The crystal structure of 5′-(furan-2-yl)-3′-((4-methylphenyl)sulfonamido)-3′,4′,5′,6′-tetrahydro-[1,1′:3′,1″-terphenyl]-4′-carboxylic acid, C30H27NO5S
Abstract
C30H27NO5S, monoclinic, P21 (no. 4), a = 10.4329(16) Å, b = 11.6208(18) Å, c = 11.8268(18) Å, β = 114.257(5)°, V = 1307.3(4) Å3, Z = 2, R gt (F) = 0.0685, wR ref (F2) = 0.1547, T = 273(2) K.
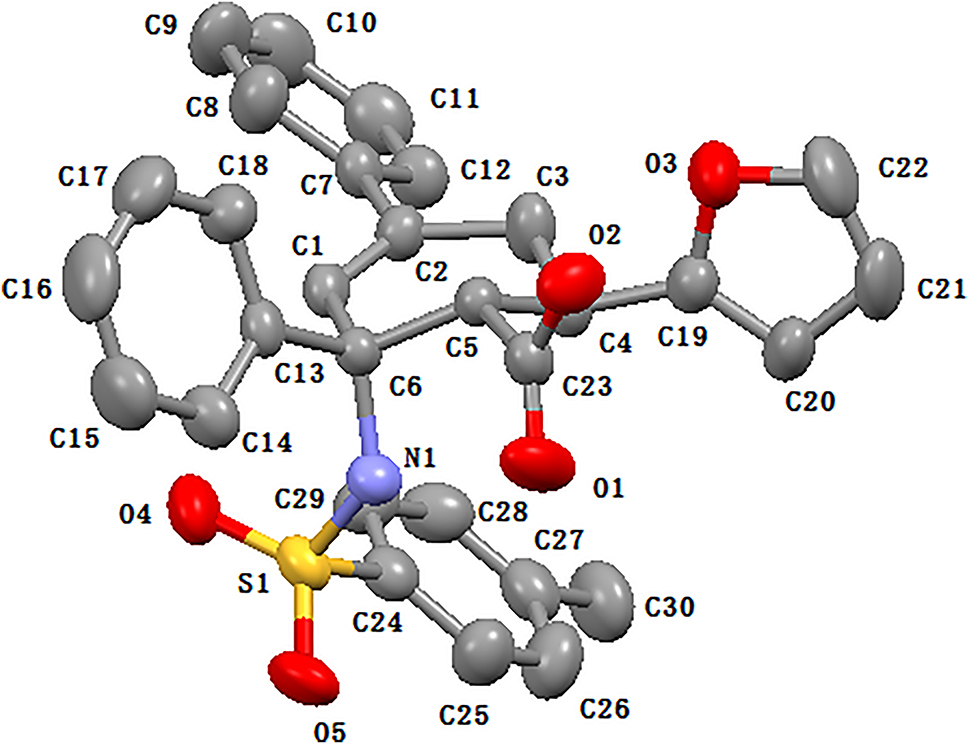
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Yellow needle |
| Size: | 0.24 × 0.19 × 0.13 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.17 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II |
| θmax, completeness: | 32.1°, 99 % |
| N (hkl)measured, N(hkl)unique, Rint: | 16,448, 8845, 0.059 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2σ(Iobs), 4334 |
| N(param)refined: | 336 |
| Programs: | Bruker [1], SHELX [2, 3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1 | 0.2876 (4) | 0.3462 (3) | 0.5984 (4) | 0.0388 (9) |
| C2 | 0.2714 (4) | 0.4295 (3) | 0.5169 (4) | 0.0404 (9) |
| C3 | 0.2906 (5) | 0.5542 (4) | 0.5545 (4) | 0.0513 (11) |
| C4 | 0.3520 (5) | 0.5723 (3) | 0.6941 (4) | 0.0428 (10) |
| C5 | 0.2820 (4) | 0.4877 (3) | 0.7527 (4) | 0.0355 (9) |
| C6 | 0.3209 (4) | 0.3623 (3) | 0.7354 (3) | 0.0350 (8) |
| C7 | 0.2313 (4) | 0.4042 (3) | 0.3840 (4) | 0.0395 (9) |
| C8 | 0.1254 (5) | 0.3255 (4) | 0.3217 (5) | 0.0592 (13) |
| C9 | 0.0868 (6) | 0.3023 (4) | 0.1963 (5) | 0.0717 (16) |
| C10 | 0.1545 (6) | 0.3566 (5) | 0.1330 (5) | 0.0699 (15) |
| C11 | 0.2582 (6) | 0.4341 (5) | 0.1937 (5) | 0.0622 (14) |
| C12 | 0.2948 (5) | 0.4589 (4) | 0.3170 (4) | 0.0496 (11) |
| C13 | 0.2345 (4) | 0.2771 (3) | 0.7752 (4) | 0.0382 (9) |
| C14 | 0.2943 (5) | 0.2068 (4) | 0.8767 (5) | 0.0526 (12) |
| C15 | 0.2132 (7) | 0.1312 (5) | 0.9112 (6) | 0.0708 (16) |
| C16 | 0.0712 (7) | 0.1258 (5) | 0.8425 (6) | 0.0722 (16) |
| C17 | 0.0107 (6) | 0.1961 (5) | 0.7416 (6) | 0.0672 (15) |
| C18 | 0.0911 (5) | 0.2704 (4) | 0.7071 (4) | 0.0534 (11) |
| C19 | 0.3430 (5) | 0.6956 (4) | 0.7277 (4) | 0.0474 (11) |
| C20 | 0.4383 (5) | 0.7724 (4) | 0.7932 (4) | 0.0585 (12) |
| C21 | 0.3669 (7) | 0.8738 (4) | 0.7985 (5) | 0.0702 (15) |
| C22 | 0.2322 (7) | 0.8511 (4) | 0.7373 (5) | 0.0727 (16) |
| C23 | 0.3188 (5) | 0.5232 (3) | 0.8844 (4) | 0.0419 (10) |
| C24 | 0.6632 (4) | 0.3420 (4) | 0.7127 (4) | 0.0443 (10) |
| C25 | 0.7755 (5) | 0.4122 (4) | 0.7806 (5) | 0.0591 (13) |
| C26 | 0.8407 (6) | 0.4761 (5) | 0.7209 (6) | 0.0685 (15) |
| C27 | 0.7947 (5) | 0.4712 (4) | 0.5932 (5) | 0.0545 (12) |
| C28 | 0.6822 (5) | 0.3999 (5) | 0.5281 (5) | 0.0587 (13) |
| C29 | 0.6164 (5) | 0.3352 (4) | 0.5867 (4) | 0.0528 (12) |
| C30 | 0.8677 (6) | 0.5359 (5) | 0.5261 (5) | 0.0756 (16) |
| H1A | 0.507903 | 0.391914 | 0.876635 | 0.049* |
| H2 | 0.917621 | 0.600226 | 0.575578 | 0.113* |
| H2A | 0.247937 | 0.613197 | 0.965108 | 0.084* |
| H3 | 0.200114 | 0.592562 | 0.517648 | 0.062* |
| H3A | 0.932782 | 0.485673 | 0.512137 | 0.113* |
| H4 | 0.916345 | 0.523085 | 0.767013 | 0.082* |
| H5 | 0.807135 | 0.416459 | 0.866332 | 0.071* |
| H5A | 0.179995 | 0.495575 | 0.707244 | 0.043* |
| H6 | 0.390914 | 0.209818 | 0.923429 | 0.063* |
| H7 | 0.255383 | 0.084507 | 0.980587 | 0.085* |
| H8 | 0.048270 | 0.316468 | 0.637284 | 0.064* |
| H9 | −0.086078 | 0.193629 | 0.695622 | 0.081* |
| H10 | 0.016289 | 0.074863 | 0.864225 | 0.087* |
| H10A | 0.129781 | 0.340611 | 0.049552 | 0.084* |
| H11 | 0.015171 | 0.249965 | 0.155434 | 0.086* |
| H12 | 0.079843 | 0.287904 | 0.364157 | 0.071* |
| H13 | 0.304789 | 0.470599 | 0.151492 | 0.075* |
| H14 | 0.363841 | 0.513776 | 0.355923 | 0.060* |
| H15 | 0.352032 | 0.589987 | 0.521622 | 0.062* |
| H16 | 0.160196 | 0.902788 | 0.727204 | 0.087* |
| H17 | 0.406781 | 0.942531 | 0.837317 | 0.084* |
| H18 | 0.535174 | 0.761398 | 0.829318 | 0.070* |
| H19 | 0.451920 | 0.552272 | 0.726321 | 0.051* |
| H21 | 0.277685 | 0.270986 | 0.569149 | 0.047* |
| H22 | 0.541235 | 0.287538 | 0.541057 | 0.063* |
| H23 | 0.649795 | 0.395437 | 0.442316 | 0.070* |
| H24 | 0.799205 | 0.563059 | 0.448008 | 0.113* |
| N1 | 0.4729 (3) | 0.3484 (3) | 0.8123 (3) | 0.0410 (8) |
| O1 | 0.4316 (3) | 0.5073 (3) | 0.9683 (3) | 0.0660 (10) |
| O2 | 0.2166 (3) | 0.5799 (3) | 0.8981 (3) | 0.0560 (8) |
| O3 | 0.2119 (3) | 0.7415 (3) | 0.6905 (3) | 0.0607 (9) |
| O4 | 0.5042 (3) | 0.1702 (2) | 0.7112 (3) | 0.0586 (9) |
| O5 | 0.6877 (3) | 0.2360 (3) | 0.9114 (3) | 0.0580 (9) |
| S1 | 0.58051 (11) | 0.26194 (8) | 0.79045 (11) | 0.0438 (3) |
1 Source of materials
The title compound was obtained via the following synthetic procedure: N-((E)-1,3-diphenylbut-2-en-1-ylidene)-4-methylbenzenesulfonamide (0.2 mmol, 1 eq) and (E)-3-(furan-2-yl)acrylaldehyde (2 eq) were added into a dry Schlenk tube, which was filled with (R)-diphenylprolinol trimethyl silyl ether (0.2 eq), dipotassium phosphate (1 eq), monopotassium phosphate (1 eq) and sodium chloride (1 eq). The tube was heated at 60 °C to initiate the reaction under nitrogen atmosphere with 2 mL of MeOH/H2O (99:1) as solvent. After 12 h, the reaction mixture was separated over silica gel chromatography eluted with petroleum ether/ethyl acetate (7:1) to obtain the intermediate in 55 % yield, which was further transformed to the title compound in 64 % yield using KMnO4 (2 eq) as the oxidant. The structure of the title compound was determined by NMR analyses before XRD analyses. The yellow needles were obtained via recrystallization in dichloromethane/ethyl acetate.
2 Experimental details
The structure was solved and refined using the Bruker SHELXTL Software Package [2, 3].
3 Comment
The title compound belongs to cyclic β-amino acids characterized by well-defined and controllable conformational preferences, demonstrating its potential as building blocks in the field of foldamer research [4, 5]. Additionally, cyclic β-amino acids also exhibited various bioactivities such as anti-tumor and anti-influenza [6], [7], [8]. Thus, it has attracted an increased interest in the past two decades, and several effective methods have been developed to access cyclic β-amino acids [9, 10].
There is one crystallographically independent molecule in the asymmetric unit of the title compound with all geometric parameters in normal ranges [11]. The cyclohexene skeleton was shown in its half-chair conformation with C1–C2–C3–C6 almost in the same plane. The dihedral angle between phenyl plane at C2 with C1–C2–C3 plane was determined as 44.5°. Only one intramolecular hydrogen bond was observed between N1–H1A and O1 with donor–acceptor distance of 2.078 Å, and the molecules were packed through the intermolecular hydrogen bond between O2–H2A from the carboxyl group with O5 from the sulfonyl group with donor-acceptor distance of 1.953 Å.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
-
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: Scientific and Technological Outstanding Talent Plan from Educational Commission of Guizhou Province (QJJ[2022]082), and the 1000 Talent Plan (Qian Cengci) of Guizhou Province (GZY[ZQ2018002]).
References
1. Bruker. APEX2 Ver 2.0-1; Bruker AXS Inc.: Madison, Wisconsin, USA, 2005.Suche in Google Scholar
2. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8.10.1107/S2053273314026370Suche in Google Scholar PubMed PubMed Central
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar
4. Appella, D. H., Christianson, L. A., Klein, D. A., Powell, D. R., Huang, X., Barchi, J. J., Gellman, S. H. Residue-based control of helix shape in β-peptide oligomers. Nature 1997, 387, 381–384; https://doi.org/10.1038/387381a0.Suche in Google Scholar PubMed
5. Choi, S. H., Ivancic, M., Guzei, I. A., Gellman, S. H. Structural characterization of peptide oligomers containing (1R,2S)-2-aminocyclohexanecarboxylic acid (cis-ACHC). Eur. J. Org Chem. 2013, 2013, 3464–3469; https://doi.org/10.1002/ejoc.201300118.Suche in Google Scholar
6. Cheng, M., Quail, M. R., Gingrich, D. E., Ott, G. R., Lu, L., Wan, W., Albom, M. S., Angeles, T. S., Aimone, L. D., Cristofani, F., Machiorlatti, R., Abele, C., Ator, M. A., Dorsey, B. D., Inghirami, G., Ruggeri, B. A. CEP-28122, a highly potent and selective orally active inhibitor of anaplastic lymphoma kinase with antitumor activity in experimental models of human cancers. Mol. Cancer Ther. 2012, 11, 670–679; https://doi.org/10.1158/1535-7163.mct-11-0776.Suche in Google Scholar
7. Chang, L. L., Truong, Q., Doss, G. A., MacCoss, M., Lyons, K., McCauley, E., Mumford, R., Forrest, G., Vincent, S., Schmidt, J. A., Hagmann, W. K. Highly constrained bicyclic VLA-4 antagonists. Bioorg. Med. Chem. Lett. 2007, 17, 597–601; https://doi.org/10.1016/j.bmcl.2006.11.011.Suche in Google Scholar PubMed
8. Feng, T., Li, Y., Wang, Y. Y., Cai, X. H., Liu, Y. P., Luo, X. D. Cytotoxic indole alkaloids from Melodinus tenuicaudatus. J. Nat. Prod. 2010, 73, 1075–1079; https://doi.org/10.1021/np100086x.Suche in Google Scholar PubMed
9. Kiss, L., Fülöp, F. Synthesis of carbocyclic and heterocyclic β- aminocarboxylic acids. Chem. Rev. 2014, 114, 1116–1169; https://doi.org/10.1021/cr300454h.Suche in Google Scholar PubMed
10. Luo, G., Huang, Z., Zhuo, S., Mou, C., Wu, J., Jin, Z., Chi, Y. R. Access to cyclic β-amino acids by amine-catalyzed enantioselective addition of the γ-carbon atoms of α,β-unsaturated imines to enals. Angew. Chem. Int. Ed. 2019, 58, 17189–17193; https://doi.org/10.1002/anie.201908896.Suche in Google Scholar PubMed
11. Jian, J., Ye, J., Luo, G., Lang, T. The crystal structure of N-(2′-hydroxymethyl-5′-phenyl-3′,4′-dihydro-[1,1′:3′,1″-terphenyl]- 1′(2′H)- yl)-P,P-diphenylphosphinic amide, C37H34NO2P. Z. Kristallogr. N. Cryst. Struct. 2022, 237, 579–581; https://doi.org/10.1515/ncrs-2022-0157.Suche in Google Scholar
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of poly[diaqua-(μ4-3,3′-di(1H-1,2,4-triazol-1-yl)-[1,1′-biphenyl]-4,4′-dicarboxylate-N:N′:O:O′)cadmium(II)], C18H14N6O6Cd
- Crystal structure of (8R,8′S,13S,13′R)-8,8′-bis(hydroxymethyl)-9,9′,10,10′-tetramethoxy-5,5′,6,6′,8,8′,13,13′-octahydro-[13,13′-bi[1,3]dioxolo[4,5-g]isoquinolino[3,2-a]isoquinoline]-7,7′-diium chloride-methanol (1/2), C46H58N2O14Cl2
- The crystal structure of 8-methoxy-2,2-diphenyl-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C29H25BN2O3S
- Crystal structure of aqua-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N″,N‴)copper(II) 5-carboxyisophthalate tetrahydrate, C25H50N4CuO11
- The crystal structure of 1-(naphthalen-2-ylsulfonyl)-2,2-diphenyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ij]quinoline, C31H23BN2O2S
- Crystal structure of iodido-(η6-benzene) (1-(pyridin-2-yl)-N-(p-fluoro-methanamine)-κ2N,Nʹ)ruthenium(II) hexaflourophosphate, (C18H15F7IN2RuP)
- The crystal structure of 1-(3-oxo-1-phenyl-3-(p-tolyl) propylidene)-1,3-dihydro-2H-inden-2-one, C25H20O2
- Crystal structure of tricyclohexyl[4-(4H-1,2,4-triazol-4-yl)-benzoato-κO]tin(IV), C27H39N3O2Sn
- Crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ4N,O,O,O)cadmium(II)]monohydrate, C12H15NO9Cd
- Crystal structure of ethyl 2-((4-(3,5-dimethylisoxazol-4-yl)-2,6-difluorophenyl)amino)benzoate, C20H18F2N2O3
- The crystal structure of 2-(hydroxymethyl)-2-(4H-1,2,4-triazol-4-yl)propane-1,3-diol, C6H11N3O3
- The crystal structure of 1,2-bis(2,4-dinitrophenyl) hydrazine, C12H8N6O8
- Crystal structure of 1-(2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)-1,2-dihydro-4H-benzo[d][1,3]oxazin-4-one, C19H14Cl2N2O3
- The crystal structure of 5-amino-5-oxo-4-(1-oxo-4-(2-oxopyrrolidin-1-yl)isoindolin-2-yl)pentanoic acid, C17H19N3O5
- Crystal structure of N2,N6-bis(2-(((Z)-5-bromo-2-hydroxybenzylidene)amino) phenyl)pyridine-2,6-dicarboxamide, C33H23Br2N5O4
- The crystal structure of (E)-2-methoxy-6-(((5-methyl-1,3,4-thiadiazol-2-yl)imino)methyl)phenol, C11H11N3O2S
- The crystal structure of 3-((tert-butyldiphenylsilyl)methyl)-5,5-diphenyl-6-(p-tolyl) tetrahydro-2H-pyran-2-one, C41H42O2Si
- Crystal structure of 9-fluoro-4-(6-methoxypyridin-3-yl)-5,6-dihydrobenzo[h]quinazolin-2-amine, C18H15FN4O
- The crystal structure of 2-bromo-5-(4-cyanophenoxy)benzyl 1-methyl-1,2,5,6-tetrahydropyridine-3-carboxylate, C21H19BrN2O3
- Crystal structure of 3,3′-(1,4-phenylenebis(methylene))bis(1-isopropyl-1H-imidazol-3-ium) bis(hexafluorophosphate(V)), C10H14F6N2P
- The crystal structure of 2,2-di(thiophen-3-yl)-1-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C24H19BN2O2S3
- Crystal structure of 5-bromo-1-(2-iodobenzoyl)-1H-indole-3-carbaldehyde, C16H9BrINO2
- The crystal structure of monocarbonyl-2-carboxypyridinato-κ2N,O-triphenylphosphine-rhodium(I) acetonitrile solvate, C26H20.50N1.50O3PRh
- Crystal structure of dichlorido-tetrakis(1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ1N)manganese(II), C60H68O4N12Cl10Mn
- Crystal structure of 3-(tert-butyldiphenylsilyl)-1-(2,6-dichlorophenyl)-2,2-diphenylpropan-1-ol, C37H36Cl2OSi
- Crystal structure of langite from Mine du Pradet (France)
- The crystal structure of 5′-(furan-2-yl)-3′-((4-methylphenyl)sulfonamido)-3′,4′,5′,6′-tetrahydro-[1,1′:3′,1″-terphenyl]-4′-carboxylic acid, C30H27NO5S
- Synthesis and crystal structure of bis{2-(((4-acetophenone)imino)methyl)-4-fluorophenolato-κ2N,O}zinc(II), C30H22F2N2O4Zn
- The crystal structure of poly[(tripyridine-κ3N,N′,N″) μ3-(pyridine-3,4-dicarboxylate-κ3N:O:O′) manganese(II)], C22H22N4O8Mn
- The crystal structure of (E)-4-chloro-N′-(1-(4-hydroxyphenyl)propylidene)benzohydrazide, C16H15ClN2O2
- Synthesis and crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino) ethyl)phenyl)imino)methyl)phenolato-κ2N,O}cobalt(II), C40H46CoN4O4
- Crystal structure of tetraaqua-[(1-(carboxymethyl)-1H-pyrazole-3-carboxylato-κ2N,O)cobalt(II)], C6H12CoN2O8
- (6R,7S)-2,3,13-trimethoxy-6,7-dimethyl-5,6,7,8-tetrahydrobenzo[3′,4′]cycloocta [1′,2′:4,5]benzo[1,2-d][1,3]dioxol-1-ol, C22H26O6
- Crystal structure of 2-((2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)amino)benzoic acid, C18H14Cl2N2O3
- Crystal structure of (5aS,6aS,8aR,9R,11aS, 11bS,13R,13aS)-1,1,8a,11a-tetramethyl-9-((S)-1-((S)-5-methyl-6-oxo-3,6-dihydro-2H-pyran-2-yl)ethyl)-3-oxo-1,7,8,8a,9,10,11,11a,11b,12,13,13a-dodecahydro-3H,6H-cyclopenta[5,6]cyclopropa[1,8a]naphtho[2,1-c]oxepin-13-yl acetate, C32H44O6
- Crystal structure of catena-poly[triaqua-(μ2-1-(4-carboxylatophenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylato-O,O′:O″)cobalt(II)], C12H12N2O8Co
- Crystal structure of 3-[(furan-2-ylmethyl)-amino]-2-(2,3,4,5-tetrafluoro-benzoyl)-acrylic acid ethyl ester, C17H13F4NO4
- Crystal structure of methyl 4-(2-ethoxy-2-oxoethoxy)-3-methoxybenzoate, C13H16O6
- Crystal structure of 4-bromo-2-(4-chlorophenyl)-1-methyl-5-(trifluoromethyl)-1H-pyrrole-3-carbonitrile, C13H7BrClF3N2
- The crystal structure of triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ3N,O,O)nickel(II) monohydrate, C12H15NO9Ni
- Crystal structure of dihydroxy(2,4,6-triisopro-pylphenyl)telluronium trifluoromethanesulfonate, C16H25F3O5STe
- The crystal structure of 1-(carboxymethyl)-1H-imidazole 3-oxide
- The crystal structure of 1,3,5-tris(dibromomethyl)benzene, C9H6Br6
- Crystal structure of (Z)-3-(4-methoxyphenyl)-4-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-N-phenylthiazol-2(3H)-imine, C25H21N5OS
- Crystal structure of (Z)-3-(3-(4-hydroxyphenyl)-2-(phenylimino)-2,3-dihydrothiazol-4-yl)-2H-chromen-2-one, C24H16N2O3S
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of poly[diaqua-(μ4-3,3′-di(1H-1,2,4-triazol-1-yl)-[1,1′-biphenyl]-4,4′-dicarboxylate-N:N′:O:O′)cadmium(II)], C18H14N6O6Cd
- Crystal structure of (8R,8′S,13S,13′R)-8,8′-bis(hydroxymethyl)-9,9′,10,10′-tetramethoxy-5,5′,6,6′,8,8′,13,13′-octahydro-[13,13′-bi[1,3]dioxolo[4,5-g]isoquinolino[3,2-a]isoquinoline]-7,7′-diium chloride-methanol (1/2), C46H58N2O14Cl2
- The crystal structure of 8-methoxy-2,2-diphenyl-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C29H25BN2O3S
- Crystal structure of aqua-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N″,N‴)copper(II) 5-carboxyisophthalate tetrahydrate, C25H50N4CuO11
- The crystal structure of 1-(naphthalen-2-ylsulfonyl)-2,2-diphenyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ij]quinoline, C31H23BN2O2S
- Crystal structure of iodido-(η6-benzene) (1-(pyridin-2-yl)-N-(p-fluoro-methanamine)-κ2N,Nʹ)ruthenium(II) hexaflourophosphate, (C18H15F7IN2RuP)
- The crystal structure of 1-(3-oxo-1-phenyl-3-(p-tolyl) propylidene)-1,3-dihydro-2H-inden-2-one, C25H20O2
- Crystal structure of tricyclohexyl[4-(4H-1,2,4-triazol-4-yl)-benzoato-κO]tin(IV), C27H39N3O2Sn
- Crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ4N,O,O,O)cadmium(II)]monohydrate, C12H15NO9Cd
- Crystal structure of ethyl 2-((4-(3,5-dimethylisoxazol-4-yl)-2,6-difluorophenyl)amino)benzoate, C20H18F2N2O3
- The crystal structure of 2-(hydroxymethyl)-2-(4H-1,2,4-triazol-4-yl)propane-1,3-diol, C6H11N3O3
- The crystal structure of 1,2-bis(2,4-dinitrophenyl) hydrazine, C12H8N6O8
- Crystal structure of 1-(2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)-1,2-dihydro-4H-benzo[d][1,3]oxazin-4-one, C19H14Cl2N2O3
- The crystal structure of 5-amino-5-oxo-4-(1-oxo-4-(2-oxopyrrolidin-1-yl)isoindolin-2-yl)pentanoic acid, C17H19N3O5
- Crystal structure of N2,N6-bis(2-(((Z)-5-bromo-2-hydroxybenzylidene)amino) phenyl)pyridine-2,6-dicarboxamide, C33H23Br2N5O4
- The crystal structure of (E)-2-methoxy-6-(((5-methyl-1,3,4-thiadiazol-2-yl)imino)methyl)phenol, C11H11N3O2S
- The crystal structure of 3-((tert-butyldiphenylsilyl)methyl)-5,5-diphenyl-6-(p-tolyl) tetrahydro-2H-pyran-2-one, C41H42O2Si
- Crystal structure of 9-fluoro-4-(6-methoxypyridin-3-yl)-5,6-dihydrobenzo[h]quinazolin-2-amine, C18H15FN4O
- The crystal structure of 2-bromo-5-(4-cyanophenoxy)benzyl 1-methyl-1,2,5,6-tetrahydropyridine-3-carboxylate, C21H19BrN2O3
- Crystal structure of 3,3′-(1,4-phenylenebis(methylene))bis(1-isopropyl-1H-imidazol-3-ium) bis(hexafluorophosphate(V)), C10H14F6N2P
- The crystal structure of 2,2-di(thiophen-3-yl)-1-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C24H19BN2O2S3
- Crystal structure of 5-bromo-1-(2-iodobenzoyl)-1H-indole-3-carbaldehyde, C16H9BrINO2
- The crystal structure of monocarbonyl-2-carboxypyridinato-κ2N,O-triphenylphosphine-rhodium(I) acetonitrile solvate, C26H20.50N1.50O3PRh
- Crystal structure of dichlorido-tetrakis(1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ1N)manganese(II), C60H68O4N12Cl10Mn
- Crystal structure of 3-(tert-butyldiphenylsilyl)-1-(2,6-dichlorophenyl)-2,2-diphenylpropan-1-ol, C37H36Cl2OSi
- Crystal structure of langite from Mine du Pradet (France)
- The crystal structure of 5′-(furan-2-yl)-3′-((4-methylphenyl)sulfonamido)-3′,4′,5′,6′-tetrahydro-[1,1′:3′,1″-terphenyl]-4′-carboxylic acid, C30H27NO5S
- Synthesis and crystal structure of bis{2-(((4-acetophenone)imino)methyl)-4-fluorophenolato-κ2N,O}zinc(II), C30H22F2N2O4Zn
- The crystal structure of poly[(tripyridine-κ3N,N′,N″) μ3-(pyridine-3,4-dicarboxylate-κ3N:O:O′) manganese(II)], C22H22N4O8Mn
- The crystal structure of (E)-4-chloro-N′-(1-(4-hydroxyphenyl)propylidene)benzohydrazide, C16H15ClN2O2
- Synthesis and crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino) ethyl)phenyl)imino)methyl)phenolato-κ2N,O}cobalt(II), C40H46CoN4O4
- Crystal structure of tetraaqua-[(1-(carboxymethyl)-1H-pyrazole-3-carboxylato-κ2N,O)cobalt(II)], C6H12CoN2O8
- (6R,7S)-2,3,13-trimethoxy-6,7-dimethyl-5,6,7,8-tetrahydrobenzo[3′,4′]cycloocta [1′,2′:4,5]benzo[1,2-d][1,3]dioxol-1-ol, C22H26O6
- Crystal structure of 2-((2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)amino)benzoic acid, C18H14Cl2N2O3
- Crystal structure of (5aS,6aS,8aR,9R,11aS, 11bS,13R,13aS)-1,1,8a,11a-tetramethyl-9-((S)-1-((S)-5-methyl-6-oxo-3,6-dihydro-2H-pyran-2-yl)ethyl)-3-oxo-1,7,8,8a,9,10,11,11a,11b,12,13,13a-dodecahydro-3H,6H-cyclopenta[5,6]cyclopropa[1,8a]naphtho[2,1-c]oxepin-13-yl acetate, C32H44O6
- Crystal structure of catena-poly[triaqua-(μ2-1-(4-carboxylatophenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylato-O,O′:O″)cobalt(II)], C12H12N2O8Co
- Crystal structure of 3-[(furan-2-ylmethyl)-amino]-2-(2,3,4,5-tetrafluoro-benzoyl)-acrylic acid ethyl ester, C17H13F4NO4
- Crystal structure of methyl 4-(2-ethoxy-2-oxoethoxy)-3-methoxybenzoate, C13H16O6
- Crystal structure of 4-bromo-2-(4-chlorophenyl)-1-methyl-5-(trifluoromethyl)-1H-pyrrole-3-carbonitrile, C13H7BrClF3N2
- The crystal structure of triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ3N,O,O)nickel(II) monohydrate, C12H15NO9Ni
- Crystal structure of dihydroxy(2,4,6-triisopro-pylphenyl)telluronium trifluoromethanesulfonate, C16H25F3O5STe
- The crystal structure of 1-(carboxymethyl)-1H-imidazole 3-oxide
- The crystal structure of 1,3,5-tris(dibromomethyl)benzene, C9H6Br6
- Crystal structure of (Z)-3-(4-methoxyphenyl)-4-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-N-phenylthiazol-2(3H)-imine, C25H21N5OS
- Crystal structure of (Z)-3-(3-(4-hydroxyphenyl)-2-(phenylimino)-2,3-dihydrothiazol-4-yl)-2H-chromen-2-one, C24H16N2O3S

