Abstract
C13H12ClN3, orthorhombic, Fdd2 (no. 43), a = 28.7760(11) Å, b = 41.7393(17) Å, c = 8.5929(3) Å, V = 10,320.8(7) Å3, Z = 32, R gt (F) = 0.0477, wR ref (F2) = 0.0976, T = 296 K.
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colorless block |
| Size: | 0.86 × 0.69 × 0.28 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.28 mm−1 |
| Scan mode: | Ω-scans |
| θmax, completeness: | 25.0°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 9647, 4032, 0.054 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 2155 |
| N(param)refined: | 309 |
| Programs: | Olex2 [1], SHELX [2, 3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| Cl1 | 0.14907 (5) | 0.10676 (4) | 0.97745 (18) | 0.0832 (6) |
| N1 | 0.25519 (17) | 0.15579 (12) | 1.1510 (6) | 0.0668 (13) |
| N2 | 0.18239 (14) | 0.14320 (11) | 1.2509 (5) | 0.0653 (13) |
| H2 | 0.1559 | 0.1338 | 1.2421 | 0.078* |
| N3 | 0.19270 (15) | 0.16015 (10) | 1.3844 (6) | 0.0578 (12) |
| C1 | 0.20317 (17) | 0.12420 (13) | 0.9980 (7) | 0.0590 (15) |
| C2 | 0.2350 (2) | 0.12183 (14) | 0.8815 (7) | 0.0713 (17) |
| H2A | 0.2279 | 0.1107 | 0.7908 | 0.086* |
| C3 | 0.2776 (2) | 0.13613 (16) | 0.9000 (8) | 0.0774 (19) |
| H3 | 0.3002 | 0.1346 | 0.8231 | 0.093* |
| C4 | 0.2858 (2) | 0.15269 (16) | 1.0346 (9) | 0.0770 (19) |
| H4 | 0.3146 | 0.1625 | 1.0460 | 0.092* |
| C5 | 0.2140 (2) | 0.14132 (13) | 1.1326 (7) | 0.0565 (15) |
| C6 | 0.16139 (18) | 0.15986 (13) | 1.4911 (7) | 0.0580 (15) |
| H6 | 0.1343 | 0.1481 | 1.4755 | 0.070* |
| C7 | 0.1674 (2) | 0.17739 (13) | 1.6348 (6) | 0.0516 (14) |
| C8 | 0.20846 (19) | 0.19301 (13) | 1.6705 (6) | 0.0606 (16) |
| H8 | 0.2329 | 0.1927 | 1.5997 | 0.073* |
| C9 | 0.2135 (2) | 0.20902 (14) | 1.8104 (7) | 0.0688 (17) |
| H9 | 0.2414 | 0.2193 | 1.8313 | 0.083* |
| C10 | 0.1786 (2) | 0.21023 (14) | 1.9196 (7) | 0.0689 (18) |
| C11 | 0.1843 (2) | 0.22825 (17) | 2.0705 (7) | 0.102 (3) |
| H11A | 0.1744 | 0.2500 | 2.0567 | 0.154* |
| H11B | 0.2164 | 0.2279 | 2.1013 | 0.154* |
| H11C | 0.1657 | 0.2183 | 2.1498 | 0.154* |
| C12 | 0.1377 (2) | 0.19496 (16) | 1.8823 (8) | 0.0790 (18) |
| H12 | 0.1132 | 0.1956 | 1.9529 | 0.095* |
| C13 | 0.1319 (2) | 0.17874 (14) | 1.7433 (7) | 0.0708 (16) |
| H13 | 0.1038 | 0.1686 | 1.7224 | 0.085* |
| Cl2 | 0.05160 (6) | 0.02663 (4) | 1.1392 (2) | 0.1052 (7) |
| N4 | 0.11658 (18) | 0.08678 (12) | 1.4173 (7) | 0.0792 (15) |
| N5 | 0.06095 (15) | 0.09503 (12) | 1.2282 (5) | 0.0636 (13) |
| H5 | 0.0409 | 0.0878 | 1.1630 | 0.076* |
| N6 | 0.06571 (16) | 0.12764 (12) | 1.2492 (5) | 0.0578 (12) |
| C14 | 0.0864 (2) | 0.04140 (16) | 1.2876 (7) | 0.0622 (16) |
| C15 | 0.1106 (2) | 0.02108 (16) | 1.3774 (10) | 0.089 (2) |
| H15 | 0.1084 | −0.0010 | 1.3634 | 0.107* |
| C16 | 0.1392 (3) | 0.0341 (2) | 1.4923 (10) | 0.110 (2) |
| H16 | 0.1567 | 0.0210 | 1.5575 | 0.132* |
| C17 | 0.1407 (2) | 0.0665 (2) | 1.5059 (9) | 0.093 (2) |
| H17 | 0.1599 | 0.0751 | 1.5822 | 0.112* |
| C18 | 0.0882 (2) | 0.07418 (15) | 1.3112 (7) | 0.0573 (15) |
| C19 | 0.0356 (2) | 0.14463 (15) | 1.1803 (7) | 0.0636 (17) |
| H19 | 0.0120 | 0.1343 | 1.1257 | 0.076* |
| C20 | 0.0362 (2) | 0.17938 (15) | 1.1827 (7) | 0.0627 (16) |
| C21 | 0.0651 (2) | 0.19768 (19) | 1.2714 (9) | 0.088 (2) |
| H21 | 0.0856 | 0.1876 | 1.3395 | 0.105* |
| C22 | 0.0649 (3) | 0.2307 (2) | 1.2628 (11) | 0.105 (3) |
| H22 | 0.0852 | 0.2425 | 1.3242 | 0.127* |
| C23 | 0.0345 (4) | 0.24642 (19) | 1.1632 (11) | 0.107 (3) |
| C24 | 0.0060 (3) | 0.2281 (2) | 1.0782 (10) | 0.134 (3) |
| H24 | −0.0151 | 0.2379 | 1.0111 | 0.161* |
| C25 | 0.0070 (2) | 0.1959 (2) | 1.0874 (9) | 0.108 (3) |
| H25 | −0.0134 | 0.1842 | 1.0251 | 0.129* |
| C26 | 0.0362 (4) | 0.28315 (18) | 1.1560 (12) | 0.181 (4) |
| H26A | 0.0330 | 0.2900 | 1.0499 | 0.272* |
| H26B | 0.0654 | 0.2905 | 1.1967 | 0.272* |
| H26C | 0.0113 | 0.2919 | 1.2168 | 0.272* |
Source of material
According to our previous work [4], the 3-chloro-2-hydrazinylpyridine and 4-methylbenzaldehyde was stirred in EtOH (50 mL) at room temperature overnight. The obtained solid was, filtered and dried with 75% yield. The solid was recrystallized from ethanol to obtain colorless block crystals.
Experimentaldetails
Coordinates of hydrogen atoms were added using the standard AFIX options of the SHELX system. Their Uiso values were set to 1.2Ueq of the parent atoms. Using Olex2 [1], the structure was refined with the ShelXL [3].
Comment
Some pyridine derivatives possess biological activity, such as herbicidal activity [5], insecticidal activity [6], fungicidal activity [7]. While hydrazone is also a key group in some drugs or pesticides [8].
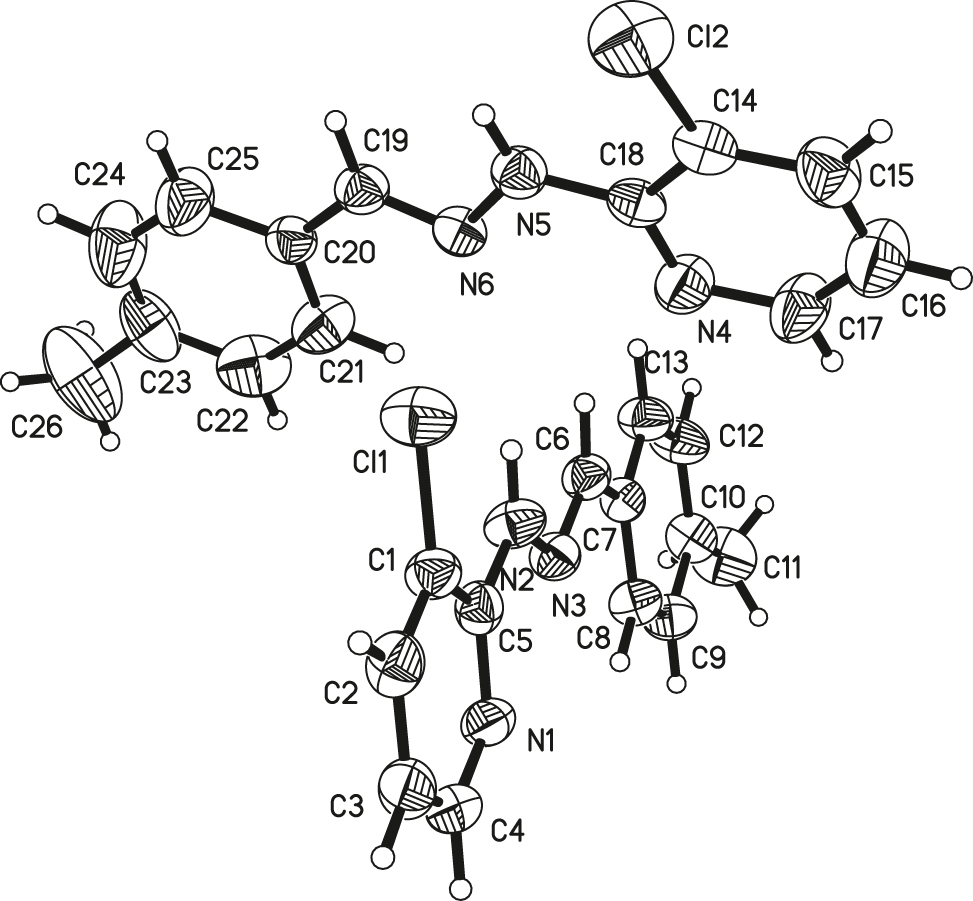
There are two crystallographically independent molecules in the asymmetric unit of the title structure (see the figure). The bond lengths and bond angles of ring systems (pyridine ring and phenyl ring) are in the normal ranges [9, 10]. The C6=N3 (C19–N6) double bond indicated the E configuration which is according to the normal double bond length [11], and the E configuration is the same as in some reported structures [12–14]. The torsion angles of C7–C6–N3–N2 and C6–N3–N2–C5 are −178.3(4)° and −178.1(4)° (2. molecule: C18–N5–N6–C19–172.6(5)° and N5–N6–C19–C20–177.0(5)°).
Funding source: Zhejiang Provincial Natural Science Foundation of China
Award Identifier / Grant number: LY19C140002
Award Identifier / Grant number: LY19B020009
Funding source: Chemical Company for Research
Award Identifier / Grant number: KYY-HX-20210140
Award Identifier / Grant number: KYY-HX-20190720
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work was funded by Zhejiang Provincial Natural Science Foundation of China (Nos. LY19C140002, LY19B020009), the Chemical Company for Research (KYY-HX-20210140, KYY-HX-20190720).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
2. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Sun, G. X., Shi, Y. X., Zhai, Z. W., Yang, M. Y., Weng, J. Q., Tan, C. X., Liu, X. H., Li, B. J. Synthesis, crystal structure and biological activity of (E)-3-Chloro-2-(2-(2-methylbenzylidene)-hydrazinyl)pyridine. Chin. J. Struct. Chem. 2016, 35, 640–644.Search in Google Scholar
5. Yu, C. S., Wang, Q., Bajsa-Hirschel, J., Cantrell, C., Duke, S. O., Liu, X. H. Synthesis, crystal structure, herbicidal activity and SAR study of novel N-(arylmethoxy)-2-chloronicotinamides derived from nicotinic acid. J. Agric. Food Chem. 2021, 69, 6423–6430; https://doi.org/10.1021/acs.jafc.0c07538.Search in Google Scholar
6. Liu, X. H., Wen, Y. H., Cheng, L., Xu, T. M., Wu, N. J. Design, synthesis, pesticidal activities of pyrimidin-4-amine derivatives bearing a 5-(trifluoromethyl)-1,2,4-oxadiazole moiety. J. Agric. Food Chem. 2021, 69, 6968–6980; https://doi.org/10.1021/acs.jafc.1c00236.Search in Google Scholar
7. Min, L. J., Wang, Q., Tan, C. X., Weng, J. Q., Liu, X. H. Synthesis, crystal structure and fungicidal activity of 2-chloro-N-(o-tolylcarbamoyl)nicotinamide. Chin. J. Struct. Chem. 2020, 39, 452–458.Search in Google Scholar
8. Shen, Z. H., Sun, Z. H., Becnel, J. J., Estep, A., Wedge, D. E., Tan, C. X., Weng, J. Q., Han, L., Liu, X. H. Synthesis and mosquiticidal activity of novel hydrazone containing pyrimidine derivatives against Aedes aegypti. Lett. Drug Des. Discov. 2018, 15, 951–956; https://doi.org/10.2174/1570180815666180102141640.Search in Google Scholar
9. Wu, H. Z., Min, L. J., Han, L., Liu, X. H., Sun, N. B. The crystal structure of (E)-3-chloro-2-(2-(2-fluorobenzylidene)hydrazinyl) pyridine, C12H9ClFN3. Z. Kristallogr. NCS 2021, 236, 953–955.10.1515/ncrs-2021-0164Search in Google Scholar
10. Dilek Ozcelik, N., Tunc, T., Catak Celik, R., Erzengin, M., Ozisik, H. Synthesis, spectroscopic, crystal structure, biological activities and theoretical studies of 2-[(2E)-2-(2-chloro-6-fluorobenzylidene) hydrazinyl]pyridine. J. Mol. Struct. 2017, 1135, 98–105; https://doi.org/10.1016/j.molstruc.2017.01.060.Search in Google Scholar
11. Jin, Y., Fu, T., Ma, B. Y., Wei, P., Zhao, J. X., Zhao, L. Synthesis and crystal structure of 1-{4-[(3-bromo-2-hydroxy-benzylidene)amino]phenyl} ethanone, C15H12BrNO2. Z. Kristallogr. NCS 2020, 235, 1091–1092; https://doi.org/10.1515/ncrs-2020-0181.Search in Google Scholar
12. Sowmya, H. B. V., Suresha Kumara, T. H., Jasinski, J. P., Millikan, S. P., Glidewell, C. Crystal structure of (E)-2-fluorobenzaldehyde(pyridin-2-yl)hydrazone. Acta Crystallogr. 2015, E71, o362–o363; https://doi.org/10.1107/s2056989015007823.Search in Google Scholar
13. Ozcelik, N., Tunc, T., Celik, R. C., Erzengin, M., Ozisik, H. 2-[(2E)-2-(3-chloro-2-fluorobenzylidene)hydrazinyl]pyridine: synthesis, spectroscopic, structural properties, biological activity and theoretical analysis. J. Mol. Struct. 2021, 1227, 129570; https://doi.org/10.1016/j.molstruc.2020.129570.Search in Google Scholar
14. Yuvaraj, H., Sundaramoorthy, S., Velmurugan, D., Kalkhambkar, R. G. (Z)-2-[2-(4-Methylbenzylidene)hydrazinyl]pyridine. Acta Crystallogr. 2011, E67, o178; https://doi.org/10.1107/s1600536810052372.Search in Google Scholar
© 2021 Guo-Xiang Sun et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of (E)-7-hydroxy-2-((6-methoxypyridin-3-yl)methylene)-3, 4-dihydronaphthalen-1(2H)-one, C17H15NO3
- Crystal structure of (E)-7-methoxy-2-((2-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1 (2H)-one, C18H17NO3
- The crystal structure of N 6,N 6′-di(pyridin-2-yl)-[2,2′-bipyridine]-6,6′-diamine, C20H16N6
- The crystal structure of {N 1,N 2-bis[2,4-dimethyl-6-(4-(tert-butyl)phenyl)(phenyl)methyl]acenaphthylene-1,2-diimino-κ2 N, N′}-dibromido-nickel(II) – dichloromethane(1/2), C64H64Br2Cl4N2Ni
- Synthesis and crystal structure of nonacarbonyltris[(2-thia-1,3,5-triaza-7-phosphatricylco[3.3.1.1]decane-κ1 P)-2,2-dioxide]triruthenium(0) – acetonitrile (7/6), C25.71H32.57N9.86O15P3S3Ru3
- A new polymorph of 1-(4-nitrophenyl)-1H-benzimidazole (C13H9N3O2)
- The crystal structure of 2,2′-((1E,1′E)-(naphthalene-2,3 diylbis(azanylylidene)) bis(methanylylidene))bis(4-methylphenol), C26H22N2O2
- The crystal structure of bis(μ2-iodido)-bis(η6-benzene)-bis(iodido)-diosmium(II), C12H12I4Os2
- Redetermination of the crystal structure of bis{hydridotris(3,5-dimethylpyrazol-1-yl-κN 3)borato}copper(II), C30H44B2CuN12
- Crystal structure of (E)-3-((4-(tert-butyl)phenyl)thio)-4-hydroxypent-3-en-2-one, C15H20O2S
- Crystal structure of 2,2′-(p-tolylazanediyl)bis(1-phenylethan-1-one), C23H21NO2
- Redetermination of the crystal structure of the crystal sponge the poly[tetrakis(μ3-2,4,6-tris(pyridin-4-yl)-1,3,5-triazine)-dodecaiodidohexazinc(II) nitrobenzene solvate], C72H48I12N24Zn6⋅10(C6H5NO2)
- Crystal structure of (4′E)-6′-(diethylamino)-2-[(E)-[(6-methylpyridin-2-yl)methylidene]amino]-4′-{2-[(2E)-1,3,3-trimethyl-2,3-dihydro-1H-indol-2-ylidene]ethylidene}-1′,2,2′,3,3′,4′-hexahydrospiro[isoindole-1,9′-xanthene]-3-one, C44H45N5O2
- Crystal structure of (E)-7-fluoro-2-(3-fluorobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C17H12F2O1
- Crystal structure of tetrabutylammonium sulfanilate – 1-(diaminomethylene)thiourea (1/2)
- Crystal structure of [2,2′-{azanediyl)bis[(propane-3,1-diyl)(azanylylidene)methylylidene]} bis(3,5-dichlorophenolato)-κ2O,O′]-isothiocyanato-κN-iron(III), C21H19Cl4FeN4O2S
- Crystal structure of (4-chlorophenyl)(4-hydroxyphenyl)methanone, C13H9ClO2
- Crystal structure of 6,6′-((pentane-1,3-diylbis(azaneylylidene))bis(methaneylylidene))bis(2,4-dibromolphenolato-κ4 N,N′,O,O′)copper(II),) C19H16Br4CuN2O2
- Chlorido-(2,2′-(ethane-bis(5-methoxyphenolato))-κ4 N,N′,O,O′)manganese(III) monohydrate, C19H18Cl2CuN2O2
- Crystal structure of 2,6-di-tert-butyl-4-(4-methoxybenzylidene)cyclohexa-2,5-dien-1-one, C22H28O2
- Crystal structure of [6,6′-(((2,2-dimethylpropane-1,3-diyl)bis(azanylylidene))bis(methanylylidene))bis(2-chlorophenolato)-κ4N,N′,O,O′]copper(II)
- Crystal structure of 2-chloro-3-((thiophen-2-ylmethyl)amino)naphthalene-1,4-dione, C30H20O4N2Cl2S2
- Crystal structure of bis{hydridotris(3-trifluoromethyl-5-methylpyrazolyl-1-yl)borato-κN 3}manganese(II), C30H26B2F18MnN12
- Crystal structure of 1-(2-methylphenyl)-2-(2-methylbenzo[b]thienyl)-3,3,4,4,5,5-hexafluorocyclopent-ene, C21H14F6S
- Crystal structure of 2-(3-((carbamimidoylthio)methyl)benzyl)isothiouronium hexafluorophosphate monohydrate, C10H17F6N4OPS2
- Crystal structure of 4,5-diiodo-1,3-dimesityl-1H-1,2,3-triazol-3-ium chloride – chloroform (1/1), C21H23Cl4I2N3
- Crystal structure of azido-k1 N-{6,6′-((((methylazanediyl)bis(propane-3,1-diyl))bis(azanylylidene))bis(methanylylidene))bis(2,4-dibromophenolato)k5 N,N′,N″,O,O′}cobalt(III)-methanol (1/1)), C21H23Br4CoN6O3
- The crystal structure of 2-(4-((carbamimidoylthio)methyl)benzyl)isothiouronium hexafluorophosphate monohydrate, C10H17F6N4OPS2
- Crystal structure of 1,1′-(methane-1,1-diyl)bis(3-methyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C9H14F12N4P2
- Crystal structure of (4′E)-6′-(diethylamino)-2-[(E)-[(pyren-1-yl)methylidene]amino]-4′-{2-[(2E)-1,3,3-trimethyl-2,3-dihydro-1H-indol-2-ylidene]ethylidene}-1′,2,2′,3,3′,4′-hexahydrospiro[isoindole-1,9′-xanthene]-3-one, C54H48N4O2
- Crystal structure of poly[bis(μ2-2,6-bis(1-imidazoly)pyridine-κ2 N,N′)-bis(thiocyanato-κ1 N)copper(II)] dithiocyanate, C24H18CuN12S2
- Cones with a three-fold symmetry constructed from three hydrogen bonded theophyllinium cations that coat [FeCl4]− anions in the crystal structure of tris(theophyllinium) bis(tetrachloridoferrate(III)) chloride trihydrate, C21H33Cl9Fe2N12O9
- Crystal structure of 14-O-[(4-(4-hydroxypiperidine-1-yl)-6-methylpyrimidine-2-yl)thioacetyl]-mutilin monohydrate, C32H49N3O6S
- The crystal structure of (E)-3-chloro-2-(2-(4-methylbenzylidene)hydrazinyl)pyridine, C13H12ClN3
- The crystal structure of 4-phenyl-4-[2-(pyridine-4-carbonyl)hydrazinylidene]butanoic acid, C16H15N3O3
- The crystal structure of 6-amino-5-carboxypyridin-1-ium pentaiodide monohydrate C6H9I5N2O3
- Crystal structure of bis(μ3-oxido)-bis(μ2-2-formylbenzoato-k2O:O′)-bis(2-(dimethoxymethyl)-benzoato-κO)-oktakismethyl-tetratin(IV)
- Crystal structure of 2-((E)-(((E)-2-hydroxy-4-methylbenzylidene) hydrazineylidene)methyl)-4-methylphenol, C16H16N2O2
- Crystal structure of (E)-amino(2-((5-methylfuran-2-yl)methylene)hydrazinyl) methaniminium nitrate monohydrate, C14H26N10O10
- The crystal structure of N′-(2-chloro-6-hydroxybenzylidene)thiophene-2-carbohydrazide monohydrate, C12H11ClN2O3S
- Crystal structure of catena-poly[(μ2-1,1′-(biphenyl-4,4′-diyl)bis(1H-imidazol)-κ2N:N′)-bis(4-bromobenzoate-κ1O)zinc(II)], C64H44Br4N8O8Zn2
- The crystal structure of catena-poly[(1-(4-carboxybenzyl)pyridin-1-ium-4-carboxylato-κ1O)-(μ2-oxalato-κ4 O:O′:O″:O‴)dioxidouranium(VI)], C16H11NO10U
- Crystal structure of 3-allyl-4-(2-bromoethyl)-5-(4-methoxyphenyl)-2-phenylfuran, C22H21BrO2
- Halogen bonds in the crystal structure of 4,3′:5′,4″-terpyridine — 1,3-diiodotetrafluorobenzene (1/1), C21H11F4I2N3
- Crystal structure of 2-(1H-indol-3-yl)ethan-1-aminium 2-(4-acetylphenoxy)acetate, C20H22N2O4
- Chalcogen bonds in the crystal structure of 4,7-dibromo-2,1,3-benzoselenadiazole, C6H2Br2N2Se
- The crystal structure of 1,4-bis((1H-benzimidazol-2-yl)methyl)-piperazine-2,5-dione dihydrate, C20H22N6O4
- The crystal structure of C19H20O8
- The crystal structure of KNa3Te8O18·5H2O exhibiting a ∞2[Te4O9]2− layer
- Erratum
- Erratum to: Crystal structure of (Z)-3-(6-bromo-1H-indol-3-yl)-1,3-diphenylprop-2-en-1-one, C23H16BrNO
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of (E)-7-hydroxy-2-((6-methoxypyridin-3-yl)methylene)-3, 4-dihydronaphthalen-1(2H)-one, C17H15NO3
- Crystal structure of (E)-7-methoxy-2-((2-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1 (2H)-one, C18H17NO3
- The crystal structure of N 6,N 6′-di(pyridin-2-yl)-[2,2′-bipyridine]-6,6′-diamine, C20H16N6
- The crystal structure of {N 1,N 2-bis[2,4-dimethyl-6-(4-(tert-butyl)phenyl)(phenyl)methyl]acenaphthylene-1,2-diimino-κ2 N, N′}-dibromido-nickel(II) – dichloromethane(1/2), C64H64Br2Cl4N2Ni
- Synthesis and crystal structure of nonacarbonyltris[(2-thia-1,3,5-triaza-7-phosphatricylco[3.3.1.1]decane-κ1 P)-2,2-dioxide]triruthenium(0) – acetonitrile (7/6), C25.71H32.57N9.86O15P3S3Ru3
- A new polymorph of 1-(4-nitrophenyl)-1H-benzimidazole (C13H9N3O2)
- The crystal structure of 2,2′-((1E,1′E)-(naphthalene-2,3 diylbis(azanylylidene)) bis(methanylylidene))bis(4-methylphenol), C26H22N2O2
- The crystal structure of bis(μ2-iodido)-bis(η6-benzene)-bis(iodido)-diosmium(II), C12H12I4Os2
- Redetermination of the crystal structure of bis{hydridotris(3,5-dimethylpyrazol-1-yl-κN 3)borato}copper(II), C30H44B2CuN12
- Crystal structure of (E)-3-((4-(tert-butyl)phenyl)thio)-4-hydroxypent-3-en-2-one, C15H20O2S
- Crystal structure of 2,2′-(p-tolylazanediyl)bis(1-phenylethan-1-one), C23H21NO2
- Redetermination of the crystal structure of the crystal sponge the poly[tetrakis(μ3-2,4,6-tris(pyridin-4-yl)-1,3,5-triazine)-dodecaiodidohexazinc(II) nitrobenzene solvate], C72H48I12N24Zn6⋅10(C6H5NO2)
- Crystal structure of (4′E)-6′-(diethylamino)-2-[(E)-[(6-methylpyridin-2-yl)methylidene]amino]-4′-{2-[(2E)-1,3,3-trimethyl-2,3-dihydro-1H-indol-2-ylidene]ethylidene}-1′,2,2′,3,3′,4′-hexahydrospiro[isoindole-1,9′-xanthene]-3-one, C44H45N5O2
- Crystal structure of (E)-7-fluoro-2-(3-fluorobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C17H12F2O1
- Crystal structure of tetrabutylammonium sulfanilate – 1-(diaminomethylene)thiourea (1/2)
- Crystal structure of [2,2′-{azanediyl)bis[(propane-3,1-diyl)(azanylylidene)methylylidene]} bis(3,5-dichlorophenolato)-κ2O,O′]-isothiocyanato-κN-iron(III), C21H19Cl4FeN4O2S
- Crystal structure of (4-chlorophenyl)(4-hydroxyphenyl)methanone, C13H9ClO2
- Crystal structure of 6,6′-((pentane-1,3-diylbis(azaneylylidene))bis(methaneylylidene))bis(2,4-dibromolphenolato-κ4 N,N′,O,O′)copper(II),) C19H16Br4CuN2O2
- Chlorido-(2,2′-(ethane-bis(5-methoxyphenolato))-κ4 N,N′,O,O′)manganese(III) monohydrate, C19H18Cl2CuN2O2
- Crystal structure of 2,6-di-tert-butyl-4-(4-methoxybenzylidene)cyclohexa-2,5-dien-1-one, C22H28O2
- Crystal structure of [6,6′-(((2,2-dimethylpropane-1,3-diyl)bis(azanylylidene))bis(methanylylidene))bis(2-chlorophenolato)-κ4N,N′,O,O′]copper(II)
- Crystal structure of 2-chloro-3-((thiophen-2-ylmethyl)amino)naphthalene-1,4-dione, C30H20O4N2Cl2S2
- Crystal structure of bis{hydridotris(3-trifluoromethyl-5-methylpyrazolyl-1-yl)borato-κN 3}manganese(II), C30H26B2F18MnN12
- Crystal structure of 1-(2-methylphenyl)-2-(2-methylbenzo[b]thienyl)-3,3,4,4,5,5-hexafluorocyclopent-ene, C21H14F6S
- Crystal structure of 2-(3-((carbamimidoylthio)methyl)benzyl)isothiouronium hexafluorophosphate monohydrate, C10H17F6N4OPS2
- Crystal structure of 4,5-diiodo-1,3-dimesityl-1H-1,2,3-triazol-3-ium chloride – chloroform (1/1), C21H23Cl4I2N3
- Crystal structure of azido-k1 N-{6,6′-((((methylazanediyl)bis(propane-3,1-diyl))bis(azanylylidene))bis(methanylylidene))bis(2,4-dibromophenolato)k5 N,N′,N″,O,O′}cobalt(III)-methanol (1/1)), C21H23Br4CoN6O3
- The crystal structure of 2-(4-((carbamimidoylthio)methyl)benzyl)isothiouronium hexafluorophosphate monohydrate, C10H17F6N4OPS2
- Crystal structure of 1,1′-(methane-1,1-diyl)bis(3-methyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C9H14F12N4P2
- Crystal structure of (4′E)-6′-(diethylamino)-2-[(E)-[(pyren-1-yl)methylidene]amino]-4′-{2-[(2E)-1,3,3-trimethyl-2,3-dihydro-1H-indol-2-ylidene]ethylidene}-1′,2,2′,3,3′,4′-hexahydrospiro[isoindole-1,9′-xanthene]-3-one, C54H48N4O2
- Crystal structure of poly[bis(μ2-2,6-bis(1-imidazoly)pyridine-κ2 N,N′)-bis(thiocyanato-κ1 N)copper(II)] dithiocyanate, C24H18CuN12S2
- Cones with a three-fold symmetry constructed from three hydrogen bonded theophyllinium cations that coat [FeCl4]− anions in the crystal structure of tris(theophyllinium) bis(tetrachloridoferrate(III)) chloride trihydrate, C21H33Cl9Fe2N12O9
- Crystal structure of 14-O-[(4-(4-hydroxypiperidine-1-yl)-6-methylpyrimidine-2-yl)thioacetyl]-mutilin monohydrate, C32H49N3O6S
- The crystal structure of (E)-3-chloro-2-(2-(4-methylbenzylidene)hydrazinyl)pyridine, C13H12ClN3
- The crystal structure of 4-phenyl-4-[2-(pyridine-4-carbonyl)hydrazinylidene]butanoic acid, C16H15N3O3
- The crystal structure of 6-amino-5-carboxypyridin-1-ium pentaiodide monohydrate C6H9I5N2O3
- Crystal structure of bis(μ3-oxido)-bis(μ2-2-formylbenzoato-k2O:O′)-bis(2-(dimethoxymethyl)-benzoato-κO)-oktakismethyl-tetratin(IV)
- Crystal structure of 2-((E)-(((E)-2-hydroxy-4-methylbenzylidene) hydrazineylidene)methyl)-4-methylphenol, C16H16N2O2
- Crystal structure of (E)-amino(2-((5-methylfuran-2-yl)methylene)hydrazinyl) methaniminium nitrate monohydrate, C14H26N10O10
- The crystal structure of N′-(2-chloro-6-hydroxybenzylidene)thiophene-2-carbohydrazide monohydrate, C12H11ClN2O3S
- Crystal structure of catena-poly[(μ2-1,1′-(biphenyl-4,4′-diyl)bis(1H-imidazol)-κ2N:N′)-bis(4-bromobenzoate-κ1O)zinc(II)], C64H44Br4N8O8Zn2
- The crystal structure of catena-poly[(1-(4-carboxybenzyl)pyridin-1-ium-4-carboxylato-κ1O)-(μ2-oxalato-κ4 O:O′:O″:O‴)dioxidouranium(VI)], C16H11NO10U
- Crystal structure of 3-allyl-4-(2-bromoethyl)-5-(4-methoxyphenyl)-2-phenylfuran, C22H21BrO2
- Halogen bonds in the crystal structure of 4,3′:5′,4″-terpyridine — 1,3-diiodotetrafluorobenzene (1/1), C21H11F4I2N3
- Crystal structure of 2-(1H-indol-3-yl)ethan-1-aminium 2-(4-acetylphenoxy)acetate, C20H22N2O4
- Chalcogen bonds in the crystal structure of 4,7-dibromo-2,1,3-benzoselenadiazole, C6H2Br2N2Se
- The crystal structure of 1,4-bis((1H-benzimidazol-2-yl)methyl)-piperazine-2,5-dione dihydrate, C20H22N6O4
- The crystal structure of C19H20O8
- The crystal structure of KNa3Te8O18·5H2O exhibiting a ∞2[Te4O9]2− layer
- Erratum
- Erratum to: Crystal structure of (Z)-3-(6-bromo-1H-indol-3-yl)-1,3-diphenylprop-2-en-1-one, C23H16BrNO

