Abstract
C18H15FN4O, triclinic, P
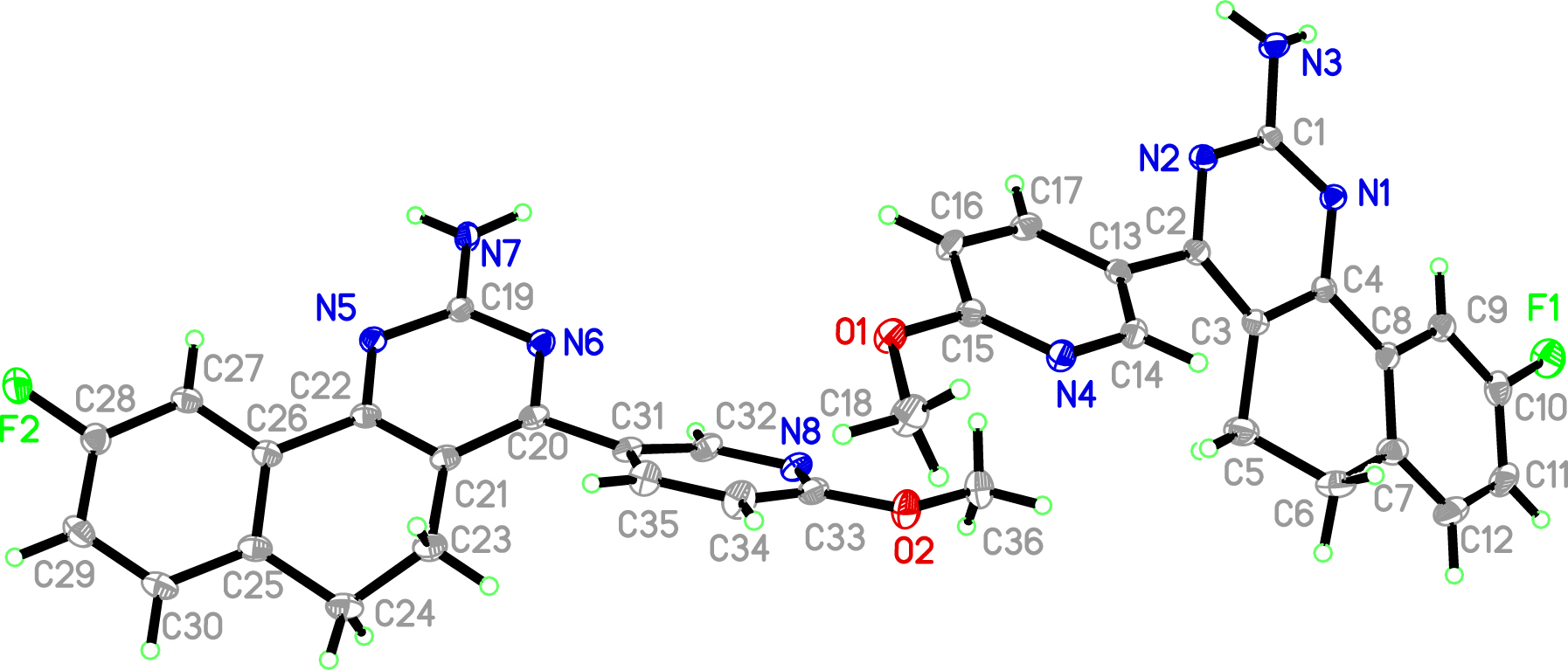
Displacement ellipsoids are drawn at the 35 % probability level.
Tables 1 and 2 contain details on crystal structure and measurement conditions and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.16 × 0.13 × 0.11 mm |
| Wavelength: | MoKα radiation (0.71073 Å) |
| μ: | 0.10 mm−1 |
| Diffractometer, scan mode: | SuperNova |
| θ max, completeness: | 25.5°, >99 % |
| N(hkl)measured, N(hkl)unique, R int: | 10,179, 5599, 0.030 |
| Criterion for I obs, N(hkl)gt: | I obs > 2σ(I obs), 4494 |
| N(param)refined: | 440 |
| Programs: | CrysAlis PRO [1], Shelx [2, 3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| C1 | 0.26540 (19) | 0.18423 (17) | 1.10189 (10) | 0.0183 (4) |
| C2 | 0.13470 (19) | 0.19304 (18) | 0.97155 (10) | 0.0189 (4) |
| C3 | 0.18043 (19) | 0.07452 (18) | 0.94379 (11) | 0.0203 (4) |
| C4 | 0.26424 (18) | 0.01364 (17) | 1.00386 (10) | 0.0182 (4) |
| C5 | 0.1636 (2) | 0.0154 (2) | 0.85344 (11) | 0.0318 (5) |
| H5A | 0.076983 | 0.046594 | 0.820888 | 0.038* |
| H5B | 0.255009 | 0.042272 | 0.832042 | 0.038* |
| C6 | 0.1404 (2) | −0.1272 (2) | 0.84412 (12) | 0.0336 (5) |
| H6A | 0.144710 | −0.161290 | 0.787410 | 0.040* |
| H6B | 0.039351 | −0.154372 | 0.855064 | 0.040* |
| C7 | 0.2588 (2) | −0.18059 (19) | 0.90193 (11) | 0.0251 (4) |
| C8 | 0.31510 (19) | −0.11256 (17) | 0.98074 (11) | 0.0195 (4) |
| C9 | 0.4192 (2) | −0.16534 (19) | 1.03621 (12) | 0.0250 (4) |
| H9 | 0.458509 | −0.120912 | 1.088524 | 0.030* |
| C10 | 0.4631 (2) | −0.2830 (2) | 1.01316 (13) | 0.0285 (5) |
| C11 | 0.4120 (2) | −0.3511 (2) | 0.93618 (13) | 0.0312 (5) |
| H11 | 0.445484 | −0.430085 | 0.921555 | 0.037* |
| C12 | 0.3094 (2) | −0.2989 (2) | 0.88114 (13) | 0.0318 (5) |
| H12 | 0.272884 | −0.343908 | 0.828687 | 0.038* |
| C13 | 0.03909 (19) | 0.26973 (18) | 0.91804 (10) | 0.0186 (4) |
| C14 | −0.09203 (19) | 0.21652 (18) | 0.86401 (11) | 0.0214 (4) |
| H14 | −0.120283 | 0.129156 | 0.861618 | 0.026* |
| C15 | −0.14095 (19) | 0.40626 (18) | 0.81912 (10) | 0.0196 (4) |
| C16 | −0.0148 (2) | 0.47209 (18) | 0.87403 (11) | 0.0230 (4) |
| H16 | 0.007696 | 0.560286 | 0.876966 | 0.028* |
| C17 | 0.0747 (2) | 0.40233 (18) | 0.92339 (11) | 0.0223 (4) |
| H17 | 0.159627 | 0.443267 | 0.960712 | 0.027* |
| C18 | −0.3480 (2) | 0.4057 (2) | 0.70810 (13) | 0.0370 (5) |
| H18A | −0.309646 | 0.336326 | 0.677510 | 0.056* |
| H18B | −0.390182 | 0.461695 | 0.670650 | 0.056* |
| H18C | −0.426324 | 0.371993 | 0.735959 | 0.056* |
| C19 | 0.47970 (19) | 1.00190 (18) | 0.69198 (11) | 0.0205 (4) |
| C20 | 0.31757 (19) | 0.82867 (18) | 0.62392 (11) | 0.0201 (4) |
| C21 | 0.27263 (19) | 0.89708 (18) | 0.55814 (11) | 0.0203 (4) |
| C22 | 0.34151 (19) | 1.02257 (18) | 0.56620 (11) | 0.0199 (4) |
| C23 | 0.1578 (2) | 0.8453 (2) | 0.48013 (11) | 0.0265 (5) |
| H23A | 0.154733 | 0.752722 | 0.470465 | 0.032* |
| H23B | 0.056397 | 0.865996 | 0.487593 | 0.032* |
| C24 | 0.2007 (2) | 0.9019 (2) | 0.40554 (11) | 0.0288 (5) |
| H24A | 0.291492 | 0.866458 | 0.391388 | 0.035* |
| H24B | 0.118006 | 0.877205 | 0.358585 | 0.035* |
| C25 | 0.23120 (19) | 1.04474 (19) | 0.42057 (11) | 0.0232 (4) |
| C26 | 0.30411 (19) | 1.10340 (18) | 0.49993 (11) | 0.0202 (4) |
| C27 | 0.3421 (2) | 1.23586 (19) | 0.51477 (11) | 0.0232 (4) |
| H27 | 0.390105 | 1.275567 | 0.567035 | 0.028* |
| C28 | 0.3074 (2) | 1.30623 (19) | 0.45103 (11) | 0.0258 (4) |
| C29 | 0.2370 (2) | 1.2521 (2) | 0.37235 (11) | 0.0273 (5) |
| H29 | 0.214948 | 1.302413 | 0.330162 | 0.033* |
| C30 | 0.2003 (2) | 1.1212 (2) | 0.35816 (11) | 0.0278 (5) |
| H30 | 0.153599 | 1.082991 | 0.305318 | 0.033* |
| C31 | 0.25351 (19) | 0.69567 (18) | 0.62823 (11) | 0.0212 (4) |
| C32 | 0.3457 (2) | 0.61100 (18) | 0.66334 (11) | 0.0238 (4) |
| H32 | 0.450046 | 0.637857 | 0.678601 | 0.029* |
| C33 | 0.1484 (2) | 0.45684 (19) | 0.65526 (11) | 0.0239 (4) |
| C34 | 0.0450 (2) | 0.5316 (2) | 0.61701 (13) | 0.0302 (5) |
| H34 | −0.058129 | 0.500929 | 0.600805 | 0.036* |
| C35 | 0.0981 (2) | 0.65146 (19) | 0.60363 (12) | 0.0272 (5) |
| H35 | 0.030791 | 0.703453 | 0.578210 | 0.033* |
| C36 | 0.1951 (2) | 0.2708 (2) | 0.71844 (13) | 0.0349 (5) |
| H36A | 0.280549 | 0.256293 | 0.690986 | 0.052* |
| H36B | 0.142651 | 0.189925 | 0.724408 | 0.052* |
| H36C | 0.231450 | 0.318768 | 0.772260 | 0.052* |
| F1 | 0.56469 (13) | −0.33313 (12) | 1.06894 (7) | 0.0410 (3) |
| F2 | 0.34106 (13) | 1.43584 (11) | 0.46579 (7) | 0.0358 (3) |
| N1 | 0.30484 (16) | 0.06622 (14) | 1.08372 (9) | 0.0192 (3) |
| N2 | 0.17884 (16) | 0.24908 (15) | 1.05054 (9) | 0.0197 (3) |
| N3 | 0.31187 (18) | 0.24239 (16) | 1.18122 (9) | 0.0255 (4) |
| H3A | 0.386145 | 0.209600 | 1.213918 | 0.031 (6)* |
| H3B | 0.296836 | 0.324981 | 1.188727 | 0.027 (6)* |
| N4 | −0.18196 (16) | 0.28250 (15) | 0.81446 (9) | 0.0222 (4) |
| N5 | 0.44263 (16) | 1.07706 (15) | 0.63332 (9) | 0.0207 (4) |
| N6 | 0.42334 (16) | 0.87946 (15) | 0.69065 (9) | 0.0221 (4) |
| N7 | 0.58239 (18) | 1.05512 (16) | 0.75950 (9) | 0.0278 (4) |
| H7B | 0.627386 | 1.136515 | 0.761179 | 0.027 (5)* |
| H7A | 0.614897 | 1.010344 | 0.802938 | 0.044 (7)* |
| N8 | 0.29704 (17) | 0.49333 (15) | 0.67718 (9) | 0.0247 (4) |
| O1 | −0.22509 (14) | 0.47574 (12) | 0.76812 (8) | 0.0261 (3) |
| O2 | 0.09185 (14) | 0.34128 (13) | 0.67023 (8) | 0.0312 (3) |
1 Source of material
7-Fluoro-3,4-dihydronaphthalen-1(2H)-one (1.76 g, 10.0 mmol) and 2-methoxybenzaldehyde (1.36 g, 10.0 mmol) were dissolved in 15 mL of methanol. Then 5.0 mL of 10 % sodium hydroxide solution was added to the mixture and stirred for 3 h at room temperature. The reaction endpoint was detected by thin layer chromatography (TLC). The crude product was concentrated under reduced pressure and purified by silica gel column chromatography to obtain (E)-7-fluoro-2-((6-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one.
The (E)-7-fluoro-2-((6-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one (1.00 g, 2.9 mmol), guanidine hydrochloride (1.39 g, 14.50 mmol), potassium hydroxide (0.81 g, 14.50 mmol), and potassium carbonate (0.80 g, 5.80 mmol) were added to anhydrous ethanol (15 mL) and 1,2-dichloroethane (15 mL). The mixture was heated to reflux for about 1.5 h. The reaction endpoint was detected by TLC. When the reaction was completed, the mixture was cooled to room temperature, filtered, concentrated under pressure and purified by silica gel column chromatography (petroleum ether: ethyl acetate = 8:1, v:v). Crystals of the title compound were obtained by slow evaporation of a methanol solution of the target at room temperature.
2 Experimental details
The H atoms were placed in idealized positions and treated as riding on their parent atoms, with d(C–H) = 0.96 Å (methyl), U iso(H) = 1.5U eq(C), and d(C–H) = 0.97 Å (methylene), U iso(H) = 1.2U eq(C), and d(C–H) = 0.93 Å (aromatic), U iso(H) = 1.2U eq(C).
3 Comment
3,4–Dihydronaphthalen-1(2H)-one (DHN) molecules are aromatic bicyclic compounds [4, 5]. Pyridine ring was obtained by Claisen–Schmidt condensation of DHN, then the pyrimidine ring with amino substituent was prepared by condensation with guanidine hydrochloride, and finally 5,6-dihydrobenzo[h]quinazolin-2-amine (BQA) derivative was synthesized [6]. The synthesis and single crystal X-ray diffraction of 9-(2-chloroethoxy)-4-(4-methoxy-3-(trifluoromethyl)phenyl)-5,6-dihydrobenzo[h]quinazolin-2-amine and 9-bromo-4-(6-methoxypyridin-2-yl)-5,6-dihydrobenzo[h]quinazolin-2-amine (BQA derivatives) have been studied in our group [7, 8].
Single-crystal structure analysis revealed that the title compound crystallized with one BQA molecule in the asymmetric unit (cf. the figure). The parent structure is 5,6-dihydrobenzo[h]quinazolin-2-amine. The bond lengths of C(14)=N(4) and C(15)=N(4) in pyridine ring are 1.346(2) Å and 1.317(2) Å, respectively. In addition, the bond length of C(1)=N(1) and C(1)=N(2) on pyrimidine ring is 1.339(2) Å, and the bond length of C(1)–N(3) on amino substituent is 1.360(2) Å [9, 10]. At the same time, the benzene ring group was replaced by fluorine atom, and the length of C(10)–F(1) bond was 1.367(2) Å. Under the pull of the two methylene groups, C(5)–C(6), the (9-fluoro)phenyl ring has better coplanarity with the central pyrimidine ring, whose dihedral angle is only 8.4°. Due to the space effect of the title compound, the 4-methoxy-pyridine ring is not coplanar with the central pyrimidine ring, with the dihedral angle of about 43.5°. Bond lengths and angles are all in the expected ranges [11]. Compared with DHN derivatives [12], the substituents in the title compound are more comprehensive. With –NH2, –F, and –OMe groups as hydrogen bond acceptors and donors, it is easy to form a three-dimensional structure through hydrogen bond extension [13, 14].
Funding source: Science and Technology Innovation Development Plan of Yantai
Award Identifier / Grant number: 2020XDRH105
Funding source: National Natural Science Foundation of China
Award Identifier / Grant number: 81473104
-
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: Science and Technology Innovation Development Plan of Yantai (No. 2020XDRH105) and the National Natural Science Foundation of China (No. 81473104).
-
Conflict of interest: The authors declare no conflicts of interest regarding this article.
References
1. Rigaku, O. D. CrysAlisp̂ro; Rigaku Oxford Diffraction Ltd: Yarnton, Oxfordshire, England, 2017.Suche in Google Scholar
2. Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Suche in Google Scholar PubMed
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar
4. Sun, Y., Zhou, Y. Q., Liu, Y. K., Zhang, H. Q., Hou, G. G., Meng, Q. G., Hou, Y. Potential anti-neuroinflammatory NF-κB inhibitors based on 3,4-dihydronaphthalen-1(2H)-one derivative. J. Enzyme Inhib. Med. Chem. 2020, 35, 1631–1640; https://doi.org/10.1080/14756366.2020.1804899.Suche in Google Scholar PubMed PubMed Central
5. Wang, C., Wang, L., Meng, Q. G., Huang, Z. X., Ma, N. N., Wang, C. H. Crystal structure of (E)-2-((3-fluoropyridin-4-yl)methylene)-7-methoxy-3,4-dihydronaphthalen1(2H)-one, C17H14FNO2. Z. Kristallogr. N. Cryst. Struct. 2021, 236, 1073–1075; https://doi.org/10.1515/ncrs-2021-0223.Suche in Google Scholar
6. Zhang, X. F., Luan, M. Z., Yan, W. B., Zhao, F. L., Hou, Y., Hou, G. G., Meng, Q. G. Anti-neuroinflammatory effects of novel 5,6-dihydrobenzo [h]quinazolin-2-amine derivatives in lipopolysaccharide-stimulated BV2 microglial cells. Eur. J. Med. Chem. 2022, 235, 114322; https://doi.org/10.1016/j.ejmech.2022.114322.Suche in Google Scholar PubMed
7. Li, W. X., Wang, L., Jiang, N., Hou, G. G., Liu, Y. J. Crystal structure of 9-bromo-4-(6-methoxypyridin-2-yl)-5,6-dihydrobenzo[h]quinazolin-2-amine, C18H15BrN4O. Z. Kristallogr. N. Cryst. Struct. 2022, 237, 991–993; https://doi.org/10.1515/ncrs-2022-0339.Suche in Google Scholar
8. Li, R. K., Wang, C. H., Hou, G. G., Li, C. B. Crystal structure of 9-(2-chloroethoxy)-4-(4-methoxy-3-(trifluoromethyl)phenyl)-5,6-dihydrobenzo[h]quinazolin-2-amine, C22H19ClF3N3O2. Z. Kristallogr. N. Cryst. Struct. 2023, 238, 243–245; https://doi.org/10.1515/ncrs-2022-0589.Suche in Google Scholar
9. Zhou, Y. Q., Sun, Y., Luo, H. L., Gao, Z. F., Zhang, H. Q., Meng, Q. G., Bai, X. Y., Hou, G. G., Hou, Y. Discovery of anti-hepatoma agents from 1,4,5,6,7,8-hexahydropyrido[4,3-d]pyrimidine by inhibiting PI3K/AKT/NF-κB pathway activation. Eur. J. Med. Chem. 2021, 225, 113796; https://doi.org/10.1016/j.ejmech.2021.113796.Suche in Google Scholar PubMed
10. Yang, Y., Gao, Z. F., Hou, G. G., Meng, Q. G., Hou, Y. Discovery of anti-neuroinflammatory agents from 1,4,5,6-tetrahydrobenzo[2,3]oxepino[4,5-d]pyrimidin-2-amine derivatives by regulating microglia polarization. Eur. J. Med. Chem. 2023, 259, 115688; https://doi.org/10.1016/j.ejmech.2023.115688.Suche in Google Scholar PubMed
11. Luan, M. Z., Zhang, X. F., Yang, Y., Meng, Q. G., Hou, G. G. Anti-inflammatory activity of fluorine-substituted benzo[h]quinazoline-2-amine derivatives as NF-κB inhibitors. Bioorg. Chem. 2023, 132, 106360; https://doi.org/10.1016/j.bioorg.2023.106360.Suche in Google Scholar PubMed
12. Zhang, Y. L., Liu, S. L., Hou, G. G., Zhang, X. F., Wang, L., Xin, W. Y. Crystal structure of (E)-7-bromo-2-(4-methoxybenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C18H15BrO2. Z. Kristallogr. N. Cryst. Struct. 2022, 237, 945–947; https://doi.org/10.1515/ncrs-2022-0315.Suche in Google Scholar
13. Sun, Y., Gao, Z. F., Wang, C. H., Hou, G. G. Synthesis, crystal structures and anti-inflammatory activity of fluorine-substituted 1,4,5,6-tetrahydrobenzo[h]quinazolin-2-amine derivatives. Acta Crystallogr. 2019, C75, 1157–1165; https://doi.org/10.1107/s2053229619010118.Suche in Google Scholar
14. Bonacorso, H. G., Rosa, W. C., Oliveira, S. M., Brusco, I., Pozza, C. C. D., Nogara, P. A., Wiethan, C. W., Rodrigues, M. B., Frizzo, C. P., Zanatta, N. Synthesis and antinociceptive activity of new 2-substituted 4-(trifluoromethyl)-5,6-dihydrobenzo[h]quinazolines. Bioorg. Med. Chem. Lett. 2016, 26, 4808–4814; https://doi.org/10.1016/j.bmcl.2016.08.021.Suche in Google Scholar PubMed
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of poly[diaqua-(μ4-3,3′-di(1H-1,2,4-triazol-1-yl)-[1,1′-biphenyl]-4,4′-dicarboxylate-N:N′:O:O′)cadmium(II)], C18H14N6O6Cd
- Crystal structure of (8R,8′S,13S,13′R)-8,8′-bis(hydroxymethyl)-9,9′,10,10′-tetramethoxy-5,5′,6,6′,8,8′,13,13′-octahydro-[13,13′-bi[1,3]dioxolo[4,5-g]isoquinolino[3,2-a]isoquinoline]-7,7′-diium chloride-methanol (1/2), C46H58N2O14Cl2
- The crystal structure of 8-methoxy-2,2-diphenyl-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C29H25BN2O3S
- Crystal structure of aqua-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N″,N‴)copper(II) 5-carboxyisophthalate tetrahydrate, C25H50N4CuO11
- The crystal structure of 1-(naphthalen-2-ylsulfonyl)-2,2-diphenyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ij]quinoline, C31H23BN2O2S
- Crystal structure of iodido-(η6-benzene) (1-(pyridin-2-yl)-N-(p-fluoro-methanamine)-κ2N,Nʹ)ruthenium(II) hexaflourophosphate, (C18H15F7IN2RuP)
- The crystal structure of 1-(3-oxo-1-phenyl-3-(p-tolyl) propylidene)-1,3-dihydro-2H-inden-2-one, C25H20O2
- Crystal structure of tricyclohexyl[4-(4H-1,2,4-triazol-4-yl)-benzoato-κO]tin(IV), C27H39N3O2Sn
- Crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ4N,O,O,O)cadmium(II)]monohydrate, C12H15NO9Cd
- Crystal structure of ethyl 2-((4-(3,5-dimethylisoxazol-4-yl)-2,6-difluorophenyl)amino)benzoate, C20H18F2N2O3
- The crystal structure of 2-(hydroxymethyl)-2-(4H-1,2,4-triazol-4-yl)propane-1,3-diol, C6H11N3O3
- The crystal structure of 1,2-bis(2,4-dinitrophenyl) hydrazine, C12H8N6O8
- Crystal structure of 1-(2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)-1,2-dihydro-4H-benzo[d][1,3]oxazin-4-one, C19H14Cl2N2O3
- The crystal structure of 5-amino-5-oxo-4-(1-oxo-4-(2-oxopyrrolidin-1-yl)isoindolin-2-yl)pentanoic acid, C17H19N3O5
- Crystal structure of N2,N6-bis(2-(((Z)-5-bromo-2-hydroxybenzylidene)amino) phenyl)pyridine-2,6-dicarboxamide, C33H23Br2N5O4
- The crystal structure of (E)-2-methoxy-6-(((5-methyl-1,3,4-thiadiazol-2-yl)imino)methyl)phenol, C11H11N3O2S
- The crystal structure of 3-((tert-butyldiphenylsilyl)methyl)-5,5-diphenyl-6-(p-tolyl) tetrahydro-2H-pyran-2-one, C41H42O2Si
- Crystal structure of 9-fluoro-4-(6-methoxypyridin-3-yl)-5,6-dihydrobenzo[h]quinazolin-2-amine, C18H15FN4O
- The crystal structure of 2-bromo-5-(4-cyanophenoxy)benzyl 1-methyl-1,2,5,6-tetrahydropyridine-3-carboxylate, C21H19BrN2O3
- Crystal structure of 3,3′-(1,4-phenylenebis(methylene))bis(1-isopropyl-1H-imidazol-3-ium) bis(hexafluorophosphate(V)), C10H14F6N2P
- The crystal structure of 2,2-di(thiophen-3-yl)-1-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C24H19BN2O2S3
- Crystal structure of 5-bromo-1-(2-iodobenzoyl)-1H-indole-3-carbaldehyde, C16H9BrINO2
- The crystal structure of monocarbonyl-2-carboxypyridinato-κ2N,O-triphenylphosphine-rhodium(I) acetonitrile solvate, C26H20.50N1.50O3PRh
- Crystal structure of dichlorido-tetrakis(1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ1N)manganese(II), C60H68O4N12Cl10Mn
- Crystal structure of 3-(tert-butyldiphenylsilyl)-1-(2,6-dichlorophenyl)-2,2-diphenylpropan-1-ol, C37H36Cl2OSi
- Crystal structure of langite from Mine du Pradet (France)
- The crystal structure of 5′-(furan-2-yl)-3′-((4-methylphenyl)sulfonamido)-3′,4′,5′,6′-tetrahydro-[1,1′:3′,1″-terphenyl]-4′-carboxylic acid, C30H27NO5S
- Synthesis and crystal structure of bis{2-(((4-acetophenone)imino)methyl)-4-fluorophenolato-κ2N,O}zinc(II), C30H22F2N2O4Zn
- The crystal structure of poly[(tripyridine-κ3N,N′,N″) μ3-(pyridine-3,4-dicarboxylate-κ3N:O:O′) manganese(II)], C22H22N4O8Mn
- The crystal structure of (E)-4-chloro-N′-(1-(4-hydroxyphenyl)propylidene)benzohydrazide, C16H15ClN2O2
- Synthesis and crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino) ethyl)phenyl)imino)methyl)phenolato-κ2N,O}cobalt(II), C40H46CoN4O4
- Crystal structure of tetraaqua-[(1-(carboxymethyl)-1H-pyrazole-3-carboxylato-κ2N,O)cobalt(II)], C6H12CoN2O8
- (6R,7S)-2,3,13-trimethoxy-6,7-dimethyl-5,6,7,8-tetrahydrobenzo[3′,4′]cycloocta [1′,2′:4,5]benzo[1,2-d][1,3]dioxol-1-ol, C22H26O6
- Crystal structure of 2-((2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)amino)benzoic acid, C18H14Cl2N2O3
- Crystal structure of (5aS,6aS,8aR,9R,11aS, 11bS,13R,13aS)-1,1,8a,11a-tetramethyl-9-((S)-1-((S)-5-methyl-6-oxo-3,6-dihydro-2H-pyran-2-yl)ethyl)-3-oxo-1,7,8,8a,9,10,11,11a,11b,12,13,13a-dodecahydro-3H,6H-cyclopenta[5,6]cyclopropa[1,8a]naphtho[2,1-c]oxepin-13-yl acetate, C32H44O6
- Crystal structure of catena-poly[triaqua-(μ2-1-(4-carboxylatophenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylato-O,O′:O″)cobalt(II)], C12H12N2O8Co
- Crystal structure of 3-[(furan-2-ylmethyl)-amino]-2-(2,3,4,5-tetrafluoro-benzoyl)-acrylic acid ethyl ester, C17H13F4NO4
- Crystal structure of methyl 4-(2-ethoxy-2-oxoethoxy)-3-methoxybenzoate, C13H16O6
- Crystal structure of 4-bromo-2-(4-chlorophenyl)-1-methyl-5-(trifluoromethyl)-1H-pyrrole-3-carbonitrile, C13H7BrClF3N2
- The crystal structure of triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ3N,O,O)nickel(II) monohydrate, C12H15NO9Ni
- Crystal structure of dihydroxy(2,4,6-triisopro-pylphenyl)telluronium trifluoromethanesulfonate, C16H25F3O5STe
- The crystal structure of 1-(carboxymethyl)-1H-imidazole 3-oxide
- The crystal structure of 1,3,5-tris(dibromomethyl)benzene, C9H6Br6
- Crystal structure of (Z)-3-(4-methoxyphenyl)-4-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-N-phenylthiazol-2(3H)-imine, C25H21N5OS
- Crystal structure of (Z)-3-(3-(4-hydroxyphenyl)-2-(phenylimino)-2,3-dihydrothiazol-4-yl)-2H-chromen-2-one, C24H16N2O3S
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of poly[diaqua-(μ4-3,3′-di(1H-1,2,4-triazol-1-yl)-[1,1′-biphenyl]-4,4′-dicarboxylate-N:N′:O:O′)cadmium(II)], C18H14N6O6Cd
- Crystal structure of (8R,8′S,13S,13′R)-8,8′-bis(hydroxymethyl)-9,9′,10,10′-tetramethoxy-5,5′,6,6′,8,8′,13,13′-octahydro-[13,13′-bi[1,3]dioxolo[4,5-g]isoquinolino[3,2-a]isoquinoline]-7,7′-diium chloride-methanol (1/2), C46H58N2O14Cl2
- The crystal structure of 8-methoxy-2,2-diphenyl-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C29H25BN2O3S
- Crystal structure of aqua-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N″,N‴)copper(II) 5-carboxyisophthalate tetrahydrate, C25H50N4CuO11
- The crystal structure of 1-(naphthalen-2-ylsulfonyl)-2,2-diphenyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ij]quinoline, C31H23BN2O2S
- Crystal structure of iodido-(η6-benzene) (1-(pyridin-2-yl)-N-(p-fluoro-methanamine)-κ2N,Nʹ)ruthenium(II) hexaflourophosphate, (C18H15F7IN2RuP)
- The crystal structure of 1-(3-oxo-1-phenyl-3-(p-tolyl) propylidene)-1,3-dihydro-2H-inden-2-one, C25H20O2
- Crystal structure of tricyclohexyl[4-(4H-1,2,4-triazol-4-yl)-benzoato-κO]tin(IV), C27H39N3O2Sn
- Crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ4N,O,O,O)cadmium(II)]monohydrate, C12H15NO9Cd
- Crystal structure of ethyl 2-((4-(3,5-dimethylisoxazol-4-yl)-2,6-difluorophenyl)amino)benzoate, C20H18F2N2O3
- The crystal structure of 2-(hydroxymethyl)-2-(4H-1,2,4-triazol-4-yl)propane-1,3-diol, C6H11N3O3
- The crystal structure of 1,2-bis(2,4-dinitrophenyl) hydrazine, C12H8N6O8
- Crystal structure of 1-(2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)-1,2-dihydro-4H-benzo[d][1,3]oxazin-4-one, C19H14Cl2N2O3
- The crystal structure of 5-amino-5-oxo-4-(1-oxo-4-(2-oxopyrrolidin-1-yl)isoindolin-2-yl)pentanoic acid, C17H19N3O5
- Crystal structure of N2,N6-bis(2-(((Z)-5-bromo-2-hydroxybenzylidene)amino) phenyl)pyridine-2,6-dicarboxamide, C33H23Br2N5O4
- The crystal structure of (E)-2-methoxy-6-(((5-methyl-1,3,4-thiadiazol-2-yl)imino)methyl)phenol, C11H11N3O2S
- The crystal structure of 3-((tert-butyldiphenylsilyl)methyl)-5,5-diphenyl-6-(p-tolyl) tetrahydro-2H-pyran-2-one, C41H42O2Si
- Crystal structure of 9-fluoro-4-(6-methoxypyridin-3-yl)-5,6-dihydrobenzo[h]quinazolin-2-amine, C18H15FN4O
- The crystal structure of 2-bromo-5-(4-cyanophenoxy)benzyl 1-methyl-1,2,5,6-tetrahydropyridine-3-carboxylate, C21H19BrN2O3
- Crystal structure of 3,3′-(1,4-phenylenebis(methylene))bis(1-isopropyl-1H-imidazol-3-ium) bis(hexafluorophosphate(V)), C10H14F6N2P
- The crystal structure of 2,2-di(thiophen-3-yl)-1-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C24H19BN2O2S3
- Crystal structure of 5-bromo-1-(2-iodobenzoyl)-1H-indole-3-carbaldehyde, C16H9BrINO2
- The crystal structure of monocarbonyl-2-carboxypyridinato-κ2N,O-triphenylphosphine-rhodium(I) acetonitrile solvate, C26H20.50N1.50O3PRh
- Crystal structure of dichlorido-tetrakis(1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ1N)manganese(II), C60H68O4N12Cl10Mn
- Crystal structure of 3-(tert-butyldiphenylsilyl)-1-(2,6-dichlorophenyl)-2,2-diphenylpropan-1-ol, C37H36Cl2OSi
- Crystal structure of langite from Mine du Pradet (France)
- The crystal structure of 5′-(furan-2-yl)-3′-((4-methylphenyl)sulfonamido)-3′,4′,5′,6′-tetrahydro-[1,1′:3′,1″-terphenyl]-4′-carboxylic acid, C30H27NO5S
- Synthesis and crystal structure of bis{2-(((4-acetophenone)imino)methyl)-4-fluorophenolato-κ2N,O}zinc(II), C30H22F2N2O4Zn
- The crystal structure of poly[(tripyridine-κ3N,N′,N″) μ3-(pyridine-3,4-dicarboxylate-κ3N:O:O′) manganese(II)], C22H22N4O8Mn
- The crystal structure of (E)-4-chloro-N′-(1-(4-hydroxyphenyl)propylidene)benzohydrazide, C16H15ClN2O2
- Synthesis and crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino) ethyl)phenyl)imino)methyl)phenolato-κ2N,O}cobalt(II), C40H46CoN4O4
- Crystal structure of tetraaqua-[(1-(carboxymethyl)-1H-pyrazole-3-carboxylato-κ2N,O)cobalt(II)], C6H12CoN2O8
- (6R,7S)-2,3,13-trimethoxy-6,7-dimethyl-5,6,7,8-tetrahydrobenzo[3′,4′]cycloocta [1′,2′:4,5]benzo[1,2-d][1,3]dioxol-1-ol, C22H26O6
- Crystal structure of 2-((2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)amino)benzoic acid, C18H14Cl2N2O3
- Crystal structure of (5aS,6aS,8aR,9R,11aS, 11bS,13R,13aS)-1,1,8a,11a-tetramethyl-9-((S)-1-((S)-5-methyl-6-oxo-3,6-dihydro-2H-pyran-2-yl)ethyl)-3-oxo-1,7,8,8a,9,10,11,11a,11b,12,13,13a-dodecahydro-3H,6H-cyclopenta[5,6]cyclopropa[1,8a]naphtho[2,1-c]oxepin-13-yl acetate, C32H44O6
- Crystal structure of catena-poly[triaqua-(μ2-1-(4-carboxylatophenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylato-O,O′:O″)cobalt(II)], C12H12N2O8Co
- Crystal structure of 3-[(furan-2-ylmethyl)-amino]-2-(2,3,4,5-tetrafluoro-benzoyl)-acrylic acid ethyl ester, C17H13F4NO4
- Crystal structure of methyl 4-(2-ethoxy-2-oxoethoxy)-3-methoxybenzoate, C13H16O6
- Crystal structure of 4-bromo-2-(4-chlorophenyl)-1-methyl-5-(trifluoromethyl)-1H-pyrrole-3-carbonitrile, C13H7BrClF3N2
- The crystal structure of triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ3N,O,O)nickel(II) monohydrate, C12H15NO9Ni
- Crystal structure of dihydroxy(2,4,6-triisopro-pylphenyl)telluronium trifluoromethanesulfonate, C16H25F3O5STe
- The crystal structure of 1-(carboxymethyl)-1H-imidazole 3-oxide
- The crystal structure of 1,3,5-tris(dibromomethyl)benzene, C9H6Br6
- Crystal structure of (Z)-3-(4-methoxyphenyl)-4-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-N-phenylthiazol-2(3H)-imine, C25H21N5OS
- Crystal structure of (Z)-3-(3-(4-hydroxyphenyl)-2-(phenylimino)-2,3-dihydrothiazol-4-yl)-2H-chromen-2-one, C24H16N2O3S

