystal structure solving.
References
1. BRUKER. SAINT, APEX2 and SADABS; Bruker AXS Inc.: Madison, Wisconsin, USA, 2009.Search in Google Scholar
2. Sheldrick, G. M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
3. Sheldrick, G. M. Acta Crystallorgr. 2008, A64, 112–122. https://doi.org/10.1107/s0108767307043930.Search in Google Scholar PubMed
4. Sheldrick, G. M. SHELXTL-Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. 2015, A71, 3–8. https://doi.org/10.1107/S2053273314026370.Search in Google Scholar PubMed PubMed Central
5. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
6. Bochkov, A. Y.; Akchurin, I. O. A.; Dyachenko, O. A.; Traven, V. F. NIR-Fluorescent Coumarin-Fused BODIPY Dyes with Large Stokes Shifts. Chem. Commun. 2013, 49, 11653–11655; https://doi.org/10.1039/c3cc46498a.Search in Google Scholar PubMed
7. Li, X.; Liu, X.; Li, F. Configuration of Super-Fast Cu2+-Responsive Chemosensor by Attaching Diaminomaleonitrile to BODIPY Scaffold for High-Contrast Fluorescence Imaging of Living Cells. Spectrochim. Acta, Part A 2024, 304, 123377; https://doi.org/10.1016/j.saa.2023.123377.Search in Google Scholar PubMed
8. Zheng, X.; Liu, X.; Liu, L.; Li, X.; Jiang, S.; Niu, C.; Xie, P.; Liu, G.; Cao, Z.; Ren, Y.; Qin, Y.; Wang, J. Multi-Stimuli-Induced Mechanical Bending and Reversible Fluorescence Switching in a Single Organic Crystal. Angew. Chem., Int. Ed. 2022, 61, e202113073; https://doi.org/10.1002/anie.202113073.Search in Google Scholar PubMed
9. Li, X.; Li, F.; Ji, G. A Fluorescent Turn-On Sensor toward Multiple Heavy Metal Ions Based on meso-Anisole Modified BODIPY Scaffold. J. Fluoresc. 2023, 33, 631–637; https://doi.org/10.1007/s10895-022-03110-1.Search in Google Scholar PubMed
10. Li, X.; Tian, G.; Shao, D.; Xu, Y.; Wang, Y.; Ji, G.; Ryu, J.; Son, Y. A. A BODIPY Based Emission Signal Turn-On Probe toward Multiple Heavy Metals. Mol. Cryst. Liq. Cryst. 2020, 706, 38–46; https://doi.org/10.1080/15421406.2020.1743436.Search in Google Scholar
11. Li, X.; Yao, C.; Jiang, W. Emission and Energy Transfer Investigation of Non-conjugated Total Carbon Configuration between BODIPY and Naphthalimide. J. Chem. Sci. 2023, 135, 65; https://doi.org/10.1007/s12039-023-02181-2.Search in Google Scholar
12. Li, X.; Liao, M.; Sun, J.; Heo, G.; Son, Y. A. Thiophene Modulated BODIPY Dye as a Light Harvester. Mol. Cryst. Liq. Cryst. 2019, 679, 127–136; https://doi.org/10.1080/15421406.2019.1597557.Search in Google Scholar
13. Liu, Y.; Li, X.; Min, K. S.; Son, Y. A. Emission Behavior of Naphthalimide-Coumarin Cassette. Mol. Cryst. Liq. Cryst. 2018, 662, 139–146; https://doi.org/10.1080/15421406.2018.1466533.Search in Google Scholar
14. Liu, Y.; Li, X.; Kim, H.; Son, Y. A. Investigation of Fluorescent Optical Properties of Fluorine-Boron Cored Dye. Mol. Cryst. Liq. Cryst. 2018, 677, 27–33; https://doi.org/10.1080/15421406.2019.1597508.Search in Google Scholar
15. Li, X.; Liu, X. A Sensitive Probe of Meso-cyanophenyl Substituted BODIPY Derivative as Fluorescent Chemosensor for the Detection of Multiple Heavy Metal Ions. J. Fluoresc. 2025, 35, 1089–1098; https://doi.org/10.1007/s10895-024-03581-4.Search in Google Scholar PubMed
16. Malone, J. F.; Murray, C. M.; Charlton, M. H.; Docherty, R.; Lavery, A. J. X–H⋯π (Phenyl) Interactions Theoretical and Crystallographic Observations. J. Chem. Soc. Faraday Trans. 1997, 93, 3429–3436; https://doi.org/10.1039/a700669a.Search in Google Scholar
17. Xie, P.; Zhou, Y.; Li, X.; Liu, X.; Liu, L.; Cao, Z.; Wang, J.; Zheng, X. Strong Dual-State Emission of Unsymmetrical and Symmetrical Thiazolothiazole-Bridged Imidazolium Salts. Chin. Chem. Lett. 2023, 34, 107582; https://doi.org/10.1016/j.cclet.2022.06.005.Search in Google Scholar
18. Cao, Z.; Yang, F.; Wu, D.; Wu, L.; Liu, L.; Liu, G.; Li, X.; Zheng, X.; Zheng, X.; Qu, D. Supramolecular Aggregates Constructed by Pillar[5]arene-Based Host–Guest Interaction with Aggregation-Induced Emission. Polym. Chem. 2023, 14, 1318–1322; https://doi.org/10.1039/d3py00026e.Search in Google Scholar
19. Li, X.; Zhou, Q.; Heo, G.; Son, Y. A. 2,4-Dimethylpyrrole Configured Fluorine-Boron Complexes. Mol. Cryst. Liq. Cryst. 2018, 677, 34–41; https://doi.org/10.1080/15421406.2019.1597509.Search in Google Scholar
20. Li, X.; Guo, X.; Chen, Y.; Cui, T.; Xing, L. Double 3-Ethyl-2,4-Dimethylpyrrole Configured Fluorescent Dye with Fluorine-Boron as the Bridge. J. Fluoresc. 2021, 31, 1797–1803; https://doi.org/10.1007/s10895-021-02819-9.Search in Google Scholar PubMed
21. Li, X.; Han, Y.; Sun, S.; Shan, D.; Ma, X.; He, G.; Mergu, N.; Park, J.-S.; Kim, C.-H.; Son, Y. A. A Diaminomaleonitrile-Appended BODIPY Chemosensor for the Selective Detection of Cu2+ via Oxidative Cyclization and Imaging in Siha Cells and Zebrafish. Spectrochim. Acta, Part A 2020, 233, 118179; https://doi.org/10.1016/j.saa.2020.118179.Search in Google Scholar PubMed
22. He, W.; Li, X.; Kim, H.; Son, Y. A. Shifting the Emission of Proton Transfer Fluorescence with Fluorine-Boron as the Rotation Lock. Mol. Cryst. Liq. Cryst. 2020, 704, 41–47; https://doi.org/10.1080/15421406.2020.1741800.Search in Google Scholar
23. Kong, Y.; Liu, X.; Li, X. Photochromic Properties of Triangle Terthiopheneand Triphenylamine‑configured Diarylethene Type Dye with Propeller‑like Conformation. Chem. Pap. 2025, 79, 2401–2409; https://doi.org/10.1007/s11696-025-03934-8.Search in Google Scholar
24. Li, X.; Cai, Q.; Zhang, J.; Kim, H.; Son, Y. A. An “Electron Lock” toward the Photochromic Activity of Phenylacetylene Appended Bisthienylethene. Mol. Cryst. Liq. Cryst. 2020, 706, 141–149; https://doi.org/10.1080/15421406.2020.1743450.Search in Google Scholar
25. Li, X.; Zou, Y.; Heo, G.; Son, Y. A. Emission Shift of an Imidazole Bridged Diethylaminocoumarin and Diphenyl. Mol. Cryst. Liq. Cryst. 2020, 704, 48–56; https://doi.org/10.1080/15421406.2020.1741801.Search in Google Scholar
26. Ji, G.; Hou, Q.; Jiang, W.; Li, X. Investigating the Properties of Double Triangle Terthiophene Configured Dumbbell-Like Photochromic Dye with Ethyne and 1,3-Butadiene Bridge. J. Fluoresc. 2023, 33, 1495–1503; https://doi.org/10.1007/s10895-023-03171-w.Search in Google Scholar PubMed
27. Zheng, X.; Wang, G.; Liu, L.; Li, X.; Xie, P.; Fan, Y.; Cao, Z.; Ni, C.; Tian, D.; Xie, L. Hydrogen Bonding–Induced Multicolor and Thermochromic Emissions of Triphenylamines. Chem. -Eur. J. 2025, 31, e202500643; https://doi.org/10.1002/chem.202500643.Search in Google Scholar PubMed
28. Li, X.; Qian, Q.; Jiang, W. Photo-Induced Fluorochromism of a Star-Shaped Photochromic Dye with 2,4-Dimethylthiazole Attaching to Triangle Terthiophene. J. Fluoresc. 2023, 33, 1907–1915; https://doi.org/10.1007/s10895-023-03196-1.Search in Google Scholar PubMed
29. Liu, Y.; Li, X.; Sun, S.; Ji, G.; Son, Y. A. Crystal Structure of 2,7-Diiodo-1,3,6,8-tetramethyl-bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl)methylene)hydrazine, C14H14B2F4I2N4. Z. Kristallogr. -New Cryst. Struct. 2020, 235, 371–372; https://doi.org/10.1515/ncrs-2019-0678.Search in Google Scholar
30. He, W.; Liu, Y.; Sun, S.; Ji, G.; Li, X. Crystal Structure of 2-Bromo-1,3,6,8-tetramethylBOPHY (BOPHY = bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl)methylene)hydrazine), C14H15B2BrF4N4. Z. Kristallogr. -New Cryst. Struct. 2021, 236, 949–952; https://doi.org/10.1515/ncrs-2021-0163.Search in Google Scholar
31. Liu, Y.; Sun, S.; Ji, G.; Li, X.; Son, Y. A. Crystal Structure of 2-Phenylethynyl-1,3,6,8-tetramethylBOPHY (BOPHY = bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl)methylene)hydrazine), C22H20B2F4N4. Z. Kristallogr. -New Cryst. Struct. 2021, 236, 749–752; https://doi.org/10.1515/ncrs-2021-0074.Search in Google Scholar
32. Ji, G.; Hou, Q.; Jiang, W.; Li, X. Investigating the Properties of Triangle Terthiophene and Triphenylamine Configured Propeller‑like Photochromic Dye with Ethyne Bridge. J. Fluoresc. 2025, 35, 933–941; https://doi.org/10.1007/s10895-023-03557-w.Search in Google Scholar PubMed
33. Qin, X.; Li, H.; Wang, Y.; Li, Y.; Li, X. Conjugated Iminodibenzyl Dyes Incorporating Phenolic Hydroxyl Group and Strong Electron Donating or Accepting Groups for Facilitating ESIPT and Proton Transfer in Six‑ or Seven‑Membered Cycles. J. Fluoresc. 2025. https://doi.org/10.1007/s10895-025-04285-z, In press.Search in Google Scholar PubMed
34. Ji, G.; Hou, Q.; Zhang, J.; Li, X. Investigation of Triangle Terthiophene and Hydroxyphenylbenzothiazole Configured Fluorescent Dye with a Triple Bond Bridge. J. Fluoresc. 2023, 33, 153–159; https://doi.org/10.1007/s10895-022-03049-3.Search in Google Scholar PubMed
35. Li, X.; Wang, Y.; Jia, C.; Kim, H.; Son, Y. A. Photochromic Reactivity Induced by Electron Distribution: Active or Inactive. Mol. Cryst. Liq. Cryst. 2019, 689, 83–91; https://doi.org/10.1080/15421406.2019.1597556.Search in Google Scholar
36. Li, X.; Han, Y.; Min, K.; Son, Y. A. Configuration of White Light Emission by Courmarin and Naphthalimide. Mol. Cryst. Liq. Cryst. 2018, 660, 10–16; https://doi.org/10.1080/15421406.2018.1452861.Search in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of (S)-N-(10-((2,2-dimethoxyethyl)amino)-1,2,3-trimethoxy-9-oxo-5,6,7,9-tetrahydrobenzo[a]heptalen-7-yl)acetamide, C25H32N2O7
- The crystal structure of 6,6′-difluoro-3,3′-dimethyl-5,5′-di(10H-phenoxazin-10-yl)- [1,1′-biphenyl]-2,2′-dicarbonitrile, C40H24F2N4O2
- Crystal structure of poly[(di-ethylenediamine-κ2N,N′)cadmium(II) tetradedocyloxidohexavanadate] (V4+/V5+ = 2/1), C4H16CdN4O14V6
- The crystal structure of poly[bis(dimethylformamide-κ1N)-(μ4-2′,3,3″,5′-tetrakis(trifluoromethyl)-[1,1′:4′,1″-terphenyl]-4,4″-dicarboxylato-κ4 O,O′: O″,O‴)dicadmium(II)], C27H15CdF12NO5
- Crystal structure of bis(μ2-ferrocenylcarboxylato-O,O′)-(μ3-oxido-κ3O:O:O)-bis(μ2-salicyladoximato-κ2N,O,O′)-(μ2-isopropoxo)-tris(isopropoxy-κ1O trititanium(IV)), C48H55N2O13Fe2Ti3
- Crystal structure of 3-(diethylamino)-7,9,11-trimethyl-8-phenyl-6H,13H-12λ4,13λ4-chromeno[3′,4′:4,5]pyrrolo[1,2-c]pyrrolo[2,1-f][1,3,2]diazaborinin-6-one, C28H26BF2N3O2
- The crystal structure of catena-poly[aqua-μ2-2-nitro-benzene-1,3-dicarboxylato-κ2O,O′)-(1,10-phenanthroline-κ2N,N′)-zinc(II)], C20H13N3O7Zn
- Crystal structure of poly[diaqua-{μ3-1-(3-carboxylatophenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylato-κ4O,O′:O′′:O′′′′}manganese(II)] hydrate
- Crystal structure of N′-((1-hydroxycyclohexyl)(phenyl)methyl)-2-methoxybenzohydrazide methanol solvate, C22H28N2O4
- The cocrystal of caffeic acid — progesterone — water (1/2/1), C51H70O9
- Crystal structure of (((oxido(quinolin-6-yl)methoxy)triphenyl-λ5-stibanyl)oxy)(quinolin-7-yl)methanolate
- Crystal structure of [(E)-6′-(diethylamino)-2-(2-(((E)-pyren-1-ylmethylene)amino)ethyl)-4′-(2-((E)-1,3,3-trimethylindolin-2-ylidene)ethylidene)-1′,2′,3′,4′-tetrahydrospiro[isoindoline-1,9′-xanthen]-3-one]-methanol, solvate C57H56N4O3
- The crystal structure of 1-(acridin-9-yl)pyrrolidine-2,5-dione, C17H22N2O2
- Crystal structure of N-(4-acetylphenyl)-2-(6-methoxynaphthalen-2-yl)propanamide, C22H21NO3
- The crystal structure of 5,10,15,20-tetrakis(4-(1H-1,2,4-triazol-1-yl)phenyl)porphyrin, C52H34N16
- Crystal structure of hexacarbonyl-μ2-[phenylmethanedithiolato-κ4S:S,S′:S′]diiron (Fe–Fe) C13H6Fe2O6S2
- Crystal structure of diiodo-bis(1-((2-propyl-1H-benzo[d]imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κ1N)cadmium(II), C34H34CdI2N10
- Crystal structure of (E)-(3-(3-bromophenyl)acryloyl)ferrocene, C19H15BrFeO
- Crystal structure of catena-poly(μ2-6-chloropyridine-2-carboxylato-κ3N,O:O′)(6-chloropyridine-2-carboxylato-κ2O,N)copper(II), C12H6Cl2N2O4Cu
- Crystal structure of poly[diaqua-μ 3-(5-(3,5-dicarboxy-2,4,6-trimethylbenzyl)-2,4,6-trimethylisophthalato)-κ 6O,O′:O″,O‴:O‴′,O‴″) terbium(III)-monohydrate], C23H28TbO12
- Crystal structure of (E)-2-(((5-chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)methylene)amino)-3′,6′-dihydroxyspiro[isoindoline-1,9′-xanthen]-3-one – ethanol (1/2), C35H33ClN4O6
- The crystal structure of 3-(5-amino-3-phenylisoxazol-4-yl)-4-chloro-3-hydroxyindolin-2-one, C17H12ClN3O3
- The crystal structure of dimethylammonium 4-[2-(4-fluorophenyl)-4, 5-diphenyl-1H-imidazol-1-yl]benzenesulfonate, C29H26FN3O3S
- Crystal structure of (R)-2-ammonio-3-((5-carboxypentyl)thio)propanoate
- Crystal structure of 4-cyclohexyl-5-(thiophen-2-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione, C12H15N3S2
- The crystal structure of 4,6-bis(dimethylamino)-2-fluoroisophthalonitrile, C12H13FN4
- Hydrogen bonding in the crystal structure of nicotin-1,1′-dium tetrabromidomanganate(II)
- The crystal structure of bis(2-bromobenzyl)(2-((2-oxybenzylidene)amino)-4-methylpentanoato-κ3N, O,O′)tin(IV), C27H27Br2NO3Sn
- Crystal structure of (E)-(3-(p-tolyl)acryloyl)ferrocene, C20H18FeO
- Crystal structure of (E)-7-fluoro-2-((5-(4-methylpiperazin-1-yl)pyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C21H22FN3O
- Crystal structure of (E)-7-methoxy-2-((5-(4-methylpiperazin-1-yl)pyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C22H25N3O2
- The crystal structure of poly(bis(μ2-1,3,5-tri(1H-imidazol-1-yl)benzene-κ2N:N′)-(μ2-2,3,5,6-tetrafluoroterephthalato-κ2O:O′)-manganese(II), C38H24F4N12O4Mn
- Crystal structure of (3,4-dimethoxybenzyl)triphenylphosphonium bromide ethanol solvate, C29H32BrO3P
- Crystal structure of tetraethylammonium hydrogencarbonate – (diaminomethylene)thiourea – water (2/1/3)
- Crystal structure of N, N-Dimethyl-N′-tosylformimidamide, C10H14N2O2S
- The crystal structure of ethyl 2-methyl-5-oxo-4-(2-methoxyphenyl)-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C20H23N2O4
- Crystal structure of bis(μ2-1,5-bis[(E)-1-(2-hydroxyphenyl)ethylidene] thiocarbonohydrazide)-bis(dimethylformamide)-dizinc(II) dimethylformamide solvate, C40H46N10O6S2Zn2⋅C3H7NO
- Crystal structure of azido-κ1N{hydridotris(3-tert-butyl-5-methylpyrazol-1-yl)borato-κ3N,N′,N″}copper(II), C24H40BCuN9
- The crystal structure of fac-tricarbonyl(1,10-phenanthroline-κ2N,N′)-(azido-κ1N)rhenium(I), C15H8N5O3Re
- Crystal structure of 4-((triphenylphosphonio)methyl)pyridin-1-ium tetrachloridozincate(II), C24H22Cl4NPZn
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of (S)-N-(10-((2,2-dimethoxyethyl)amino)-1,2,3-trimethoxy-9-oxo-5,6,7,9-tetrahydrobenzo[a]heptalen-7-yl)acetamide, C25H32N2O7
- The crystal structure of 6,6′-difluoro-3,3′-dimethyl-5,5′-di(10H-phenoxazin-10-yl)- [1,1′-biphenyl]-2,2′-dicarbonitrile, C40H24F2N4O2
- Crystal structure of poly[(di-ethylenediamine-κ2N,N′)cadmium(II) tetradedocyloxidohexavanadate] (V4+/V5+ = 2/1), C4H16CdN4O14V6
- The crystal structure of poly[bis(dimethylformamide-κ1N)-(μ4-2′,3,3″,5′-tetrakis(trifluoromethyl)-[1,1′:4′,1″-terphenyl]-4,4″-dicarboxylato-κ4 O,O′: O″,O‴)dicadmium(II)], C27H15CdF12NO5
- Crystal structure of bis(μ2-ferrocenylcarboxylato-O,O′)-(μ3-oxido-κ3O:O:O)-bis(μ2-salicyladoximato-κ2N,O,O′)-(μ2-isopropoxo)-tris(isopropoxy-κ1O trititanium(IV)), C48H55N2O13Fe2Ti3
- Crystal structure of 3-(diethylamino)-7,9,11-trimethyl-8-phenyl-6H,13H-12λ4,13λ4-chromeno[3′,4′:4,5]pyrrolo[1,2-c]pyrrolo[2,1-f][1,3,2]diazaborinin-6-one, C28H26BF2N3O2
- The crystal structure of catena-poly[aqua-μ2-2-nitro-benzene-1,3-dicarboxylato-κ2O,O′)-(1,10-phenanthroline-κ2N,N′)-zinc(II)], C20H13N3O7Zn
- Crystal structure of poly[diaqua-{μ3-1-(3-carboxylatophenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylato-κ4O,O′:O′′:O′′′′}manganese(II)] hydrate
- Crystal structure of N′-((1-hydroxycyclohexyl)(phenyl)methyl)-2-methoxybenzohydrazide methanol solvate, C22H28N2O4
- The cocrystal of caffeic acid — progesterone — water (1/2/1), C51H70O9
- Crystal structure of (((oxido(quinolin-6-yl)methoxy)triphenyl-λ5-stibanyl)oxy)(quinolin-7-yl)methanolate
- Crystal structure of [(E)-6′-(diethylamino)-2-(2-(((E)-pyren-1-ylmethylene)amino)ethyl)-4′-(2-((E)-1,3,3-trimethylindolin-2-ylidene)ethylidene)-1′,2′,3′,4′-tetrahydrospiro[isoindoline-1,9′-xanthen]-3-one]-methanol, solvate C57H56N4O3
- The crystal structure of 1-(acridin-9-yl)pyrrolidine-2,5-dione, C17H22N2O2
- Crystal structure of N-(4-acetylphenyl)-2-(6-methoxynaphthalen-2-yl)propanamide, C22H21NO3
- The crystal structure of 5,10,15,20-tetrakis(4-(1H-1,2,4-triazol-1-yl)phenyl)porphyrin, C52H34N16
- Crystal structure of hexacarbonyl-μ2-[phenylmethanedithiolato-κ4S:S,S′:S′]diiron (Fe–Fe) C13H6Fe2O6S2
- Crystal structure of diiodo-bis(1-((2-propyl-1H-benzo[d]imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κ1N)cadmium(II), C34H34CdI2N10
- Crystal structure of (E)-(3-(3-bromophenyl)acryloyl)ferrocene, C19H15BrFeO
- Crystal structure of catena-poly(μ2-6-chloropyridine-2-carboxylato-κ3N,O:O′)(6-chloropyridine-2-carboxylato-κ2O,N)copper(II), C12H6Cl2N2O4Cu
- Crystal structure of poly[diaqua-μ 3-(5-(3,5-dicarboxy-2,4,6-trimethylbenzyl)-2,4,6-trimethylisophthalato)-κ 6O,O′:O″,O‴:O‴′,O‴″) terbium(III)-monohydrate], C23H28TbO12
- Crystal structure of (E)-2-(((5-chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)methylene)amino)-3′,6′-dihydroxyspiro[isoindoline-1,9′-xanthen]-3-one – ethanol (1/2), C35H33ClN4O6
- The crystal structure of 3-(5-amino-3-phenylisoxazol-4-yl)-4-chloro-3-hydroxyindolin-2-one, C17H12ClN3O3
- The crystal structure of dimethylammonium 4-[2-(4-fluorophenyl)-4, 5-diphenyl-1H-imidazol-1-yl]benzenesulfonate, C29H26FN3O3S
- Crystal structure of (R)-2-ammonio-3-((5-carboxypentyl)thio)propanoate
- Crystal structure of 4-cyclohexyl-5-(thiophen-2-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione, C12H15N3S2
- The crystal structure of 4,6-bis(dimethylamino)-2-fluoroisophthalonitrile, C12H13FN4
- Hydrogen bonding in the crystal structure of nicotin-1,1′-dium tetrabromidomanganate(II)
- The crystal structure of bis(2-bromobenzyl)(2-((2-oxybenzylidene)amino)-4-methylpentanoato-κ3N, O,O′)tin(IV), C27H27Br2NO3Sn
- Crystal structure of (E)-(3-(p-tolyl)acryloyl)ferrocene, C20H18FeO
- Crystal structure of (E)-7-fluoro-2-((5-(4-methylpiperazin-1-yl)pyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C21H22FN3O
- Crystal structure of (E)-7-methoxy-2-((5-(4-methylpiperazin-1-yl)pyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C22H25N3O2
- The crystal structure of poly(bis(μ2-1,3,5-tri(1H-imidazol-1-yl)benzene-κ2N:N′)-(μ2-2,3,5,6-tetrafluoroterephthalato-κ2O:O′)-manganese(II), C38H24F4N12O4Mn
- Crystal structure of (3,4-dimethoxybenzyl)triphenylphosphonium bromide ethanol solvate, C29H32BrO3P
- Crystal structure of tetraethylammonium hydrogencarbonate – (diaminomethylene)thiourea – water (2/1/3)
- Crystal structure of N, N-Dimethyl-N′-tosylformimidamide, C10H14N2O2S
- The crystal structure of ethyl 2-methyl-5-oxo-4-(2-methoxyphenyl)-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C20H23N2O4
- Crystal structure of bis(μ2-1,5-bis[(E)-1-(2-hydroxyphenyl)ethylidene] thiocarbonohydrazide)-bis(dimethylformamide)-dizinc(II) dimethylformamide solvate, C40H46N10O6S2Zn2⋅C3H7NO
- Crystal structure of azido-κ1N{hydridotris(3-tert-butyl-5-methylpyrazol-1-yl)borato-κ3N,N′,N″}copper(II), C24H40BCuN9
- The crystal structure of fac-tricarbonyl(1,10-phenanthroline-κ2N,N′)-(azido-κ1N)rhenium(I), C15H8N5O3Re
- Crystal structure of 4-((triphenylphosphonio)methyl)pyridin-1-ium tetrachloridozincate(II), C24H22Cl4NPZn

