Abstract
C28H28O7, triclinic,
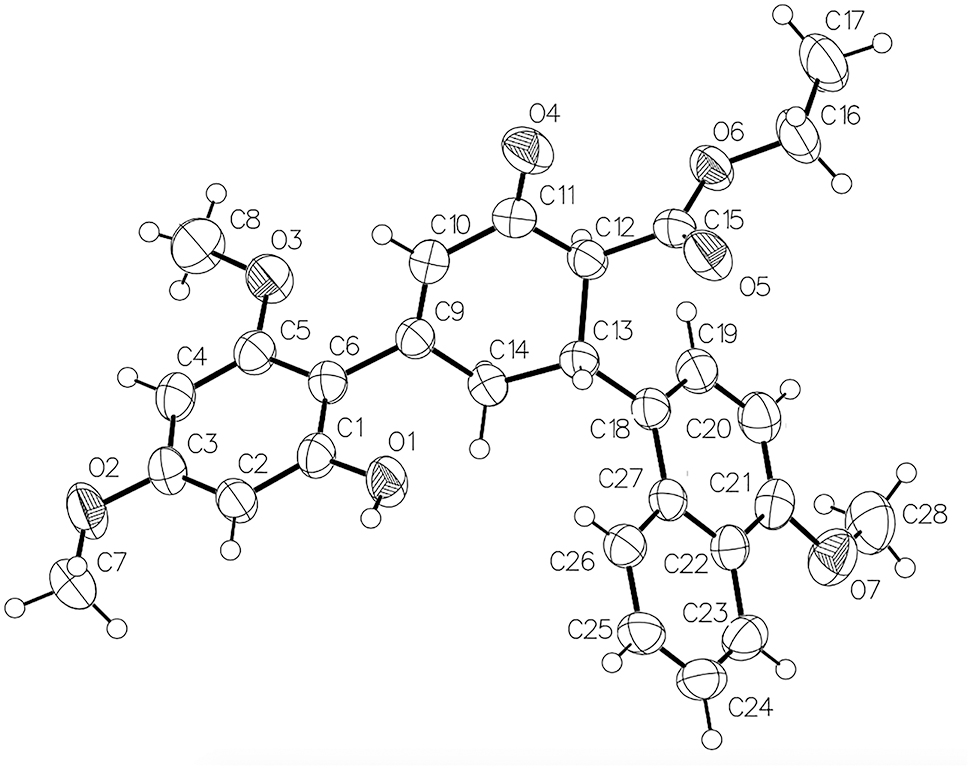
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless Block |
| Size: | 0.20 × 0.15 × 0.10 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.10 mm−1 |
| Diffractometer, scan mode: | PHOTON 100 CMOS, φ and ω |
| θ max, completeness: | 26.2°, 99% |
| N(hkl)measured, N(hkl)unique, R int: | 29,975, 4697, 0.065 |
| Criterion for I obs, N(hkl)gt: | I obs > 2σ(I obs), 2951 |
| N(param)refined: | 321 |
| Programs: | Bruker [1], SHELX [2, 3], Olex2 [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| C1 | −0.0681 (2) | 0.3211 (2) | 0.4174 (2) | 0.0449 (6) |
| O1 | −0.10339 (17) | 0.21218 (14) | 0.46434 (19) | 0.0589 (5) |
| H1 | −0.1865 | 0.2285 | 0.5235 | 0.088* |
| C2 | −0.1724 (2) | 0.4390 (2) | 0.4501 (2) | 0.0482 (6) |
| H2 | −0.2694 | 0.4457 | 0.5045 | 0.058* |
| C3 | −0.1303 (2) | 0.5454 (2) | 0.4009 (2) | 0.0488 (6) |
| C4 | 0.0126 (3) | 0.5373 (2) | 0.3221 (3) | 0.0523 (6) |
| H4 | 0.0396 | 0.6110 | 0.2898 | 0.063* |
| C5 | 0.1145 (2) | 0.4194 (2) | 0.2918 (2) | 0.0464 (6) |
| C6 | 0.0757 (2) | 0.3082 (2) | 0.3372 (2) | 0.0418 (5) |
| O2 | −0.22372 (19) | 0.66599 (15) | 0.4240 (2) | 0.0678 (6) |
| C7 | −0.3696 (3) | 0.6821 (3) | 0.5072 (3) | 0.0687 (8) |
| H7A | −0.4061 | 0.6384 | 0.4740 | 0.103* |
| H7B | −0.4240 | 0.7715 | 0.5104 | 0.103* |
| H7C | −0.3794 | 0.6474 | 0.5946 | 0.103* |
| O3 | 0.25865 (17) | 0.40044 (16) | 0.21597 (19) | 0.0626 (5) |
| C8 | 0.3042 (3) | 0.5099 (3) | 0.1669 (4) | 0.0809 (10) |
| H8A | 0.2573 | 0.5628 | 0.1117 | 0.121* |
| H8B | 0.4077 | 0.4842 | 0.1158 | 0.121* |
| H8C | 0.2784 | 0.5570 | 0.2396 | 0.121* |
| C9 | 0.1814 (2) | 0.1795 (2) | 0.3038 (2) | 0.0426 (6) |
| C10 | 0.2742 (2) | 0.1251 (2) | 0.3574 (3) | 0.0511 (6) |
| H10 | 0.2735 | 0.1705 | 0.4164 | 0.061* |
| C11 | 0.3768 (2) | −0.0017 (2) | 0.3291 (3) | 0.0505 (6) |
| O4 | 0.4554 (2) | −0.05331 (19) | 0.3844 (2) | 0.0796 (7) |
| C12 | 0.3837 (2) | −0.0686 (2) | 0.2244 (2) | 0.0429 (6) |
| H12 | 0.4482 | −0.0409 | 0.1373 | 0.051* |
| C13 | 0.2331 (2) | −0.0325 (2) | 0.2282 (2) | 0.0400 (5) |
| H13 | 0.1688 | −0.0568 | 0.3172 | 0.048* |
| C14 | 0.1765 (2) | 0.1120 (2) | 0.2085 (2) | 0.0446 (6) |
| H14A | 0.2344 | 0.1381 | 0.1188 | 0.054* |
| H14B | 0.0769 | 0.1371 | 0.2196 | 0.054* |
| C15 | 0.4474 (2) | −0.2094 (2) | 0.2457 (2) | 0.0463 (6) |
| O5 | 0.38290 (17) | −0.27433 (16) | 0.33133 (19) | 0.0634 (5) |
| O6 | 0.58315 (15) | −0.25410 (15) | 0.16003 (17) | 0.0535 (5) |
| C16 | 0.6520 (3) | −0.3910 (2) | 0.1711 (3) | 0.0701 (8) |
| H16A | 0.6061 | −0.4360 | 0.1494 | 0.084* |
| H16B | 0.6417 | −0.4174 | 0.2611 | 0.084* |
| C17 | 0.8053 (3) | −0.4213 (3) | 0.0799 (4) | 0.0863 (11) |
| H17A | 0.8146 | −0.3911 | −0.0083 | 0.129* |
| H17B | 0.8512 | −0.5122 | 0.0825 | 0.129* |
| H17C | 0.8512 | −0.3806 | 0.1054 | 0.129* |
| C18 | 0.2266 (2) | −0.0975 (2) | 0.1310 (2) | 0.0427 (6) |
| C19 | 0.3440 (2) | −0.1423 (2) | 0.0176 (3) | 0.0499 (6) |
| H19 | 0.4333 | −0.1379 | 0.0025 | 0.060* |
| C20 | 0.3364 (3) | −0.1950 (2) | −0.0775 (3) | 0.0548 (7) |
| H20 | 0.4196 | −0.2258 | −0.1537 | 0.066* |
| C21 | 0.2073 (3) | −0.2008 (2) | −0.0580 (3) | 0.0524 (6) |
| C22 | 0.0818 (2) | −0.1570 (2) | 0.0572 (2) | 0.0458 (6) |
| C23 | −0.0532 (3) | −0.1617 (2) | 0.0778 (3) | 0.0544 (7) |
| H23 | −0.0588 | −0.1956 | 0.0141 | 0.065* |
| C24 | −0.1733 (3) | −0.1185 (2) | 0.1867 (3) | 0.0580 (7) |
| H24 | −0.2619 | −0.1214 | 0.1979 | 0.070* |
| C25 | −0.1662 (2) | −0.0693 (2) | 0.2830 (3) | 0.0550 (7) |
| H25 | −0.2501 | −0.0403 | 0.3593 | 0.066* |
| C26 | −0.0380 (2) | −0.0630 (2) | 0.2672 (3) | 0.0488 (6) |
| H26 | −0.0354 | −0.0297 | 0.3331 | 0.059* |
| C27 | 0.0905 (2) | −0.1057 (2) | 0.1534 (2) | 0.0423 (5) |
| O7 | 0.1851 (2) | −0.24807 (19) | −0.14345 (19) | 0.0702 (6) |
| C28 | 0.2999 (3) | −0.2770 (3) | −0.2688 (3) | 0.0792 (9) |
| H28A | 0.3298 | −0.2028 | −0.3106 | 0.119* |
| H28B | 0.2685 | −0.3024 | −0.3221 | 0.119* |
| H28C | 0.3803 | −0.3454 | −0.2597 | 0.119* |
Source of material
To a solution of 4,6-dimethoxy-2-hydroxyacetophenone (20 mmol, 3.32 g) in 100 ml of ethanol was added 4-methoxy-1-naphthaldehyde (20 mmol, 3.72 g) and the temperature was adjusted to around 276–278 K in an ice-bath. To the reaction mixture was added 15 mL of 50% (w/v) aqueous KOH solution and the reaction mixture was stirred at room temperature for 24 h. At the end of the reaction, ice water was added to the mixture and it was acidified with 6N HCl (pH = 3–4). The resulting precipitate was filtered and washed with water and ethanol. The crude solid was purified by recrystallization from ethanol to give pure chalcone. An excess amount of ethylacetoacetate (2.6 g, 20 mmol) was added to a solution of the chalcone compound (5 mmol, 1.82 g) in 50 mL anhydrous ethanol, and 5 mL of aqueous NaOH solution was added to the reaction mixture and was refluxed at 360 K for 5 h. The reaction mixture was cooled to room temperature and was acidified with 3N HCl (pH = 3–4). The resulting precipitate was filtered and washed with water and ethanol. The crude solid product was purified by recrystallization from ethanol to afford the title compound (m.p.: 375–377 K; yield: 72%).
Experimental details
Data collections and reduction were carried out using the Bruker software Apex2 and Saint including Sadabs [1]. Hydrogen atoms were placed in their geometrically idealized positions and constrained to ride on their parent atoms.
Comment
Compounds containing a cyclohexenone moiety have been reported to inhibit acetylcholinesterase (AChE) [5], attenuate heart failure in a zebra fish model [6] and show anticancer activities in HCT116 human colon cancer cells [7]. Since cyclohexenone derivatives exhibit various biological activities, the title compound was designed and synthesized according to a previous report [8].
The molecular structure of the title compound is shown in the Figure. The central cyclohexenone ring (C9–C14) has an envelope conformation with the atom C13 as the flap. The benzene ring (C1–C6) and the naphthalene ring system (C18–C27) are attached to the central cyclohexenone ring (C9–C14) at positions C9 and C13, respectively. The dihedral angle between the benzene and ring (C1–C6) and the naphthalene ring system (C18–C27) is 65.03(3)°. The hydrogen atom H13 attached to C13 forms a trans diaxial conformation with each one of H12 atom of the C12 (H12–C12–C13–H13 = −177.2°) and H14A atom of the C14 methylene group (H14A–C14–C13–H13 = −176.1°). The hydrogen atom H13 forms a gauche conformation with the other methylene hydrogen atom H14B (H14B–C14–C13–H13 = −58.4°). The methoxy group at the ortho and para position of the benzene ring are almost coplanar with the ring [C6–C5–O3–C8 = 179.8(2)°, C2–C3–O2–C7 = 3.0(4)°]. However, the methoxy group on the naphthalene ring is tilted from the ring [C20–C21–O7–C28 = 9.1(4)°]. In the crystal, molecules are linked by pairs of O1–H1⋯O5 interactions forming inversion dimers. There are also C–H⋯O interactions present and the dimers are linked via C7–H7B⋯O4 hydrogen bonds. All derived bond lengths and angles of the title structure are in the typical ranges [9, 10].
Funding source: Basic Science Research Program
Award Identifier / Grant number: NRF-2021R1F1A1052699
Acknowledgements
The authors acknowledge financial support from the Basic Science Research Program (award No. NRF-2021R1F1A1052699). S. Y. Shin was supported by the KU Research Professor Program of Konkuk University.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: Basic Science Research Program (award No. NRF-2021R1F1A1052699).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. SAINT, APEX2 and SADABS; Bruker AXS Inc.: Madison, WI, USA, 2012.Search in Google Scholar
2. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar PubMed PubMed Central
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar PubMed PubMed Central
4. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
5. Zheng, Z. H., Dong, Y. S., Zhang, H., Lu, X. H., Ren, X., Zhao, G., He, J. G., Si, S. Y. Isolation and characterization of N98–1272 A, B and C, selective acetylcholinesterase inhibitors from metabolites of an actinomycete strain. J. Enzym. Inhib. Med. Chem. 2007, 22, 43–49; https://doi.org/10.1080/14756360600988781.Search in Google Scholar PubMed
6. Huang, C., Monte, A., Cook, J. M., Kabir, M. S., Peterson, K. P. Zebrafish heart failure models for the evaluation of chemical probes and drugs. Assay Drug Dev. Technol. 2013, 11, 561–572; https://doi.org/10.1089/adt.2013.548.Search in Google Scholar PubMed PubMed Central
7. Shin, S. Y., Park, J., Jung, Y., Lee, Y. H., Koh, D., Yoon, Y., Lim, Y. Anticancer activities of cyclohexenone derivatives. Appl. Biol. Chem. 2020, 63, 82; https://doi.org/10.1186/s13765-020-00567-1.Search in Google Scholar
8. Lee, Y., Koh, D., Lim, Y. 1H and 13C NMR spectral assignments of 25 ethyl 2-oxocyclohex-3-enecarboxylates. Magn. Reson. Chem. 2018, 56, 1188–1200; https://doi.org/10.1002/mrc.4778.Search in Google Scholar PubMed
9. Duan, J., He, X., Choy, P. Y., Wang, Q., Xie, M., Li, R., Xu, K., Shang, Y., Kwong, F. Y. Cascade lactonization/benzannulation of propargylamines with dimethyl 3-oxoglutarate for modular assembly of hydroxylated/arene-functionalized benzo[c]chromen-6-ones. Org. Lett. 2021, 23, 6455–6460; https://doi.org/10.1021/acs.orglett.1c02266.Search in Google Scholar PubMed
10. Fun, H.-K., Jebas, S. R., Girish, K. S., Kalluraya, B. Ethyl 6-(4-chlorophenyl)-4-(4-methoxyphenyl)-2-oxocyclohex-3-ene-1-carboxylate. Acta Crystallogr. 2009, E65, o1235; https://doi.org/10.1107/s1600536809016237.Search in Google Scholar PubMed PubMed Central
© 2022 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of undecacalcium decaarsenide, Ca11As10
- Crystal structure of catena-poly[diiodido-(μ2-1,5-dimethyl-2-phenyl-4-((pyridin-4- ylmethylene)amino)-1,2-dihydro-3H -pyrazol-3-one-κ2 N: O)zinc(II)], C17H16I2N4OZn
- The crystal structure of 5,10,15,20-tetrakis(4-(tert-butyl)phenyl)porphyrin-21,23-diido-κ4 N 4-naphthalocyanido-κ4 N 4-neodymium(IV) - chloroform (1/6) C114H90N12Cl18Nd
- The crystal structure of 1-(4-bromophenyl)-3-(2-chlorobenzyl)urea, C14H12BrClN2O
- Crystal structure of bis[benzyl(methyl)carbamodithioato-κ 2 S,S′]-di-n-butyltin(IV), C26H38N2S4Sn
- Crystal structure of (E)-3-(2-(4-(diethylamino)-2-hydroxystyryl)-3,3-dimethyl-3H-indol-1-ium-1-yl)propane-1-sulfonate – methanol (1/2), C25H32N2O4S⋅2CH3OH
- Synthesis and crystal structure of {(N′,N″-(((ethane-1,2-diylbis(oxy))bis(2,1-phenylene))-bis(methaneylylidene))bis(2-hydroxybenzohydrazonato)-κ6 N 2 O 4}copper(II), C30H24CuN4O6
- The crystal structure of ((E)-2,4-dibromo-6-(((5-(nitro)-2-oxidophenyl)imino)methyl)phenolato-κ 3 N,O,O′)tris(pyridine-κN)manganese(II), C28H21Br2MnN5O4
- Synthesis and crystal structure of 1-((3R,10S,13R,17S)-10,13-dimethyl-3-(p-tolylamino)hexadecahydro-1H-cyclopenta-[a]phenanthren-17-yl)ethan-1-one, C28H41NO
- The crystal structure of 3-nitrobenzene-1,2-dicarboxylic acid—pyrazine-2-carboxamide(1/1), C13H10N4O7
- Crystal structure of poly[tetrakis(μ3-2-aminonicotinato-κ3N,O,O′)-(μ2-oxalato-κ4 O,O′:O″,O′″)-(μ4-oxalato-κ6 N:N′:O,O′:O″,O′″)dicopper(I)-disamarium(III)], [SmCu(C6N2H5O2)2(C2O4)] n
- The crystal structure of 2,3,4-trihydroxybenzoic- acid—pyrazine-2-carboxamide—water (1/1/1), C12H13N3O7
- Crystal structure of N-ethyl-4-[3-(trifluoromethyl)-phenyl]piperazine-1-carbothioamide, C14H18F3N3S
- The crystal structure of 3-anilino-1,4-diphenyl-4H-1,2,4-triazol-1-ium iodide, C20H17N4I
- The crystal structure of (tris(2-benzimidazolylmethyl)amine)-benzoato-copper(II) perchlorate monohydrate, CuC31H28N7O7Cl
- Crystal structure of [2-hydroxy-3-methyl-benzoato-k1 O-triphenyltin(IV)], C26H22O3Sn
- Crystal structure of diaqua-bis(4-(hydroxymethyl)-benzoato-k1 O)zinc(II), C16H18O8Zn
- The crystal structure of dicarbonyl-(N-nitroso-N-oxido-phenylamine-κ 2 O,O)-rhodium(I), C8H5N2O4Rh
- The crystal structure of oxalic acid – 2-ethoxybenzamide (2/1), C20H24N2O8
- The crystal structure of ethyl 7-ethyl-5-methyl-4,7-dihydrotetrazolo[1,5-a]pyrimidine-6-carboxylate, C10H15N5O2
- Crystal structure of poly[(N,N-dimethylacetamide-κO) (μ4-2-nitroisophthalato-κ 4 O:O′:O″:O′″)manganese(II)], C11H10N2O7Mn
- Crystal structure of 14-O-acetyldelcosine, C26H41NO8
- The crystal structure of poly[(1,10-phenanthroline-κ2 N,N′)-(μ 4-2-chlorobenzene-1,3-dicarboxylato-κ5 O:O′:O″:O‴) cadmium(II)] monohydrate, C20H13CdClN2O5
- Crystal structure of propane-1,3-diylbis(diphenylphosphine sulfide) ethanol solvate, C27H26P2S2
- Crystal structure of bis{[(4-diethylamino-2-hydroxy-benzylidene)-hydrazinocarbonylmethyl]-trimethylammonium} tetrabromozincate, C32H54N8O4ZnBr4
- Synthesis and crystal structure of dimethyl 2,2′-(2,5-bis(4-hydroxyphenyl)-2,5-dihydrofuran-3,4-diyl)dibenzoate, C34H30O7
- Synthesis and crystal structure of 2-(2-oxo-2-phenylethyl)-4H-chromen-4-one, C17H12O3
- The crystal structure of tetra(imidazole-κ1 N)zinc(II) μ2-oxido-hexaoxido-divanadium(VI) C12H16N8O6V2Zn
- Crystal structure of S-2-(1-(5-methylpyridin-2-ylamino)octyl)-3-hydroxynaphthalene-1,4-dione, C24H28N2O3
- Crystal structure of bis(μ2-5-chloro-2-oxido-N-(1-oxido-2-oxo-2-phenylethylidene)-benzohydrazonato-κ5 N,O,O′:N′,O′′)-oktakis(pyridine-κ1 N)trinickel(II) – methanol – pyridine (1/1/1) C76H65N13Cl2Ni3O9
- The crystal structure of methyl 3,5-diaminobenzoate, C8H10N2O2
- Crystal structure of 10-(9H-carbazol-9-yl)-5H-dibenzo[a,d][7]annelen-5-one, C27H17NO
- Crystal structure of ethyl 1-(2-hydroxyethyl)-4-((4-methoxyphenyl)amino)-5-oxo-2,5-dihydro-1H-pyrrole-3-carboxylate, C16H20N2O5
- The crystal structure of 1-(4-bromophenyl)-3-cycloheptylurea, C14H19BrN2O
- The crystal structure of 1,4-bis(1,2,3,4,5-pentamethylcyclopenta-2,4-dien-1-yl)-3,6-bis ((4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)methylene)-1,4-dialuminacyclohexane – benzene (1/2), C50H72Al2B2O4
- Crystal structure of bis(μ 3-diphenylphosphinato)-tetrakis(μ 2-diphenylphosphinato)-bis(diphenylphosphinato)-bis(μ 2-hydroxo)dicopper(II)-ditin(IV), C104H100O18P8Cu2Sn2
- Crystal structure of 3-((3,4-dichloroisothiazol-5-yl)methoxy)benzo[d] isothiazole 1,1-dioxide, C11H6Cl2N2O3S2
- Synthesis and crystal structure of 2-(2-(2-fluorophenyl)-2-oxoethyl)-4H-chromen-4-one, C17H11FO3
- The crystal structure of tris(carbonyl)-bis(carbonyl)-[μ-propane-1,2- dithiolato]-(benzyldiphenylphosphine)diiron (Fe—Fe), C27H23Fe2O5PS2
- Crystal structure of 1-(2-(4-chlorophenethyl)-2-hydroxy-3,3-dimethylbutyl)-1H-1,2,4-triazol-4-ium nitrate, C16H23N4O4Cl
- The crystal structure of 3,3′-disulfanediyldi(1H-1,2,4-triazol-5-amine) monohydrate, C4H8N8OS2
- The crystal structure of trans-[bis(4-methylpyridine-κN)bis(quinoline-2-carboxylato- κ 2 N,O)cadmium(II)], C32H26CdN4O4
- The crystal structure of ethyl 2′-hydroxy-4′,6′-dimethoxy-3-(4-methoxynaphthalen-1-yl)-5-oxo-2,3,4,5-tetrahydro-[1,1′-biphenyl]-4-carboxylate, C28H28O7
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of undecacalcium decaarsenide, Ca11As10
- Crystal structure of catena-poly[diiodido-(μ2-1,5-dimethyl-2-phenyl-4-((pyridin-4- ylmethylene)amino)-1,2-dihydro-3H -pyrazol-3-one-κ2 N: O)zinc(II)], C17H16I2N4OZn
- The crystal structure of 5,10,15,20-tetrakis(4-(tert-butyl)phenyl)porphyrin-21,23-diido-κ4 N 4-naphthalocyanido-κ4 N 4-neodymium(IV) - chloroform (1/6) C114H90N12Cl18Nd
- The crystal structure of 1-(4-bromophenyl)-3-(2-chlorobenzyl)urea, C14H12BrClN2O
- Crystal structure of bis[benzyl(methyl)carbamodithioato-κ 2 S,S′]-di-n-butyltin(IV), C26H38N2S4Sn
- Crystal structure of (E)-3-(2-(4-(diethylamino)-2-hydroxystyryl)-3,3-dimethyl-3H-indol-1-ium-1-yl)propane-1-sulfonate – methanol (1/2), C25H32N2O4S⋅2CH3OH
- Synthesis and crystal structure of {(N′,N″-(((ethane-1,2-diylbis(oxy))bis(2,1-phenylene))-bis(methaneylylidene))bis(2-hydroxybenzohydrazonato)-κ6 N 2 O 4}copper(II), C30H24CuN4O6
- The crystal structure of ((E)-2,4-dibromo-6-(((5-(nitro)-2-oxidophenyl)imino)methyl)phenolato-κ 3 N,O,O′)tris(pyridine-κN)manganese(II), C28H21Br2MnN5O4
- Synthesis and crystal structure of 1-((3R,10S,13R,17S)-10,13-dimethyl-3-(p-tolylamino)hexadecahydro-1H-cyclopenta-[a]phenanthren-17-yl)ethan-1-one, C28H41NO
- The crystal structure of 3-nitrobenzene-1,2-dicarboxylic acid—pyrazine-2-carboxamide(1/1), C13H10N4O7
- Crystal structure of poly[tetrakis(μ3-2-aminonicotinato-κ3N,O,O′)-(μ2-oxalato-κ4 O,O′:O″,O′″)-(μ4-oxalato-κ6 N:N′:O,O′:O″,O′″)dicopper(I)-disamarium(III)], [SmCu(C6N2H5O2)2(C2O4)] n
- The crystal structure of 2,3,4-trihydroxybenzoic- acid—pyrazine-2-carboxamide—water (1/1/1), C12H13N3O7
- Crystal structure of N-ethyl-4-[3-(trifluoromethyl)-phenyl]piperazine-1-carbothioamide, C14H18F3N3S
- The crystal structure of 3-anilino-1,4-diphenyl-4H-1,2,4-triazol-1-ium iodide, C20H17N4I
- The crystal structure of (tris(2-benzimidazolylmethyl)amine)-benzoato-copper(II) perchlorate monohydrate, CuC31H28N7O7Cl
- Crystal structure of [2-hydroxy-3-methyl-benzoato-k1 O-triphenyltin(IV)], C26H22O3Sn
- Crystal structure of diaqua-bis(4-(hydroxymethyl)-benzoato-k1 O)zinc(II), C16H18O8Zn
- The crystal structure of dicarbonyl-(N-nitroso-N-oxido-phenylamine-κ 2 O,O)-rhodium(I), C8H5N2O4Rh
- The crystal structure of oxalic acid – 2-ethoxybenzamide (2/1), C20H24N2O8
- The crystal structure of ethyl 7-ethyl-5-methyl-4,7-dihydrotetrazolo[1,5-a]pyrimidine-6-carboxylate, C10H15N5O2
- Crystal structure of poly[(N,N-dimethylacetamide-κO) (μ4-2-nitroisophthalato-κ 4 O:O′:O″:O′″)manganese(II)], C11H10N2O7Mn
- Crystal structure of 14-O-acetyldelcosine, C26H41NO8
- The crystal structure of poly[(1,10-phenanthroline-κ2 N,N′)-(μ 4-2-chlorobenzene-1,3-dicarboxylato-κ5 O:O′:O″:O‴) cadmium(II)] monohydrate, C20H13CdClN2O5
- Crystal structure of propane-1,3-diylbis(diphenylphosphine sulfide) ethanol solvate, C27H26P2S2
- Crystal structure of bis{[(4-diethylamino-2-hydroxy-benzylidene)-hydrazinocarbonylmethyl]-trimethylammonium} tetrabromozincate, C32H54N8O4ZnBr4
- Synthesis and crystal structure of dimethyl 2,2′-(2,5-bis(4-hydroxyphenyl)-2,5-dihydrofuran-3,4-diyl)dibenzoate, C34H30O7
- Synthesis and crystal structure of 2-(2-oxo-2-phenylethyl)-4H-chromen-4-one, C17H12O3
- The crystal structure of tetra(imidazole-κ1 N)zinc(II) μ2-oxido-hexaoxido-divanadium(VI) C12H16N8O6V2Zn
- Crystal structure of S-2-(1-(5-methylpyridin-2-ylamino)octyl)-3-hydroxynaphthalene-1,4-dione, C24H28N2O3
- Crystal structure of bis(μ2-5-chloro-2-oxido-N-(1-oxido-2-oxo-2-phenylethylidene)-benzohydrazonato-κ5 N,O,O′:N′,O′′)-oktakis(pyridine-κ1 N)trinickel(II) – methanol – pyridine (1/1/1) C76H65N13Cl2Ni3O9
- The crystal structure of methyl 3,5-diaminobenzoate, C8H10N2O2
- Crystal structure of 10-(9H-carbazol-9-yl)-5H-dibenzo[a,d][7]annelen-5-one, C27H17NO
- Crystal structure of ethyl 1-(2-hydroxyethyl)-4-((4-methoxyphenyl)amino)-5-oxo-2,5-dihydro-1H-pyrrole-3-carboxylate, C16H20N2O5
- The crystal structure of 1-(4-bromophenyl)-3-cycloheptylurea, C14H19BrN2O
- The crystal structure of 1,4-bis(1,2,3,4,5-pentamethylcyclopenta-2,4-dien-1-yl)-3,6-bis ((4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)methylene)-1,4-dialuminacyclohexane – benzene (1/2), C50H72Al2B2O4
- Crystal structure of bis(μ 3-diphenylphosphinato)-tetrakis(μ 2-diphenylphosphinato)-bis(diphenylphosphinato)-bis(μ 2-hydroxo)dicopper(II)-ditin(IV), C104H100O18P8Cu2Sn2
- Crystal structure of 3-((3,4-dichloroisothiazol-5-yl)methoxy)benzo[d] isothiazole 1,1-dioxide, C11H6Cl2N2O3S2
- Synthesis and crystal structure of 2-(2-(2-fluorophenyl)-2-oxoethyl)-4H-chromen-4-one, C17H11FO3
- The crystal structure of tris(carbonyl)-bis(carbonyl)-[μ-propane-1,2- dithiolato]-(benzyldiphenylphosphine)diiron (Fe—Fe), C27H23Fe2O5PS2
- Crystal structure of 1-(2-(4-chlorophenethyl)-2-hydroxy-3,3-dimethylbutyl)-1H-1,2,4-triazol-4-ium nitrate, C16H23N4O4Cl
- The crystal structure of 3,3′-disulfanediyldi(1H-1,2,4-triazol-5-amine) monohydrate, C4H8N8OS2
- The crystal structure of trans-[bis(4-methylpyridine-κN)bis(quinoline-2-carboxylato- κ 2 N,O)cadmium(II)], C32H26CdN4O4
- The crystal structure of ethyl 2′-hydroxy-4′,6′-dimethoxy-3-(4-methoxynaphthalen-1-yl)-5-oxo-2,3,4,5-tetrahydro-[1,1′-biphenyl]-4-carboxylate, C28H28O7

