Crystal structure of ethyl 1-(2-hydroxyethyl)-4-((4-methoxyphenyl)amino)-5-oxo-2,5-dihydro-1H-pyrrole-3-carboxylate, C16H20N2O5
-
Fatin Nur Ain Abdul Rashid
, Muhamad Zulfaqar Bacho
and Mohd Fazli Mohammat
Abstract
C16H20N2O5, triclinic,
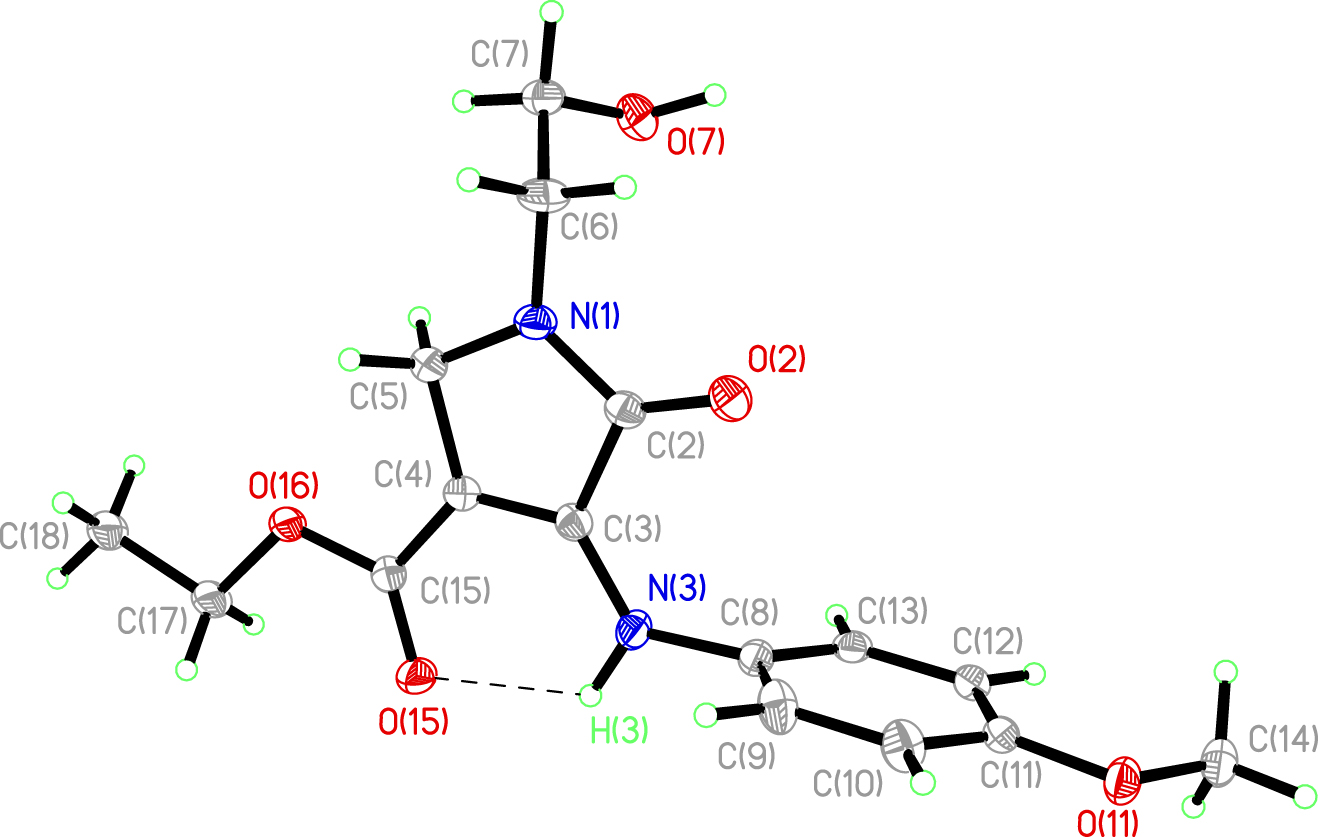
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless prism |
| Size: | 0.20 × 0.10 × 0.10 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.10 mm−1 |
| Diffractometer, scan mode: | Rigaku XtaLAB P200, ω |
| θ max, completeness: | 26.4°, >99% |
| N(hkl) measured, N(hkl) unique, R int: | 7943, 3178, 0.038 |
| Criterion for I obs, N(hkl) gt: | I obs > 2 σ(I obs), 2979 |
| N(param) refined: | 218 |
| Programs: | CrysAlisPRO [1], SHELX [2, 3], Mercury [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| O2 | 0.11051 (12) | 0.16554 (10) | 0.41846 (9) | 0.0256 (2) |
| O7 | −0.05650 (12) | −0.00798 (9) | 0.74929 (9) | 0.0252 (2) |
| O11 | 0.62501 (12) | 0.08398 (9) | 0.02766 (8) | 0.0218 (2) |
| O15 | 0.37685 (11) | 0.49239 (9) | 0.62943 (9) | 0.0227 (2) |
| O16 | 0.15173 (11) | 0.51537 (9) | 0.77205 (8) | 0.0210 (2) |
| N1 | −0.03381 (13) | 0.26014 (11) | 0.58643 (10) | 0.0192 (2) |
| N3 | 0.36381 (13) | 0.32308 (11) | 0.44944 (10) | 0.0210 (2) |
| C2 | 0.09672 (15) | 0.23665 (12) | 0.49985 (11) | 0.0180 (3) |
| C3 | 0.22057 (15) | 0.31601 (12) | 0.52269 (11) | 0.0168 (3) |
| C4 | 0.15872 (15) | 0.37848 (12) | 0.62344 (11) | 0.0171 (3) |
| C5 | −0.00996 (15) | 0.34710 (12) | 0.67152 (11) | 0.0182 (3) |
| H5A | 0.000488 | 0.295627 | 0.765591 | 0.022* |
| H5B | −0.108557 | 0.434339 | 0.660656 | 0.022* |
| C6 | −0.18845 (16) | 0.21055 (14) | 0.58967 (12) | 0.0235 (3) |
| H6A | −0.183048 | 0.162756 | 0.516621 | 0.028* |
| H6B | −0.294674 | 0.292329 | 0.574819 | 0.028* |
| C7 | −0.20545 (16) | 0.10962 (13) | 0.71960 (12) | 0.0228 (3) |
| H7A | −0.222266 | 0.159903 | 0.791731 | 0.027* |
| H7B | −0.311069 | 0.077179 | 0.714958 | 0.027* |
| C8 | 0.42331 (15) | 0.25494 (13) | 0.34363 (12) | 0.0183 (3) |
| C9 | 0.36126 (16) | 0.32034 (13) | 0.21533 (13) | 0.0237 (3) |
| H9 | 0.271743 | 0.407427 | 0.198590 | 0.028* |
| C10 | 0.42897 (16) | 0.25948 (13) | 0.11218 (12) | 0.0228 (3) |
| H10 | 0.385147 | 0.304037 | 0.024651 | 0.027* |
| C11 | 0.56138 (15) | 0.13291 (12) | 0.13611 (11) | 0.0175 (3) |
| C12 | 0.62112 (15) | 0.06565 (12) | 0.26465 (12) | 0.0176 (3) |
| H12 | 0.708970 | −0.022341 | 0.281932 | 0.021* |
| C13 | 0.55152 (15) | 0.12793 (12) | 0.36767 (11) | 0.0175 (3) |
| H13 | 0.592751 | 0.082492 | 0.455646 | 0.021* |
| C14 | 0.75390 (17) | −0.05001 (13) | 0.05126 (12) | 0.0240 (3) |
| H14A | 0.858124 | −0.044962 | 0.094595 | 0.029* |
| H14B | 0.787361 | −0.076083 | −0.033088 | 0.029* |
| H14C | 0.704607 | −0.120876 | 0.108448 | 0.029* |
| C15 | 0.24119 (15) | 0.46570 (12) | 0.67301 (11) | 0.0178 (3) |
| C17 | 0.22268 (18) | 0.60931 (14) | 0.82359 (12) | 0.0246 (3) |
| H17A | 0.228085 | 0.691792 | 0.752255 | 0.029* |
| H17B | 0.343285 | 0.559982 | 0.859765 | 0.029* |
| C18 | 0.10359 (18) | 0.65577 (14) | 0.93055 (13) | 0.0257 (3) |
| H18A | 0.100625 | 0.573518 | 1.001217 | 0.031* |
| H18B | −0.015559 | 0.703724 | 0.893858 | 0.031* |
| H18C | 0.147814 | 0.720445 | 0.966503 | 0.031* |
| H7 | −0.058 (3) | −0.0669 (17) | 0.6883 (15) | 0.047 (5)* |
| H3 | 0.431 (2) | 0.3830 (15) | 0.4679 (17) | 0.037 (4)* |
Source of materials
A mixture of 2,3-dioxopyrrolidine (2.00 g, 9.29 mmol), p-anisidine (1.37 g, 11.15 mmol) and formic acid (0.56 mL, 14.87 mmol) in ethanol was refluxed for 24 h. The solution was concentrated by distillation. The organic mixture was extracted with dichloromethane, washed with water and dried over MgSO4. The solvent was evaporated under reduced pressure and the crude was purified by column chromatography on silica gel (ethyl acetate:petroleum ether, 50:50) to afford 1 as brown solid product (2.34 g, 79%).
Experimental details
The data were indexed and processed using CrysAlisPRO [1]. A multi-scan absorption correction was performed. The structure was solved with SHELXT Version 2018/2 [2] solution program. The model was refined with SHELXL Version 2018/3 [3].
Comment
Pyrrolinones play an important role in organic synthesis and find vast applications in pharmaceutical fields due to their broad range of biological properties such as anti-inflammatory, antifungal, and antibacterial activities [5, 6]. Owing to their notable pharmaceutical effects, they have received tremendous attention over the past few years [7]. Thus, in view of diversifying the structural motifs of these classes of compounds, we decided to prepare the analogue of N-substituted pyrrolinone via amination of 2,3-dioxopyrrolidine with p-anisidine [8]. The starting material, 2,3-dioxopyrrolidine was initially prepared according to the reported procedure [9], [10], [11]. This paper describes the crystal structure of ethyl 1-(2-hydroxyethyl)-4-((4-methoxyphenyl)amino)-5-oxo-2,5-dihydro-1H-pyrrole-3-carboxylate (1).
The crystal structure of 1 is similar to previously reported structure of ethyl 1-(4-fluorophenyl)-4-phenyl-1H-pyrrole-3-carboxylate [12]. All bond angles and lengths are within the normal ranges and comparable to the related structures [12], [13], [14], [15]. The 4-methoxyaniline (N3/C8–C13/O11/C–14) and hydroxyethyl (C6/C7/O7) moieties are observed slightly twisted at N3–C8 and C6–C7 bonds, respectively. The twisted angle of C3–N3–C8–C9 is found to be 87.97° while the twisted angle for N1–C6–C7–O7 is −57.11. The ethyl acetate (O15/O16/C15/C17/C18) and 4-methoxyaniline (N3/C8–C13/O11/C14) moieties are almost perpendicular to each other, subtending a dihedral angle of 86.00°. Meanwhile, the pyrrole ring (N1/C2–C5) forms a dihedral angle of 83.17° with the phenyl ring (C8–C13). In the molecule, an intramolecular N3–H3···O15 hydrogen bond is observed, forming a pseudo six-membered ring, which further stabilizes and lock its atoms in a nearly planar arrangement. A similar feature was observed in the reported structure of ethyl 3-(4-methoxyphenyl)-5-methylcarbamoyl-1H-pyrazole-4-carboxylate [16].
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: Forest Research Institute Malaysia (FRIM) and Malaysian Government (MOHE) for the financial support (FRGS/1/2019/STG05/NRE/03/1).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Rigaku Oxford Diffraction. CrysAlisPRO; Rigaku Corp.: Tokyo, Japan, 2021.Search in Google Scholar
2. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar PubMed PubMed Central
3. Sheldrick, G. M. A short history of Shelx. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Search in Google Scholar PubMed
4. Macrae, C. F., Sovago, I., Cottrell, S. J., Galek, P. T. A., McCabe, P., Pidcock, E., Platings, M., Shields, G. P., Stevens, J. S., Towler, M., Wood, P. A. Mercury 4.0: from visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235; https://doi.org/10.1107/s1600576719014092.Search in Google Scholar PubMed PubMed Central
5. Ali, Y., Alam, M. S., Hamid, H., Hussain, A. 2(3H)Pyrrolone – a biologically active scaffold (A review). Orient. J. Chem. 2014, 30, 1–16; https://doi.org/10.13005/ojc/300101.Search in Google Scholar
6. Zali-Boeini, H., Mobin, M., Hajibabaei, K., Ghani, M. Approaches to the construction of substituted 4-amino-1H-pyrrol-2(5 H)-ones. J. Org. Chem. 2012, 77, 5808–5812; https://doi.org/10.1021/jo3004309.Search in Google Scholar PubMed
7. Tan, Q. W., Chovatia, P., Willis, M. C. Copper-catalysed synthesis of alkylidene 2-pyrrolinone derivatives from the combination of α-keto amides and alkynes. Org. Biomol. Chem. 2018, 16, 7797–7800; https://doi.org/10.1039/c8ob02205d.Search in Google Scholar PubMed
8. Bacho, M. Z., Mohammat, M. F., Shaameri, Z., Wibowo, A., Kamarulzaman, F., Hamzah, A. S. A facile synthesis of pyrrolidine-based iminosugars as potential alpha-glucosidase inhibitors. Orient. J. Chem. 2020, 36, 309–319; https://doi.org/10.13005/ojc/360214.Search in Google Scholar
9. Mohammat, M. F., Najim, N., Mansor, N. S., Sarman, S., Shaameri, Z., Zain, M. M., Hamzah, A. S. Synthesis and bioactivity of some 2-oxo-5-aryl-3-hydrazone and 2-oxo-5-aryl-4-hydrazone pyrrolidine derivatives. Arkivoc 2011, ix, 429–438; https://doi.org/10.3998/ark.5550190.0012.932.Search in Google Scholar
10. Abdul Rashid, F. N. A., Mohammat, M. F., Shaameri, Z., Hamzah, A. S. Synthesis of novel 3, 4-fused pyrazolidinone γ-lactam bicyclic moieties from 2,3-dioxo-4-carboxy-5-(substituted) pyrrolidines. Org. Commun. 2019, 12, 121–131; https://doi.org/10.25135/acg.oc.62.19.07.1323.Search in Google Scholar
11. Mohammat, M. F., Shaameri, Z., Hamzah, A. S. Synthesis of 2,3-dioxo-5-(substituted)arylpyrroles and their 2-oxo-5-aryl-3-hydrazone pyrrolidine derivatives. Molecules 2009, 14, 250–256; https://doi.org/10.3390/molecules14010250.Search in Google Scholar PubMed PubMed Central
12. Song, H.-Y., Liu, S., Zhang, B.-N., Zhong, Q.-D., Qi, Y.-J. Crystal structure of ethyl 1-(4-fluorophenyl)-4-phenyl-1H-pyrrole-3-carboxylate, C19H16FNO2. Z. Kristallogr. N. Cryst. Struct. 2021, 236, 1063–1064; https://doi.org/10.1515/ncrs-2021-0219.Search in Google Scholar
13. Mohammat, M. F., Shaameri, Z., Hamzah, A. S., Fun, H. K., Chantrapromma, S. 4-Hydroxy-5-(4-methoxyphenyl)pyrrolidin-2-one. Acta Crystallogr. 2008, E64, 6979–6981.10.1107/S1600536808003899Search in Google Scholar PubMed PubMed Central
14. Mohammat, M. F., Mansor, N. S., Shaameri, Z., Hamzah, A. S. Diastereoselective reduction of 2,3-dioxo-4-carboxy-5-substituted pyrrolidines using NaBH4/AcOH and heterogenous hydrogenation reactions. J. Kor. Chem. Soc. 2015, 59, 31–35; https://doi.org/10.5012/jkcs.2015.59.1.31.Search in Google Scholar
15. Naganagowda, G., Mahlaka, T. J., Meijboom, R. Crystal structure of methyl-2-methyl-4-(2-oxo-2-phenylethyl)-5-phenyl-1H-pyrrole-3- carboxylate, C21H19NO3. Z. Kristallogr. N. Cryst. Struct. 2017, 232, 63–65; https://doi.org/10.1515/ncrs-2016-0149.Search in Google Scholar
16. Abdul Rashid, F. N. A., Mohammat, M. F., Arshad, S., Shaameri, Z., Hamzah, A. S. Crystal structure of ethyl 3-(4-methoxyphenyl)-5-methylcarbamoyl-1H-pyrazole-4-carboxylate, C15H17N3O4. Z. Kristallogr. N. Cryst. Struct. 2019, 234, 1137–1139; https://doi.org/10.1515/ncrs-2019-0203.Search in Google Scholar
© 2022 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of undecacalcium decaarsenide, Ca11As10
- Crystal structure of catena-poly[diiodido-(μ2-1,5-dimethyl-2-phenyl-4-((pyridin-4- ylmethylene)amino)-1,2-dihydro-3H -pyrazol-3-one-κ2 N: O)zinc(II)], C17H16I2N4OZn
- The crystal structure of 5,10,15,20-tetrakis(4-(tert-butyl)phenyl)porphyrin-21,23-diido-κ4 N 4-naphthalocyanido-κ4 N 4-neodymium(IV) - chloroform (1/6) C114H90N12Cl18Nd
- The crystal structure of 1-(4-bromophenyl)-3-(2-chlorobenzyl)urea, C14H12BrClN2O
- Crystal structure of bis[benzyl(methyl)carbamodithioato-κ 2 S,S′]-di-n-butyltin(IV), C26H38N2S4Sn
- Crystal structure of (E)-3-(2-(4-(diethylamino)-2-hydroxystyryl)-3,3-dimethyl-3H-indol-1-ium-1-yl)propane-1-sulfonate – methanol (1/2), C25H32N2O4S⋅2CH3OH
- Synthesis and crystal structure of {(N′,N″-(((ethane-1,2-diylbis(oxy))bis(2,1-phenylene))-bis(methaneylylidene))bis(2-hydroxybenzohydrazonato)-κ6 N 2 O 4}copper(II), C30H24CuN4O6
- The crystal structure of ((E)-2,4-dibromo-6-(((5-(nitro)-2-oxidophenyl)imino)methyl)phenolato-κ 3 N,O,O′)tris(pyridine-κN)manganese(II), C28H21Br2MnN5O4
- Synthesis and crystal structure of 1-((3R,10S,13R,17S)-10,13-dimethyl-3-(p-tolylamino)hexadecahydro-1H-cyclopenta-[a]phenanthren-17-yl)ethan-1-one, C28H41NO
- The crystal structure of 3-nitrobenzene-1,2-dicarboxylic acid—pyrazine-2-carboxamide(1/1), C13H10N4O7
- Crystal structure of poly[tetrakis(μ3-2-aminonicotinato-κ3N,O,O′)-(μ2-oxalato-κ4 O,O′:O″,O′″)-(μ4-oxalato-κ6 N:N′:O,O′:O″,O′″)dicopper(I)-disamarium(III)], [SmCu(C6N2H5O2)2(C2O4)] n
- The crystal structure of 2,3,4-trihydroxybenzoic- acid—pyrazine-2-carboxamide—water (1/1/1), C12H13N3O7
- Crystal structure of N-ethyl-4-[3-(trifluoromethyl)-phenyl]piperazine-1-carbothioamide, C14H18F3N3S
- The crystal structure of 3-anilino-1,4-diphenyl-4H-1,2,4-triazol-1-ium iodide, C20H17N4I
- The crystal structure of (tris(2-benzimidazolylmethyl)amine)-benzoato-copper(II) perchlorate monohydrate, CuC31H28N7O7Cl
- Crystal structure of [2-hydroxy-3-methyl-benzoato-k1 O-triphenyltin(IV)], C26H22O3Sn
- Crystal structure of diaqua-bis(4-(hydroxymethyl)-benzoato-k1 O)zinc(II), C16H18O8Zn
- The crystal structure of dicarbonyl-(N-nitroso-N-oxido-phenylamine-κ 2 O,O)-rhodium(I), C8H5N2O4Rh
- The crystal structure of oxalic acid – 2-ethoxybenzamide (2/1), C20H24N2O8
- The crystal structure of ethyl 7-ethyl-5-methyl-4,7-dihydrotetrazolo[1,5-a]pyrimidine-6-carboxylate, C10H15N5O2
- Crystal structure of poly[(N,N-dimethylacetamide-κO) (μ4-2-nitroisophthalato-κ 4 O:O′:O″:O′″)manganese(II)], C11H10N2O7Mn
- Crystal structure of 14-O-acetyldelcosine, C26H41NO8
- The crystal structure of poly[(1,10-phenanthroline-κ2 N,N′)-(μ 4-2-chlorobenzene-1,3-dicarboxylato-κ5 O:O′:O″:O‴) cadmium(II)] monohydrate, C20H13CdClN2O5
- Crystal structure of propane-1,3-diylbis(diphenylphosphine sulfide) ethanol solvate, C27H26P2S2
- Crystal structure of bis{[(4-diethylamino-2-hydroxy-benzylidene)-hydrazinocarbonylmethyl]-trimethylammonium} tetrabromozincate, C32H54N8O4ZnBr4
- Synthesis and crystal structure of dimethyl 2,2′-(2,5-bis(4-hydroxyphenyl)-2,5-dihydrofuran-3,4-diyl)dibenzoate, C34H30O7
- Synthesis and crystal structure of 2-(2-oxo-2-phenylethyl)-4H-chromen-4-one, C17H12O3
- The crystal structure of tetra(imidazole-κ1 N)zinc(II) μ2-oxido-hexaoxido-divanadium(VI) C12H16N8O6V2Zn
- Crystal structure of S-2-(1-(5-methylpyridin-2-ylamino)octyl)-3-hydroxynaphthalene-1,4-dione, C24H28N2O3
- Crystal structure of bis(μ2-5-chloro-2-oxido-N-(1-oxido-2-oxo-2-phenylethylidene)-benzohydrazonato-κ5 N,O,O′:N′,O′′)-oktakis(pyridine-κ1 N)trinickel(II) – methanol – pyridine (1/1/1) C76H65N13Cl2Ni3O9
- The crystal structure of methyl 3,5-diaminobenzoate, C8H10N2O2
- Crystal structure of 10-(9H-carbazol-9-yl)-5H-dibenzo[a,d][7]annelen-5-one, C27H17NO
- Crystal structure of ethyl 1-(2-hydroxyethyl)-4-((4-methoxyphenyl)amino)-5-oxo-2,5-dihydro-1H-pyrrole-3-carboxylate, C16H20N2O5
- The crystal structure of 1-(4-bromophenyl)-3-cycloheptylurea, C14H19BrN2O
- The crystal structure of 1,4-bis(1,2,3,4,5-pentamethylcyclopenta-2,4-dien-1-yl)-3,6-bis ((4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)methylene)-1,4-dialuminacyclohexane – benzene (1/2), C50H72Al2B2O4
- Crystal structure of bis(μ 3-diphenylphosphinato)-tetrakis(μ 2-diphenylphosphinato)-bis(diphenylphosphinato)-bis(μ 2-hydroxo)dicopper(II)-ditin(IV), C104H100O18P8Cu2Sn2
- Crystal structure of 3-((3,4-dichloroisothiazol-5-yl)methoxy)benzo[d] isothiazole 1,1-dioxide, C11H6Cl2N2O3S2
- Synthesis and crystal structure of 2-(2-(2-fluorophenyl)-2-oxoethyl)-4H-chromen-4-one, C17H11FO3
- The crystal structure of tris(carbonyl)-bis(carbonyl)-[μ-propane-1,2- dithiolato]-(benzyldiphenylphosphine)diiron (Fe—Fe), C27H23Fe2O5PS2
- Crystal structure of 1-(2-(4-chlorophenethyl)-2-hydroxy-3,3-dimethylbutyl)-1H-1,2,4-triazol-4-ium nitrate, C16H23N4O4Cl
- The crystal structure of 3,3′-disulfanediyldi(1H-1,2,4-triazol-5-amine) monohydrate, C4H8N8OS2
- The crystal structure of trans-[bis(4-methylpyridine-κN)bis(quinoline-2-carboxylato- κ 2 N,O)cadmium(II)], C32H26CdN4O4
- The crystal structure of ethyl 2′-hydroxy-4′,6′-dimethoxy-3-(4-methoxynaphthalen-1-yl)-5-oxo-2,3,4,5-tetrahydro-[1,1′-biphenyl]-4-carboxylate, C28H28O7
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of undecacalcium decaarsenide, Ca11As10
- Crystal structure of catena-poly[diiodido-(μ2-1,5-dimethyl-2-phenyl-4-((pyridin-4- ylmethylene)amino)-1,2-dihydro-3H -pyrazol-3-one-κ2 N: O)zinc(II)], C17H16I2N4OZn
- The crystal structure of 5,10,15,20-tetrakis(4-(tert-butyl)phenyl)porphyrin-21,23-diido-κ4 N 4-naphthalocyanido-κ4 N 4-neodymium(IV) - chloroform (1/6) C114H90N12Cl18Nd
- The crystal structure of 1-(4-bromophenyl)-3-(2-chlorobenzyl)urea, C14H12BrClN2O
- Crystal structure of bis[benzyl(methyl)carbamodithioato-κ 2 S,S′]-di-n-butyltin(IV), C26H38N2S4Sn
- Crystal structure of (E)-3-(2-(4-(diethylamino)-2-hydroxystyryl)-3,3-dimethyl-3H-indol-1-ium-1-yl)propane-1-sulfonate – methanol (1/2), C25H32N2O4S⋅2CH3OH
- Synthesis and crystal structure of {(N′,N″-(((ethane-1,2-diylbis(oxy))bis(2,1-phenylene))-bis(methaneylylidene))bis(2-hydroxybenzohydrazonato)-κ6 N 2 O 4}copper(II), C30H24CuN4O6
- The crystal structure of ((E)-2,4-dibromo-6-(((5-(nitro)-2-oxidophenyl)imino)methyl)phenolato-κ 3 N,O,O′)tris(pyridine-κN)manganese(II), C28H21Br2MnN5O4
- Synthesis and crystal structure of 1-((3R,10S,13R,17S)-10,13-dimethyl-3-(p-tolylamino)hexadecahydro-1H-cyclopenta-[a]phenanthren-17-yl)ethan-1-one, C28H41NO
- The crystal structure of 3-nitrobenzene-1,2-dicarboxylic acid—pyrazine-2-carboxamide(1/1), C13H10N4O7
- Crystal structure of poly[tetrakis(μ3-2-aminonicotinato-κ3N,O,O′)-(μ2-oxalato-κ4 O,O′:O″,O′″)-(μ4-oxalato-κ6 N:N′:O,O′:O″,O′″)dicopper(I)-disamarium(III)], [SmCu(C6N2H5O2)2(C2O4)] n
- The crystal structure of 2,3,4-trihydroxybenzoic- acid—pyrazine-2-carboxamide—water (1/1/1), C12H13N3O7
- Crystal structure of N-ethyl-4-[3-(trifluoromethyl)-phenyl]piperazine-1-carbothioamide, C14H18F3N3S
- The crystal structure of 3-anilino-1,4-diphenyl-4H-1,2,4-triazol-1-ium iodide, C20H17N4I
- The crystal structure of (tris(2-benzimidazolylmethyl)amine)-benzoato-copper(II) perchlorate monohydrate, CuC31H28N7O7Cl
- Crystal structure of [2-hydroxy-3-methyl-benzoato-k1 O-triphenyltin(IV)], C26H22O3Sn
- Crystal structure of diaqua-bis(4-(hydroxymethyl)-benzoato-k1 O)zinc(II), C16H18O8Zn
- The crystal structure of dicarbonyl-(N-nitroso-N-oxido-phenylamine-κ 2 O,O)-rhodium(I), C8H5N2O4Rh
- The crystal structure of oxalic acid – 2-ethoxybenzamide (2/1), C20H24N2O8
- The crystal structure of ethyl 7-ethyl-5-methyl-4,7-dihydrotetrazolo[1,5-a]pyrimidine-6-carboxylate, C10H15N5O2
- Crystal structure of poly[(N,N-dimethylacetamide-κO) (μ4-2-nitroisophthalato-κ 4 O:O′:O″:O′″)manganese(II)], C11H10N2O7Mn
- Crystal structure of 14-O-acetyldelcosine, C26H41NO8
- The crystal structure of poly[(1,10-phenanthroline-κ2 N,N′)-(μ 4-2-chlorobenzene-1,3-dicarboxylato-κ5 O:O′:O″:O‴) cadmium(II)] monohydrate, C20H13CdClN2O5
- Crystal structure of propane-1,3-diylbis(diphenylphosphine sulfide) ethanol solvate, C27H26P2S2
- Crystal structure of bis{[(4-diethylamino-2-hydroxy-benzylidene)-hydrazinocarbonylmethyl]-trimethylammonium} tetrabromozincate, C32H54N8O4ZnBr4
- Synthesis and crystal structure of dimethyl 2,2′-(2,5-bis(4-hydroxyphenyl)-2,5-dihydrofuran-3,4-diyl)dibenzoate, C34H30O7
- Synthesis and crystal structure of 2-(2-oxo-2-phenylethyl)-4H-chromen-4-one, C17H12O3
- The crystal structure of tetra(imidazole-κ1 N)zinc(II) μ2-oxido-hexaoxido-divanadium(VI) C12H16N8O6V2Zn
- Crystal structure of S-2-(1-(5-methylpyridin-2-ylamino)octyl)-3-hydroxynaphthalene-1,4-dione, C24H28N2O3
- Crystal structure of bis(μ2-5-chloro-2-oxido-N-(1-oxido-2-oxo-2-phenylethylidene)-benzohydrazonato-κ5 N,O,O′:N′,O′′)-oktakis(pyridine-κ1 N)trinickel(II) – methanol – pyridine (1/1/1) C76H65N13Cl2Ni3O9
- The crystal structure of methyl 3,5-diaminobenzoate, C8H10N2O2
- Crystal structure of 10-(9H-carbazol-9-yl)-5H-dibenzo[a,d][7]annelen-5-one, C27H17NO
- Crystal structure of ethyl 1-(2-hydroxyethyl)-4-((4-methoxyphenyl)amino)-5-oxo-2,5-dihydro-1H-pyrrole-3-carboxylate, C16H20N2O5
- The crystal structure of 1-(4-bromophenyl)-3-cycloheptylurea, C14H19BrN2O
- The crystal structure of 1,4-bis(1,2,3,4,5-pentamethylcyclopenta-2,4-dien-1-yl)-3,6-bis ((4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)methylene)-1,4-dialuminacyclohexane – benzene (1/2), C50H72Al2B2O4
- Crystal structure of bis(μ 3-diphenylphosphinato)-tetrakis(μ 2-diphenylphosphinato)-bis(diphenylphosphinato)-bis(μ 2-hydroxo)dicopper(II)-ditin(IV), C104H100O18P8Cu2Sn2
- Crystal structure of 3-((3,4-dichloroisothiazol-5-yl)methoxy)benzo[d] isothiazole 1,1-dioxide, C11H6Cl2N2O3S2
- Synthesis and crystal structure of 2-(2-(2-fluorophenyl)-2-oxoethyl)-4H-chromen-4-one, C17H11FO3
- The crystal structure of tris(carbonyl)-bis(carbonyl)-[μ-propane-1,2- dithiolato]-(benzyldiphenylphosphine)diiron (Fe—Fe), C27H23Fe2O5PS2
- Crystal structure of 1-(2-(4-chlorophenethyl)-2-hydroxy-3,3-dimethylbutyl)-1H-1,2,4-triazol-4-ium nitrate, C16H23N4O4Cl
- The crystal structure of 3,3′-disulfanediyldi(1H-1,2,4-triazol-5-amine) monohydrate, C4H8N8OS2
- The crystal structure of trans-[bis(4-methylpyridine-κN)bis(quinoline-2-carboxylato- κ 2 N,O)cadmium(II)], C32H26CdN4O4
- The crystal structure of ethyl 2′-hydroxy-4′,6′-dimethoxy-3-(4-methoxynaphthalen-1-yl)-5-oxo-2,3,4,5-tetrahydro-[1,1′-biphenyl]-4-carboxylate, C28H28O7

