Abstract
C17H11FO3, monoclinic, P21/c (no. 14), a = 9.1987(2) Å, b = 17.5458(3) Å, c = 8.5802(2) Å, β = 108.193(3)°, V = 1315.60(5) Å3, Z = 4, R gt (F) = 0.0346, wR ref (F 2) = 0.1196, T = 293(2) K.
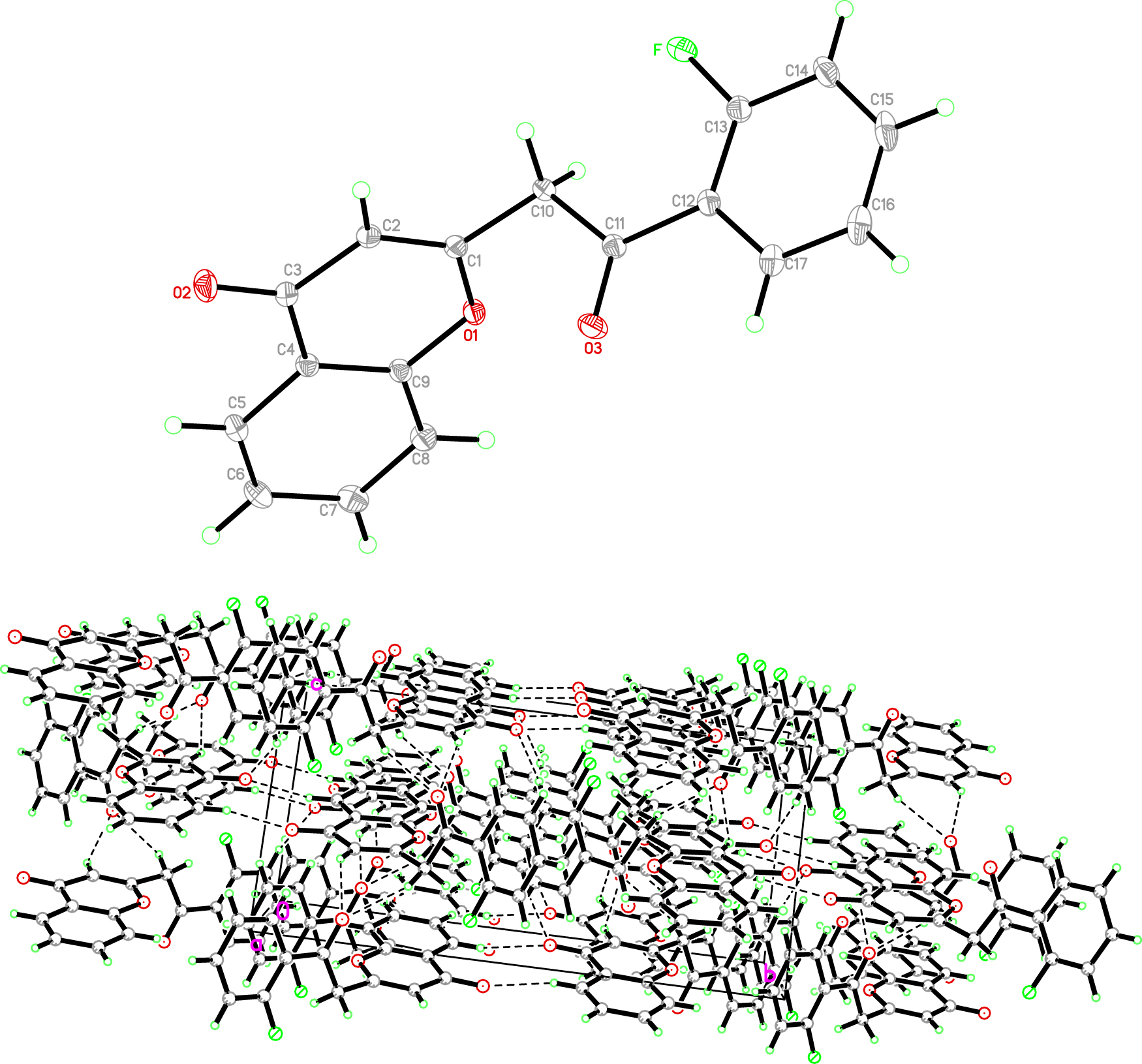
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colorless block |
| Size: | 0.20 × 0.20 × 0.10 mm |
| Wavelength: | Cu Kα radiation (1.54178 Å) |
| μ: | 0.90 mm−1 |
| Diffractometer, scan mode: | SuperNova, ω |
| θ max, completeness: | 74.4°, >99% |
| N(hkl)measured, N(hkl)unique, R int: | 8927, 2636, 0.025 |
| Criterion for I obs, N(hkl)gt: | I obs > 2 σ(I obs), 2282 |
| N(param)refined: | 191 |
| Programs: | CrysAlis Pro [1], Shelx [2, 3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| O1 | 0.13907 (9) | 0.21053 (4) | 0.96057 (10) | 0.0208 (2) |
| C1 | 0.24833 (12) | 0.24545 (6) | 0.90922 (13) | 0.0189 (3) |
| O2 | 0.16022 (11) | 0.44262 (5) | 0.92851 (12) | 0.0308 (2) |
| C2 | 0.25961 (12) | 0.32140 (6) | 0.89753 (14) | 0.0207 (3) |
| H2A | 0.3371 | 0.3416 | 0.8617 | 0.025* |
| O3 | 0.47612 (10) | 0.18074 (5) | 1.15504 (10) | 0.0272 (2) |
| C3 | 0.15398 (13) | 0.37269 (7) | 0.93941 (14) | 0.0212 (3) |
| F | 0.49913 (10) | 0.09392 (4) | 0.71310 (9) | 0.0345 (2) |
| C4 | 0.03775 (12) | 0.33442 (6) | 0.99640 (13) | 0.0188 (3) |
| C5 | −0.07314 (13) | 0.37558 (7) | 1.04359 (14) | 0.0225 (3) |
| H5A | −0.0746 | 0.4285 | 1.0380 | 0.027* |
| C6 | −0.17909 (14) | 0.33804 (7) | 1.09783 (16) | 0.0264 (3) |
| H6A | −0.2520 | 0.3656 | 1.1287 | 0.032* |
| C7 | −0.17765 (13) | 0.25808 (7) | 1.10688 (16) | 0.0267 (3) |
| H7A | −0.2488 | 0.2331 | 1.1454 | 0.032* |
| C8 | −0.07178 (13) | 0.21637 (7) | 1.05926 (15) | 0.0234 (3) |
| H8A | −0.0720 | 0.1634 | 1.0635 | 0.028* |
| C9 | 0.03548 (12) | 0.25497 (7) | 1.00475 (13) | 0.0187 (3) |
| C10 | 0.35136 (13) | 0.18836 (6) | 0.86772 (14) | 0.0201 (3) |
| H10A | 0.4041 | 0.2123 | 0.7989 | 0.024* |
| H10B | 0.2901 | 0.1469 | 0.8059 | 0.024* |
| C11 | 0.46848 (12) | 0.15642 (6) | 1.02013 (14) | 0.0190 (3) |
| C12 | 0.57831 (12) | 0.09619 (6) | 1.00437 (15) | 0.0200 (3) |
| C13 | 0.59231 (14) | 0.06808 (7) | 0.85820 (15) | 0.0241 (3) |
| C14 | 0.70054 (15) | 0.01417 (7) | 0.85227 (18) | 0.0310 (3) |
| H14A | 0.7070 | −0.0033 | 0.7523 | 0.037* |
| C15 | 0.79879 (15) | −0.01304 (7) | 0.9988 (2) | 0.0345 (3) |
| H15A | 0.8725 | −0.0491 | 0.9975 | 0.041* |
| C16 | 0.78827 (15) | 0.01296 (7) | 1.14764 (19) | 0.0335 (3) |
| H16A | 0.8546 | −0.0056 | 1.2456 | 0.040* |
| C17 | 0.67896 (14) | 0.06658 (7) | 1.14991 (16) | 0.0259 (3) |
| H17A | 0.6720 | 0.0834 | 1.2501 | 0.031* |
Source of material
A mixture of 2-hydroxyacetophenone, carbon disulfide, potassium carbonate and bromoethane was dissolved in DMSO in 250 mL round bottom flask and stirred at 35 °C (monitored by thin layer chromatography). After completion of the reaction, ice water was poured into the reaction mixture and the crude product was extracted by ethyl acetate. Organic layers were combined, washed with water and brine and dried over anhydrous MgSO4. The solvent was removed in vacuo and the product was purified by column chromatography (petroleum ether/ethyl acetate 5:1) to give the title compound as a light yellow solid.
Experimental details
Hydrogen atoms were placed in their geometrically idealized positions and constrained to ride on their parent atoms, with isotropic displacement parameters constrained as U iso(H) = 1.2U eq(C).
Comment
Chromone derivatives are widely distributed in nature and have excellent biological activity and pharmacological properties and are widely used in biomedical fields, such as anti-inflammatory [4], antioxidant [5], cardiovascular and liver protection [6]. At the same time, chromones are also important fluorophores for the production of fluorescent probes, which can be used for the development of fluorescent probes and solvochromic dyes [7, 8]. In the title compound (see the figure), the bond length and bond angle of the compound are within the normal range [9], [10], [11], [12]. The molecule consists of chromone skeleton, fluoro-substituted benzene ring and acetyl. The detailed analysis of the molecular structure showed that the bicyclic fragment is planar with the accuracy of 0.009 Å, and the dihedral angle between the benzene ring C12/C13/C14/C15/C16/C17) and the chromone skeleton is 75°. The bond length of C–F is 1.351(1) Å. No strong hydrogen bond was observed in the compound.
Funding source: Nanjing Senega Medical Technology Co. Ltd.
Acknowledgements
This work was supported by Nanjing Senega Medical Technology Co. Ltd. Also, we appreciate Wang Huaqin in Nanjing University and Wu Wenyuan in Nanjing Tech University for some data analysis.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work was supported by Nanjing Senega Medical Technology Co. Ltd.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Agilent Technologies. CrysAlisPro; Agilent Technologies: Santa Clara, CA, USA, 2017.Search in Google Scholar
2. Sheldrick, G. M. Crystal structure refinement with SHELX. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
3. Sheldrick, G. M. SHELX – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar PubMed PubMed Central
4. Borghi, S. M., Carvalho, T. T., Staurengo-Ferrari, L., Hohmann, M. S., Pinge-Filho, P., Casagrande, R., Verri, W. A.Jr. Vitexin inhibits inflammatory pain in mice by targeting TRPV1, oxidative stress, and cytokines. J. Nat. Prod. 2013, 76, 1141–1149; https://doi.org/10.1021/np400222v.Search in Google Scholar PubMed
5. Stefanachi, A., Leonetti, F., Pisani, L., Catto, M., Carotti, A. Coumarin: a natural, privileged and versatile scaffold for bioactive compounds. Molecules 2018, 23, 250; https://doi.org/10.3390/molecules23020250.Search in Google Scholar PubMed PubMed Central
6. Gaspar, A., Matos, M. J., Garrido, J., Uriarte, E., Borges, F. Chromone: a valid scaffold in medicinal chemistry. Chem. Rev. 2014, 114, 4960–4992; https://doi.org/10.1021/cr400265z.Search in Google Scholar PubMed
7. Giordano, L., Shvadchak, V. V., Fauerbach, J. A., Jares-Erijman, E. A., Jovin, T. M. Highly solvatochromic 7-aryl-3-hydroxychromones. J. Phys. Chem. Lett. 2012, 3, 1011–1016; https://doi.org/10.1021/jz3002019.Search in Google Scholar PubMed
8. Kucherak, O. A., Richert, L., Mely, Y., Klymchenko, A. S. Dipolar 3- methoxychromones as bright and highly solvatochromic fluorescent dyes. Phys. Chem. Chem. Phys. 2012, 14, 2292–2300; https://doi.org/10.1039/c2cp23037b.Search in Google Scholar PubMed
9. Dziewulska-Kułaczkowska, A., Mazur, L. Structural studies and characterization of 3-formylchromone and products of its reactions with chosen primary aromatic amines. J. Mol. Struct. 2010, 985, 233–242.10.1016/j.molstruc.2010.10.049Search in Google Scholar
10. Binbuga, N., Schultz, T. P., Henry, W. P. Intra- and intermolecular hydrogen bonding in 3-hydroxy- and 5-hydroxychromone. Tetrahedron Lett. 2008, 49, 5762–5765; https://doi.org/10.1016/j.tetlet.2008.07.100.Search in Google Scholar
11. Yagishita, F., Baba, N., Ueda, Y., Katabira, S., Kasashima, Y., Mino, T., Sakamoto, M. Diastereoselective photodimerization reactions of chromone-2- carboxamides to construct a C2-chiral scaffold. Org. Biomol. Chem. 2014, 12, 9644–9649; https://doi.org/10.1039/c4ob01827c.Search in Google Scholar PubMed
12. Gomes, L. R., Low, J. N., Cagide, F., Borges, F. The crystal structures of four N-(4-halophenyl)-4-oxo-4H-chromene-3-carboxamides. Acta Crystallogr. 2015, E71, 88–93; https://doi.org/10.1107/s2056989014027054.Search in Google Scholar
© 2022 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of undecacalcium decaarsenide, Ca11As10
- Crystal structure of catena-poly[diiodido-(μ2-1,5-dimethyl-2-phenyl-4-((pyridin-4- ylmethylene)amino)-1,2-dihydro-3H -pyrazol-3-one-κ2 N: O)zinc(II)], C17H16I2N4OZn
- The crystal structure of 5,10,15,20-tetrakis(4-(tert-butyl)phenyl)porphyrin-21,23-diido-κ4 N 4-naphthalocyanido-κ4 N 4-neodymium(IV) - chloroform (1/6) C114H90N12Cl18Nd
- The crystal structure of 1-(4-bromophenyl)-3-(2-chlorobenzyl)urea, C14H12BrClN2O
- Crystal structure of bis[benzyl(methyl)carbamodithioato-κ 2 S,S′]-di-n-butyltin(IV), C26H38N2S4Sn
- Crystal structure of (E)-3-(2-(4-(diethylamino)-2-hydroxystyryl)-3,3-dimethyl-3H-indol-1-ium-1-yl)propane-1-sulfonate – methanol (1/2), C25H32N2O4S⋅2CH3OH
- Synthesis and crystal structure of {(N′,N″-(((ethane-1,2-diylbis(oxy))bis(2,1-phenylene))-bis(methaneylylidene))bis(2-hydroxybenzohydrazonato)-κ6 N 2 O 4}copper(II), C30H24CuN4O6
- The crystal structure of ((E)-2,4-dibromo-6-(((5-(nitro)-2-oxidophenyl)imino)methyl)phenolato-κ 3 N,O,O′)tris(pyridine-κN)manganese(II), C28H21Br2MnN5O4
- Synthesis and crystal structure of 1-((3R,10S,13R,17S)-10,13-dimethyl-3-(p-tolylamino)hexadecahydro-1H-cyclopenta-[a]phenanthren-17-yl)ethan-1-one, C28H41NO
- The crystal structure of 3-nitrobenzene-1,2-dicarboxylic acid—pyrazine-2-carboxamide(1/1), C13H10N4O7
- Crystal structure of poly[tetrakis(μ3-2-aminonicotinato-κ3N,O,O′)-(μ2-oxalato-κ4 O,O′:O″,O′″)-(μ4-oxalato-κ6 N:N′:O,O′:O″,O′″)dicopper(I)-disamarium(III)], [SmCu(C6N2H5O2)2(C2O4)] n
- The crystal structure of 2,3,4-trihydroxybenzoic- acid—pyrazine-2-carboxamide—water (1/1/1), C12H13N3O7
- Crystal structure of N-ethyl-4-[3-(trifluoromethyl)-phenyl]piperazine-1-carbothioamide, C14H18F3N3S
- The crystal structure of 3-anilino-1,4-diphenyl-4H-1,2,4-triazol-1-ium iodide, C20H17N4I
- The crystal structure of (tris(2-benzimidazolylmethyl)amine)-benzoato-copper(II) perchlorate monohydrate, CuC31H28N7O7Cl
- Crystal structure of [2-hydroxy-3-methyl-benzoato-k1 O-triphenyltin(IV)], C26H22O3Sn
- Crystal structure of diaqua-bis(4-(hydroxymethyl)-benzoato-k1 O)zinc(II), C16H18O8Zn
- The crystal structure of dicarbonyl-(N-nitroso-N-oxido-phenylamine-κ 2 O,O)-rhodium(I), C8H5N2O4Rh
- The crystal structure of oxalic acid – 2-ethoxybenzamide (2/1), C20H24N2O8
- The crystal structure of ethyl 7-ethyl-5-methyl-4,7-dihydrotetrazolo[1,5-a]pyrimidine-6-carboxylate, C10H15N5O2
- Crystal structure of poly[(N,N-dimethylacetamide-κO) (μ4-2-nitroisophthalato-κ 4 O:O′:O″:O′″)manganese(II)], C11H10N2O7Mn
- Crystal structure of 14-O-acetyldelcosine, C26H41NO8
- The crystal structure of poly[(1,10-phenanthroline-κ2 N,N′)-(μ 4-2-chlorobenzene-1,3-dicarboxylato-κ5 O:O′:O″:O‴) cadmium(II)] monohydrate, C20H13CdClN2O5
- Crystal structure of propane-1,3-diylbis(diphenylphosphine sulfide) ethanol solvate, C27H26P2S2
- Crystal structure of bis{[(4-diethylamino-2-hydroxy-benzylidene)-hydrazinocarbonylmethyl]-trimethylammonium} tetrabromozincate, C32H54N8O4ZnBr4
- Synthesis and crystal structure of dimethyl 2,2′-(2,5-bis(4-hydroxyphenyl)-2,5-dihydrofuran-3,4-diyl)dibenzoate, C34H30O7
- Synthesis and crystal structure of 2-(2-oxo-2-phenylethyl)-4H-chromen-4-one, C17H12O3
- The crystal structure of tetra(imidazole-κ1 N)zinc(II) μ2-oxido-hexaoxido-divanadium(VI) C12H16N8O6V2Zn
- Crystal structure of S-2-(1-(5-methylpyridin-2-ylamino)octyl)-3-hydroxynaphthalene-1,4-dione, C24H28N2O3
- Crystal structure of bis(μ2-5-chloro-2-oxido-N-(1-oxido-2-oxo-2-phenylethylidene)-benzohydrazonato-κ5 N,O,O′:N′,O′′)-oktakis(pyridine-κ1 N)trinickel(II) – methanol – pyridine (1/1/1) C76H65N13Cl2Ni3O9
- The crystal structure of methyl 3,5-diaminobenzoate, C8H10N2O2
- Crystal structure of 10-(9H-carbazol-9-yl)-5H-dibenzo[a,d][7]annelen-5-one, C27H17NO
- Crystal structure of ethyl 1-(2-hydroxyethyl)-4-((4-methoxyphenyl)amino)-5-oxo-2,5-dihydro-1H-pyrrole-3-carboxylate, C16H20N2O5
- The crystal structure of 1-(4-bromophenyl)-3-cycloheptylurea, C14H19BrN2O
- The crystal structure of 1,4-bis(1,2,3,4,5-pentamethylcyclopenta-2,4-dien-1-yl)-3,6-bis ((4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)methylene)-1,4-dialuminacyclohexane – benzene (1/2), C50H72Al2B2O4
- Crystal structure of bis(μ 3-diphenylphosphinato)-tetrakis(μ 2-diphenylphosphinato)-bis(diphenylphosphinato)-bis(μ 2-hydroxo)dicopper(II)-ditin(IV), C104H100O18P8Cu2Sn2
- Crystal structure of 3-((3,4-dichloroisothiazol-5-yl)methoxy)benzo[d] isothiazole 1,1-dioxide, C11H6Cl2N2O3S2
- Synthesis and crystal structure of 2-(2-(2-fluorophenyl)-2-oxoethyl)-4H-chromen-4-one, C17H11FO3
- The crystal structure of tris(carbonyl)-bis(carbonyl)-[μ-propane-1,2- dithiolato]-(benzyldiphenylphosphine)diiron (Fe—Fe), C27H23Fe2O5PS2
- Crystal structure of 1-(2-(4-chlorophenethyl)-2-hydroxy-3,3-dimethylbutyl)-1H-1,2,4-triazol-4-ium nitrate, C16H23N4O4Cl
- The crystal structure of 3,3′-disulfanediyldi(1H-1,2,4-triazol-5-amine) monohydrate, C4H8N8OS2
- The crystal structure of trans-[bis(4-methylpyridine-κN)bis(quinoline-2-carboxylato- κ 2 N,O)cadmium(II)], C32H26CdN4O4
- The crystal structure of ethyl 2′-hydroxy-4′,6′-dimethoxy-3-(4-methoxynaphthalen-1-yl)-5-oxo-2,3,4,5-tetrahydro-[1,1′-biphenyl]-4-carboxylate, C28H28O7
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of undecacalcium decaarsenide, Ca11As10
- Crystal structure of catena-poly[diiodido-(μ2-1,5-dimethyl-2-phenyl-4-((pyridin-4- ylmethylene)amino)-1,2-dihydro-3H -pyrazol-3-one-κ2 N: O)zinc(II)], C17H16I2N4OZn
- The crystal structure of 5,10,15,20-tetrakis(4-(tert-butyl)phenyl)porphyrin-21,23-diido-κ4 N 4-naphthalocyanido-κ4 N 4-neodymium(IV) - chloroform (1/6) C114H90N12Cl18Nd
- The crystal structure of 1-(4-bromophenyl)-3-(2-chlorobenzyl)urea, C14H12BrClN2O
- Crystal structure of bis[benzyl(methyl)carbamodithioato-κ 2 S,S′]-di-n-butyltin(IV), C26H38N2S4Sn
- Crystal structure of (E)-3-(2-(4-(diethylamino)-2-hydroxystyryl)-3,3-dimethyl-3H-indol-1-ium-1-yl)propane-1-sulfonate – methanol (1/2), C25H32N2O4S⋅2CH3OH
- Synthesis and crystal structure of {(N′,N″-(((ethane-1,2-diylbis(oxy))bis(2,1-phenylene))-bis(methaneylylidene))bis(2-hydroxybenzohydrazonato)-κ6 N 2 O 4}copper(II), C30H24CuN4O6
- The crystal structure of ((E)-2,4-dibromo-6-(((5-(nitro)-2-oxidophenyl)imino)methyl)phenolato-κ 3 N,O,O′)tris(pyridine-κN)manganese(II), C28H21Br2MnN5O4
- Synthesis and crystal structure of 1-((3R,10S,13R,17S)-10,13-dimethyl-3-(p-tolylamino)hexadecahydro-1H-cyclopenta-[a]phenanthren-17-yl)ethan-1-one, C28H41NO
- The crystal structure of 3-nitrobenzene-1,2-dicarboxylic acid—pyrazine-2-carboxamide(1/1), C13H10N4O7
- Crystal structure of poly[tetrakis(μ3-2-aminonicotinato-κ3N,O,O′)-(μ2-oxalato-κ4 O,O′:O″,O′″)-(μ4-oxalato-κ6 N:N′:O,O′:O″,O′″)dicopper(I)-disamarium(III)], [SmCu(C6N2H5O2)2(C2O4)] n
- The crystal structure of 2,3,4-trihydroxybenzoic- acid—pyrazine-2-carboxamide—water (1/1/1), C12H13N3O7
- Crystal structure of N-ethyl-4-[3-(trifluoromethyl)-phenyl]piperazine-1-carbothioamide, C14H18F3N3S
- The crystal structure of 3-anilino-1,4-diphenyl-4H-1,2,4-triazol-1-ium iodide, C20H17N4I
- The crystal structure of (tris(2-benzimidazolylmethyl)amine)-benzoato-copper(II) perchlorate monohydrate, CuC31H28N7O7Cl
- Crystal structure of [2-hydroxy-3-methyl-benzoato-k1 O-triphenyltin(IV)], C26H22O3Sn
- Crystal structure of diaqua-bis(4-(hydroxymethyl)-benzoato-k1 O)zinc(II), C16H18O8Zn
- The crystal structure of dicarbonyl-(N-nitroso-N-oxido-phenylamine-κ 2 O,O)-rhodium(I), C8H5N2O4Rh
- The crystal structure of oxalic acid – 2-ethoxybenzamide (2/1), C20H24N2O8
- The crystal structure of ethyl 7-ethyl-5-methyl-4,7-dihydrotetrazolo[1,5-a]pyrimidine-6-carboxylate, C10H15N5O2
- Crystal structure of poly[(N,N-dimethylacetamide-κO) (μ4-2-nitroisophthalato-κ 4 O:O′:O″:O′″)manganese(II)], C11H10N2O7Mn
- Crystal structure of 14-O-acetyldelcosine, C26H41NO8
- The crystal structure of poly[(1,10-phenanthroline-κ2 N,N′)-(μ 4-2-chlorobenzene-1,3-dicarboxylato-κ5 O:O′:O″:O‴) cadmium(II)] monohydrate, C20H13CdClN2O5
- Crystal structure of propane-1,3-diylbis(diphenylphosphine sulfide) ethanol solvate, C27H26P2S2
- Crystal structure of bis{[(4-diethylamino-2-hydroxy-benzylidene)-hydrazinocarbonylmethyl]-trimethylammonium} tetrabromozincate, C32H54N8O4ZnBr4
- Synthesis and crystal structure of dimethyl 2,2′-(2,5-bis(4-hydroxyphenyl)-2,5-dihydrofuran-3,4-diyl)dibenzoate, C34H30O7
- Synthesis and crystal structure of 2-(2-oxo-2-phenylethyl)-4H-chromen-4-one, C17H12O3
- The crystal structure of tetra(imidazole-κ1 N)zinc(II) μ2-oxido-hexaoxido-divanadium(VI) C12H16N8O6V2Zn
- Crystal structure of S-2-(1-(5-methylpyridin-2-ylamino)octyl)-3-hydroxynaphthalene-1,4-dione, C24H28N2O3
- Crystal structure of bis(μ2-5-chloro-2-oxido-N-(1-oxido-2-oxo-2-phenylethylidene)-benzohydrazonato-κ5 N,O,O′:N′,O′′)-oktakis(pyridine-κ1 N)trinickel(II) – methanol – pyridine (1/1/1) C76H65N13Cl2Ni3O9
- The crystal structure of methyl 3,5-diaminobenzoate, C8H10N2O2
- Crystal structure of 10-(9H-carbazol-9-yl)-5H-dibenzo[a,d][7]annelen-5-one, C27H17NO
- Crystal structure of ethyl 1-(2-hydroxyethyl)-4-((4-methoxyphenyl)amino)-5-oxo-2,5-dihydro-1H-pyrrole-3-carboxylate, C16H20N2O5
- The crystal structure of 1-(4-bromophenyl)-3-cycloheptylurea, C14H19BrN2O
- The crystal structure of 1,4-bis(1,2,3,4,5-pentamethylcyclopenta-2,4-dien-1-yl)-3,6-bis ((4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)methylene)-1,4-dialuminacyclohexane – benzene (1/2), C50H72Al2B2O4
- Crystal structure of bis(μ 3-diphenylphosphinato)-tetrakis(μ 2-diphenylphosphinato)-bis(diphenylphosphinato)-bis(μ 2-hydroxo)dicopper(II)-ditin(IV), C104H100O18P8Cu2Sn2
- Crystal structure of 3-((3,4-dichloroisothiazol-5-yl)methoxy)benzo[d] isothiazole 1,1-dioxide, C11H6Cl2N2O3S2
- Synthesis and crystal structure of 2-(2-(2-fluorophenyl)-2-oxoethyl)-4H-chromen-4-one, C17H11FO3
- The crystal structure of tris(carbonyl)-bis(carbonyl)-[μ-propane-1,2- dithiolato]-(benzyldiphenylphosphine)diiron (Fe—Fe), C27H23Fe2O5PS2
- Crystal structure of 1-(2-(4-chlorophenethyl)-2-hydroxy-3,3-dimethylbutyl)-1H-1,2,4-triazol-4-ium nitrate, C16H23N4O4Cl
- The crystal structure of 3,3′-disulfanediyldi(1H-1,2,4-triazol-5-amine) monohydrate, C4H8N8OS2
- The crystal structure of trans-[bis(4-methylpyridine-κN)bis(quinoline-2-carboxylato- κ 2 N,O)cadmium(II)], C32H26CdN4O4
- The crystal structure of ethyl 2′-hydroxy-4′,6′-dimethoxy-3-(4-methoxynaphthalen-1-yl)-5-oxo-2,3,4,5-tetrahydro-[1,1′-biphenyl]-4-carboxylate, C28H28O7

