Abstract
C17H15NO3, triclinic,
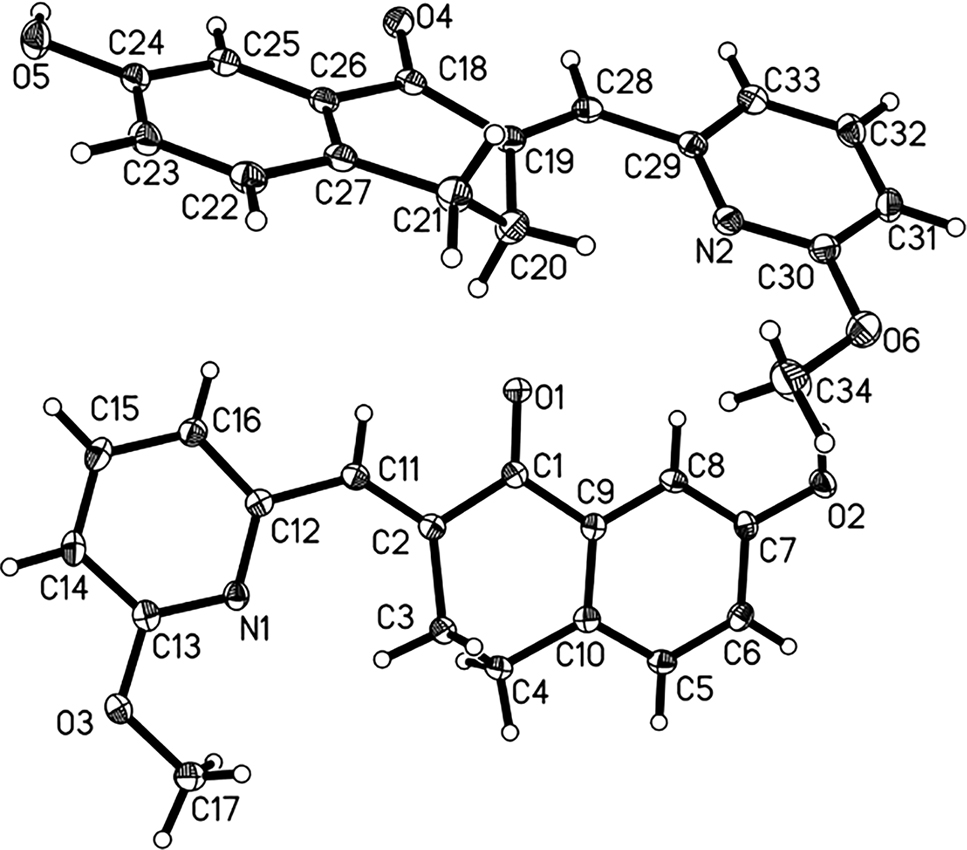
The molecular structure is shown in the figure. Displacement ellipsoids are drawn at the 40% probability level. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Yellow block |
| Size: | 0.13 × 0.12 × 0.10 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.10 mm−1 |
| Diffractometer, scan mode: | SuperNova |
| θmax, completeness: | 25.5°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 9244, 5038, 0.025 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 4080 |
| N(param)refined: | 383 |
| Programs: | CrysAlisPRO [1], SHELX [2], [3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1 | 0.6511 (2) | 0.37693 (13) | 0.02118 (11) | 0.0175 (3) |
| C2 | 0.4853 (2) | 0.33607 (13) | 0.05593 (11) | 0.0182 (4) |
| C3 | 0.4599 (2) | 0.21732 (14) | 0.03643 (12) | 0.0228 (4) |
| H3A | 0.334953 | 0.194165 | 0.042426 | 0.027* |
| H3B | 0.534525 | 0.209552 | 0.086724 | 0.027* |
| C4 | 0.5094 (2) | 0.14321 (14) | −0.06736 (12) | 0.0227 (4) |
| H4A | 0.504387 | 0.069222 | −0.073809 | 0.027* |
| H4B | 0.421911 | 0.141177 | −0.117786 | 0.027* |
| C5 | 0.8061 (2) | 0.11105 (14) | −0.14522 (12) | 0.0208 (4) |
| H5 | 0.765646 | 0.035969 | −0.173143 | 0.025* |
| C6 | 0.9747 (2) | 0.14846 (14) | −0.16421 (12) | 0.0212 (4) |
| H6 | 1.046478 | 0.098841 | −0.203713 | 0.025* |
| C7 | 1.0359 (2) | 0.26065 (14) | −0.12384 (11) | 0.0188 (4) |
| C8 | 0.9297 (2) | 0.33332 (13) | −0.06384 (11) | 0.0183 (4) |
| H8 | 0.970838 | 0.408287 | −0.036556 | 0.022* |
| C9 | 0.7604 (2) | 0.29542 (13) | −0.04355 (11) | 0.0174 (4) |
| C10 | 0.6955 (2) | 0.18273 (13) | −0.08550 (11) | 0.0188 (4) |
| C11 | 0.3764 (2) | 0.40996 (14) | 0.10176 (11) | 0.0191 (4) |
| H11 | 0.418808 | 0.479991 | 0.108651 | 0.023* |
| C12 | 0.2038 (2) | 0.40092 (13) | 0.14284 (11) | 0.0177 (4) |
| C13 | −0.0323 (2) | 0.29892 (14) | 0.17238 (12) | 0.0203 (4) |
| C14 | −0.1197 (2) | 0.38971 (15) | 0.22199 (12) | 0.0239 (4) |
| H14 | −0.228044 | 0.383070 | 0.249224 | 0.029* |
| C15 | −0.0391 (2) | 0.48863 (15) | 0.22875 (12) | 0.0240 (4) |
| H15 | −0.092044 | 0.551250 | 0.260781 | 0.029* |
| C16 | 0.1232 (2) | 0.49391 (14) | 0.18691 (12) | 0.0210 (4) |
| H16 | 0.177496 | 0.559801 | 0.188568 | 0.025* |
| C17 | −0.0400 (2) | 0.10651 (15) | 0.10537 (14) | 0.0297 (4) |
| H17A | −0.030161 | 0.099968 | 0.038791 | 0.045* |
| H17B | −0.114177 | 0.042343 | 0.102317 | 0.045* |
| H17C | 0.078006 | 0.113579 | 0.136486 | 0.045* |
| C18 | 0.7915 (2) | 0.87256 (13) | 0.49960 (11) | 0.0183 (4) |
| C19 | 0.9319 (2) | 0.79937 (13) | 0.47370 (11) | 0.0184 (4) |
| C20 | 0.9193 (2) | 0.71556 (15) | 0.51506 (13) | 0.0258 (4) |
| H20A | 1.033228 | 0.688049 | 0.510952 | 0.031* |
| H20B | 0.826312 | 0.654267 | 0.474908 | 0.031* |
| C21 | 0.8751 (2) | 0.76514 (15) | 0.62350 (12) | 0.0245 (4) |
| H21A | 0.859672 | 0.708543 | 0.646730 | 0.029* |
| H21B | 0.974653 | 0.820803 | 0.664974 | 0.029* |
| C22 | 0.5825(2) | 0.81246 (14) | 0.70147 (12) | 0.0219 (4) |
| H22 | 0.602239 | 0.775285 | 0.739679 | 0.026* |
| C23 | 0.4317(2) | 0.86352 (14) | 0.71286 (12) | 0.0225 (4) |
| H23 | 0.351119 | 0.860032 | 0.758138 | 0.027* |
| C24 | 0.3998 (2) | 0.92010 (13) | 0.65701 (12) | 0.0198 (4) |
| C25 | 0.5171 (2) | 0.92188 (13) | 0.58793 (11) | 0.0186 (4) |
| H25 | 0.495005 | 0.958266 | 0.549320 | 0.022* |
| C26 | 0.6691 (2) | 0.86950 (13) | 0.57534 (11) | 0.0176 (4) |
| C27 | 0.7060 (2) | 0.81539 (13) | 0.63387 (12) | 0.0193 (4) |
| C28 | 1.0580 (2) | 0.81543 (13) | 0.41681 (11) | 0.0172 (3) |
| H28 | 1.041033 | 0.868530 | 0.395372 | 0.021* |
| C29 | 1.2178 (2) | 0.76286 (13) | 0.38312 (11) | 0.0176 (4) |
| C30 | 1.4004 (2) | 0.63949 (14) | 0.37863 (13) | 0.0224 (4) |
| C31 | 1.5203 (2) | 0.66874 (14) | 0.32111 (13) | 0.0249 (4) |
| H31 | 1.622063 | 0.635261 | 0.301572 | 0.030* |
| C32 | 1.4822 (2) | 0.74872 (14) | 0.29457 (12) | 0.0229 (4) |
| H32 | 1.558203 | 0.770683 | 0.256145 | 0.027* |
| C33 | 1.3285 (2) | 0.79681 (14) | 0.32566 (11) | 0.0203 (4) |
| H33 | 1.300573 | 0.851035 | 0.308087 | 0.024* |
| C34 | 1.3078 (2) | 0.52660 (18) | 0.45777 (18) | 0.0454 (6) |
| H34A | 1.305991 | 0.587024 | 0.522152 | 0.068* |
| H34B | 1.340584 | 0.465398 | 0.466487 | 0.068* |
| H34C | 1.190225 | 0.506202 | 0.421826 | 0.068* |
| N1 | 0.12467 (16) | 0.30214 (11) | 0.13616 (9) | 0.0189 (3) |
| N2 | 1.25470 (17) | 0.68363 (11) | 0.40945 (10) | 0.0201 (3) |
| O1 | 0.69851 (14) | 0.47535 (9) | 0.04534 (8) | 0.0232 (3) |
| O2 | 1.20095 (14) | 0.29427 (10) | −0.14626 (9) | 0.0258 (3) |
| H2 | 1.227398 | 0.360985 | −0.114419 | 0.039* |
| O3 | −0.11985 (14) | 0.20131 (10) | 0.16273 (9) | 0.0255 (3) |
| O4 | 0.77477 (14) | 0.93468 (9) | 0.46041 (8) | 0.0217 (3) |
| O5 | 0.25137 (14) | 0.97224 (10) | 0.67322 (9) | 0.0251 (3) |
| H5A | 0.241687 | 0.998242 | 0.633132 | 0.038* |
| O6 | 1.43737 (15) | 0.55831 (10) | 0.40217 (10) | 0.0338 (3) |
Source of material
Drop 5 mL (25%) sodium hydroxide aqueous solution to 7-hydroxy-3,4-dihydronaphthalen-1(2H)-one and 6-meth-oxypicolinaldehyde, then add 10 mL methanol and stir at room temperature for 3 h. Silica gel thin layer method was used to monitor the process control chromatography (TLC, 254 nm). When the reaction was stopped, the precipitate was filtered from the reaction and dissolved with ethyl acetate. The organic phase was washed successively by water and brine, and dried with anhydrous sodium sulfate. After filtration, the ethyl acetate was condensed under vacuo to yield a white solid, which was purified by silica gel column chromatography (petroleum ether: ethyl acetate = 1:2, v/v). Appropriate crystals of the title compound were obtained by recrystallization from dichloromethane and methanol (1:1, v/v) and dried in vacuo at 65 °C for 3 h.
Experimental details
The H atoms were placed in idealized positions and treated as riding on their parent atoms, with d (C–H) = 0.97 Å (methylene), Uiso(H) = 1.2Ueq(C), and d(C–H) = 0.93 Å (aromatic), Uiso(H) = 1.2Ueq(C).
Comment
In inflammatory neurodegenerative diseases of the central nervous system (CNS), the microglia are activated and polarized into pro-inflammatory M1 phenotype. Neuroinflammation is a mediator and key factor in the progression of brain diseases [4]. The inflammatory process may lead to excessive release of inflammatory mediators or cytokines, such as tumor necrosis factor-α (TNF-α), interleukin (IL)-6, and so on [5]. Existing studies have showed that 3,4-dihydronaphthalene-1(2H-1)-one (DHN) derivatives have anti-tumor and anti-inflammatory activity as modifiers for allergic and inflammatory responses, however, few studies on DHN derivatives against neuroinflammation have been conducted. Thus it is promising to synthesize novel pyridine substituted derivatives to fight neuroinflammation [6]. Some DHN derivatives had been designed and synthesized as anti-inflammatory agents [7], [8]. Our group investigated DHN derivatives based on pyridine groups and tested their anti-neuroinflammatory activity. The results showed that the methoxy substituted compounds have better activity.
The title compound crystallizes in the triclinic space group
Funding source: Science and Technology Innovation Development Plan of Yantai
Award Identifier / Grant number: 2020XDRH105
Funding source: National Natural Science Foundation of China doi.org/10.13039/501100001809
Award Identifier / Grant number: 81473104
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work was supported by Science and Technology Innovation Development Plan of Yantai (No. 2020XDRH105) and the National Natural Science Foundation of China (No. 81473104).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Rigaku OD. CrysAlisPRO; Rigaku Oxford Diffraction Ltd: Yarnton, Oxfordshire, England, 2017.Suche in Google Scholar
2. Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Suche in Google Scholar PubMed
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar
4. Gao, C. L., Hou, G. G., Liu, J., Ru, T., Xu, Y. Z., Zhao, S. Y., Ye, H., Zhang, L. Y., Chen, K. X., Guo, Y. W., Pang, T., Li, X. W. Synthesis and target identification of benzoxepane derivatives as potential anti-neuroinflammatory agents for ischemic stroke. Angew. Chem. Int. Ed. 2020, 59, 2429–2439; https://doi.org/10.1002/anie.201912489.Suche in Google Scholar PubMed
5. Li, N., Xin, W. Y., Yao, B. R., Cong, W., Wang, C. H., Hou, G. G. N-Phenylsulfonyl-3,5-bis(arylidene)-4-piperidone derivatives as activation NF-κB inhibitors in hepatic carcinoma cell lines. Eur. J. Med. Chem. 2018, 155, 531–544; https://doi.org/10.1016/j.ejmech.2018.06.027.Suche in Google Scholar PubMed
6. Zhou, Y. Q., Hou, G. G., Meng, Q. G., Hou, Y. Crystal structure and anti-inflammatory activity of (3E,5E)-1-((4-chlorophenyl)sulfonyl)-3-(pyridin-4-ylmethylene)-5-(2-(trifluoromethyl) benzylidene)piperidin-4-one, C25H18ClF3N2O3S. Z. Kristallogr. N. Cryst. Struct. 2020, 235, 411–413; https://doi.org/10.1515/ncrs-2019-0716.Suche in Google Scholar
7. Zhang, X. F., Meng, Q. G. Crystal structure of (3E,5E)-2-((2-methoxy-3-pyridyl) methylene)-7-fluoro-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2. Z. Kristallogr. N. Cryst. Struct. 2021, 236, 507–509; https://doi.org/10.1515/ncrs-2020-0603.Suche in Google Scholar
8. Luan, M. Z., Wang, H. Y., Zhang, M., Song, J., Hou, G. G., Zhao, F. L., Meng, Q. G. Crystal structure of (E)-2-(3,5-bis(trifluoromethyl)benzylidene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C20H14F6O2. Z. Kristallogr. N. Cryst. Struct. 2021, 236, 61–63; https://doi.org/10.1515/ncrs-2020-0446.Suche in Google Scholar
9. Xiong, C. L., Lan, Y. D., Song, X. Y., Xiong, W. M., Nie, X. L. Crystal structure of methyl 4-acetoxy-3,5-dimethoxybenzoate, C12H14O6. Z. Kristallogr. N. Cryst. Struct. 2021, 236, 573–575; https://doi.org/10.1515/ncrs-2020-0632.Suche in Google Scholar
10. Sun, Y., Liu, Y. K., Li, J. D., Meng, Q. G., Hou, G. G. Crystal structure and anti-inflammatory activity of (3E,5E)-3-(2-fluorobenzylidene)-1- ((4-fluorophenyl)sulfonyl)-5-(pyridin-3-ylmethylene)piperidin-4-one, C24H18F2N2O3S. Z. Kristallogr. N. Cryst. Struct. 2020, 235, 377–379; https://doi.org/10.1515/ncrs-2019-0683.Suche in Google Scholar
© 2021 Lun-Hai Liang et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of [aqua-(4-iodopyridine-2,6-dicarboxylato-κ3 O,N,O′)-(1,10-phenanothroline-κ2 N,N′)copper(II)] dihydrate, C19H16O7N3CuI
- The crystal structure of tetrakis(1-isopropyl-1H-imidazolium) octamolybdate, C24H44Mo8N8O26
- Crystal structure of catena-poly[bis(µ2-3,5-bis(1-imidazolyl)pyridine-κ2 N:N′)-(µ2-3-nitrophthalato-k3 O,O′:O″)cadmium(II)] dihydrate, C30H25N11O8Cd
- The crystal structure of diaqua-bis(2-(3-(1H-pyrazol-4-yl)-1H-1,2,4-triazol-5-yl)pyridine-κ2 N:N′)-bis(3,5-dicarboxybenzoato-κ1 O)cobalt(II), C38H30CoN12O14
- Crystal structure of the nickel(II) complex aqua-(2,6-di(pyrazin-2-yl)-4,4′-bipyridine-κ3 N,N′,N′′)-(phthalato-κ2 O,O′)nickel(II) tetrahydrate, C26H26N6O9Ni
- The crystal structure of 1-[5-(2-fluorophenyl)-1-(pyridine-3-sulfonyl)-1H-pyrrol-3-yl]-N-methylmethanaminium 3-carboxyprop-2-enoate, C21H20FN3O6S
- The crystal structure of 1,2-bis(4-pyridyl)ethane - 4,4-dihydroxydiphenylmethane (1/1), C25H21N2O2
- Crystal structure of bis(2-((E)-5-chloro-2-hydroxybenzylidene)hydrazineyl)methaniminium trifluoroacetate dihydrate, C34H36Cl4N10O12
- Crystal structure of 1-cyclopropyl-7-ethoxy-6,8-difluoro-4-oxo-1,4-dihydro-quinoline-3-carboxylic acid, C15H13F2NO4
- Crystal structure of methyl 3-(1H-naphtho[1,8-de][1,3,2]diazaborinin-2(3H)-yl)benzoate, C18H15BN2O2
- Crystal structure of (E)-N′-(2-chloro-6-hydroxybenzylidene)-2-hydroxybenzohydrazide, C14H11ClN2O3
- Crystal structure of Al-rich fluorophlogopite, K1.0(Mg2.8Al0.2)(Si2.8Al1.2)O10F2
- The crystal structure of 4,5-diiodo-1,3-dimesityl-1H-1,2,3-triazol-3-ium hexafluoridoantimonate(V), C20H22F6I2N3Sb
- Crystal structure of tris(3-iodopyridin-1-ium) catena-poly[(hexachlorido-κ1 Cl)-(μ2-trichlorido-κ2 Cl:Cl)diantimony(III)], C15H15Cl9I3N3Sb2
- Crystal structure of methyl 2-(1H-naphtho[1,8-de][1.3.2]diazaborinin-2(3H-yl)benzoate C18H15BN2O2
- The crystal structure of 1,8-bis(4-methoxybenzoyl)naphthalene-2,7-diyl dibenzoate, C40H28O8
- Crystal structure of 2-bromo-1,3,6,8-tetramethylBOPHY (BOPHY = bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl)methylene)hydrazine), C14H15B2BrF4N4
- The crystal structure of (E)-3-chloro-2-(2-(2-fluorobenzylidene)hydrazinyl)pyridine, C12H9ClFN3
- Crystal structure of bis(µ2- 4-iodopyridine-2,6-dicarboxylato-κ3O:N:O′)-bis(4-iodopyridine-2,6-dicarboxylato-κ3O:N:O′)-bis(µ2-1-(4-pyridyl)piperazine-κ2N:N′)-hexa-aqua-tetra-copper(II), C46H46Cu4I4N10O22
- Crystal structure of poly[diaqua-(μ2-2,5-dihydroxyterephthalato-κ2O:O′)(μ2-bis(4-pyridylformyl)piperazine-κ2N:N′)cadmium(II)] dihydrate, C24H28CdN4O12
- Crystal structure of poly[aqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)-(μ3-2,3,5,6-tetrafluoroterephthalato-κ3O:O′:O′′)cadmium(II)], C17H14N4O5F4Cd
- Crystal structure of 6-(quinolin-8-yl)benzo[a]phenanthridin-5(6H)-one, C26H16N2O
- The crystal structure of aqua-bis(6-chloropicolinato-κ2N,O)copper(II), C12H8Cl2N2O5Cu
- Crystal structure of catena-poly[diaqua-bis(μ2-4,4′-bipyridyl-κ2N:N′) disilver(I)] 4-oxidopyridine-3-sulfonate trihydrate, C25H29Ag2N5O9S
- The crystal structure of 4-(3-bromophenyl)pyrimidin-2-amine, C10H8BrN3
- Crystal structure of 6-oxo-4-phenyl-1-propyl-1,6-dihydropyridine-3-carbonitrile, C15H14N2O
- Crystal structure of 4-(2,2-difluoroethyl)-2,4-dimethyl-6-(trifluoromethyl)isoquinoline-1,3(2H,4H)-dione, C14H12F5NO2
- Crystal structure of dibromido-(1-methyl-1H-imidazole-κ1N)-(3-(3-methyl-1H-imidazol-3-ium-1-yl)propanoato-κ1O)zinc(II), C11H16Br2N4O2Zn
- The crystal structure of 1,1′-(((2 (dimethylamino)ethyl)azanediyl)bis(methylene)) bis(naphthalen-2-olato-κ4 N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)- titanium(IV) ─ dichloromethane (2/1), C33H29N3O6Ti
- The layered crystal structure of bis(theophyllinium) hexachloridostannate (IV), C14H18N8O8SnCl6
- The crystal structre of 3-(1-ethenyl-1H-imidazol-3-ium-3-yl)propane-1-sulfonate, C8H12N2O3S
- Synthesis and crystal structure of di-tert-butyl 1″-acetyl-2,2″,9′-trioxo-4a′,9a′-dihydro-1′H,3′H,9′H-dispiro[indoline-3,2′-xanthene-4′,3″-indoline]-1,3′-dicarboxylate, C39H38N2O9
- The crystal structure of 4-chloro-2-(quinolin-8-yl)isoindoline-1,3-dione, C17H9ClN2O2
- The crystal structure of 1-fluoro-4-(p-tolylethynyl)benzene, C15H11F
- The crystal structure of bis[4-bromo-2-(1H-pyrazol-3-yl) phenolato-κ2N,O] copper(II), C18H12Br2CuN4O2
- The crystal structure of poly[(μ 3-imidazolato-κ 3 N:N:N′)(tetrahydrofuran- κ 1 O)lithium(I)], C7H11LiN2O
- Crystal structure of N′,N′′′-((1E,1′E)-(propane-2,2-diylbis(1H-pyrrole-5,2diyl))bis(methaneylylidene))di(nicotinohydrazide) pentahydrate, C25H24N8O2·5H2O
- Crystal structure of 3-(2-ethoxy-2-oxoethyl)-1-ethyl-1H-imidazol-3-ium hexafluoridophos-phate(V), C9H15F6N2O2P
- Crystal structure of (1,10-phenanthroline-κ2N,N′)-bis(3-thiophenecarboxylato-κ2O,O′)copper(II), C22H14N2O4S2Cu
- The crystal structure of 2-amino-3-carboxypyridin-1-ium iodide hemihydrate, C6H8IN2O2.5
- Crystal structure of (E)-7-methoxy-2-((6-methoxypyridin-2-yl)methylene)-tetralone, C18H17NO3
- The crystal structure of [μ-hydroxido-bis[(5,5′-dimethyl-2,2′-bipyridine-κ2N,N′)-tricarbonylrhenium(I)] bromide hemihydrate, C30H26N4O9Re2Br
- The crystal structure of 2,5-bis(3,5-dimethylphenyl)thiazolo[5,4-d]thiazole, C20H18N2S2
- The crystal structure of 5-benzoyl-1-[(E)-(4-fluorobenzylidene)amino]-4-phenylpyrimidin-2(1H)-one, C24H16FN3O2
- Crystal structure of monocarbonyl(N-nitroso-N-oxido-phenylamine-κ 2 O,O′)(tricyclohexylphosphine-κP)rhodium(I), C25H39N2O3PRh
- Crystal structure of poly[bis[μ3-1,3,5-tris[(1H-imidazol-1-yl)methyl]benzene-κ3N:N′:N″]nickel(II)] hexafluorosilicate, C36H36N12NiSiF6
- The crystal structure of 13-(pyrazole-1-yl-4-carbonitrile)-matrine, C19H25N5O
- Crystal structure of 3,5-bis((E)-4-methoxy-2-(trifluoromethyl)benzylidene)-1-methylpiperidin-4-one, C24H21F6NO3
- The crystal structure of N,N′-(Disulfanediyldi-2,1-phenylene)di(6′-methylpyridine)-2-carboxamide, C26H22N4O2S2
- Crystal structure of (E)-7-fluoro-2-(4-methoxy-2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C19H14F4O2
- Crystal structure of ethyl 1-(4-fluorophenyl)-4-phenyl-1H-pyrrole-3-carboxylate, C19H16FNO2
- The crystal structure of cis-diaqua-bis (N-butyl-N-(pyridin-2-yl)pyridin-2-amine-κ2N,N′)cobalt(II)] dichloride trihydrate, C28H44Cl2N6O5Co
- Crystal structure of (E)-7-methoxy-2-((6-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C18H17NO3
- Crystal structure of (E)-2-((3-fluoropyridin-4-yl)methylene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- The crystal structure of 6-bromohexanoic acid, C6H11BrO2
- The crystal structure of 4-chloro-thiophenol, C6H5ClS
- The crystal structure of 4-bromobenzyl chloride, C7H6BrCl
- The crystal structure of di-tert-butyl dicarbonate, C10H18O5
- The crystal structure of (2-(4-chlorophenyl)-5-methyl-1,3-dioxan-5-yl)methanol, C12H15ClO3
- The crystal structure of the co-crystal: 2-hydroxybenzoic acid – N′-(butan-2-ylidene)pyridine-4-carbohydrazide, C10H13N3O·C7H6O3
- Crystal structure and anti-inflammatory activity of (E)-7-fluoro-2-((5-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- Crystal structure of (E)-7-fluoro-2-((6-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- Crystal structure of 1,1′-(butane-1,4-diyl)bis(3-propyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C32H56F24N8P4
- The crystal structure of dichlorido-bis(3-methyl-3-imidazolium-1-ylpropionato-κ2)-cadmium(II), C14H20CdCl2N4O4
- Crystal structure of 1-(2-cyanobenzyl)-3-cyano-4-phenyl-4-(2-cyanobenzyl)-1,4-dihydropyridine monohydrate, C56H42N8O
- The crystal structure of 3-(carboxymethyl)-1-ethenyl-1H-imidazol-3-ium chloride, C7H9N2O2Cl
- The crystal structure of adamantylmethoxydiphenylsilane, C23H28OSi
- Redetermination of the crystal structure of (2E,4Z,13E,15Z)-3,5,14,16-tetramethyl-2,6,13,17-tetraazatricyclo[16.4.0.07,12]docosa-1(22),2,4,7,9,11,13,15,18,20-decaene, C22H24N4
- Crystal structure of (E)-7-hydroxy-2-((6-methoxypyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H15NO3
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2 N:N′)cobalt(II)] dinitrate, C18H28N10O8Co
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of [aqua-(4-iodopyridine-2,6-dicarboxylato-κ3 O,N,O′)-(1,10-phenanothroline-κ2 N,N′)copper(II)] dihydrate, C19H16O7N3CuI
- The crystal structure of tetrakis(1-isopropyl-1H-imidazolium) octamolybdate, C24H44Mo8N8O26
- Crystal structure of catena-poly[bis(µ2-3,5-bis(1-imidazolyl)pyridine-κ2 N:N′)-(µ2-3-nitrophthalato-k3 O,O′:O″)cadmium(II)] dihydrate, C30H25N11O8Cd
- The crystal structure of diaqua-bis(2-(3-(1H-pyrazol-4-yl)-1H-1,2,4-triazol-5-yl)pyridine-κ2 N:N′)-bis(3,5-dicarboxybenzoato-κ1 O)cobalt(II), C38H30CoN12O14
- Crystal structure of the nickel(II) complex aqua-(2,6-di(pyrazin-2-yl)-4,4′-bipyridine-κ3 N,N′,N′′)-(phthalato-κ2 O,O′)nickel(II) tetrahydrate, C26H26N6O9Ni
- The crystal structure of 1-[5-(2-fluorophenyl)-1-(pyridine-3-sulfonyl)-1H-pyrrol-3-yl]-N-methylmethanaminium 3-carboxyprop-2-enoate, C21H20FN3O6S
- The crystal structure of 1,2-bis(4-pyridyl)ethane - 4,4-dihydroxydiphenylmethane (1/1), C25H21N2O2
- Crystal structure of bis(2-((E)-5-chloro-2-hydroxybenzylidene)hydrazineyl)methaniminium trifluoroacetate dihydrate, C34H36Cl4N10O12
- Crystal structure of 1-cyclopropyl-7-ethoxy-6,8-difluoro-4-oxo-1,4-dihydro-quinoline-3-carboxylic acid, C15H13F2NO4
- Crystal structure of methyl 3-(1H-naphtho[1,8-de][1,3,2]diazaborinin-2(3H)-yl)benzoate, C18H15BN2O2
- Crystal structure of (E)-N′-(2-chloro-6-hydroxybenzylidene)-2-hydroxybenzohydrazide, C14H11ClN2O3
- Crystal structure of Al-rich fluorophlogopite, K1.0(Mg2.8Al0.2)(Si2.8Al1.2)O10F2
- The crystal structure of 4,5-diiodo-1,3-dimesityl-1H-1,2,3-triazol-3-ium hexafluoridoantimonate(V), C20H22F6I2N3Sb
- Crystal structure of tris(3-iodopyridin-1-ium) catena-poly[(hexachlorido-κ1 Cl)-(μ2-trichlorido-κ2 Cl:Cl)diantimony(III)], C15H15Cl9I3N3Sb2
- Crystal structure of methyl 2-(1H-naphtho[1,8-de][1.3.2]diazaborinin-2(3H-yl)benzoate C18H15BN2O2
- The crystal structure of 1,8-bis(4-methoxybenzoyl)naphthalene-2,7-diyl dibenzoate, C40H28O8
- Crystal structure of 2-bromo-1,3,6,8-tetramethylBOPHY (BOPHY = bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl)methylene)hydrazine), C14H15B2BrF4N4
- The crystal structure of (E)-3-chloro-2-(2-(2-fluorobenzylidene)hydrazinyl)pyridine, C12H9ClFN3
- Crystal structure of bis(µ2- 4-iodopyridine-2,6-dicarboxylato-κ3O:N:O′)-bis(4-iodopyridine-2,6-dicarboxylato-κ3O:N:O′)-bis(µ2-1-(4-pyridyl)piperazine-κ2N:N′)-hexa-aqua-tetra-copper(II), C46H46Cu4I4N10O22
- Crystal structure of poly[diaqua-(μ2-2,5-dihydroxyterephthalato-κ2O:O′)(μ2-bis(4-pyridylformyl)piperazine-κ2N:N′)cadmium(II)] dihydrate, C24H28CdN4O12
- Crystal structure of poly[aqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)-(μ3-2,3,5,6-tetrafluoroterephthalato-κ3O:O′:O′′)cadmium(II)], C17H14N4O5F4Cd
- Crystal structure of 6-(quinolin-8-yl)benzo[a]phenanthridin-5(6H)-one, C26H16N2O
- The crystal structure of aqua-bis(6-chloropicolinato-κ2N,O)copper(II), C12H8Cl2N2O5Cu
- Crystal structure of catena-poly[diaqua-bis(μ2-4,4′-bipyridyl-κ2N:N′) disilver(I)] 4-oxidopyridine-3-sulfonate trihydrate, C25H29Ag2N5O9S
- The crystal structure of 4-(3-bromophenyl)pyrimidin-2-amine, C10H8BrN3
- Crystal structure of 6-oxo-4-phenyl-1-propyl-1,6-dihydropyridine-3-carbonitrile, C15H14N2O
- Crystal structure of 4-(2,2-difluoroethyl)-2,4-dimethyl-6-(trifluoromethyl)isoquinoline-1,3(2H,4H)-dione, C14H12F5NO2
- Crystal structure of dibromido-(1-methyl-1H-imidazole-κ1N)-(3-(3-methyl-1H-imidazol-3-ium-1-yl)propanoato-κ1O)zinc(II), C11H16Br2N4O2Zn
- The crystal structure of 1,1′-(((2 (dimethylamino)ethyl)azanediyl)bis(methylene)) bis(naphthalen-2-olato-κ4 N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)- titanium(IV) ─ dichloromethane (2/1), C33H29N3O6Ti
- The layered crystal structure of bis(theophyllinium) hexachloridostannate (IV), C14H18N8O8SnCl6
- The crystal structre of 3-(1-ethenyl-1H-imidazol-3-ium-3-yl)propane-1-sulfonate, C8H12N2O3S
- Synthesis and crystal structure of di-tert-butyl 1″-acetyl-2,2″,9′-trioxo-4a′,9a′-dihydro-1′H,3′H,9′H-dispiro[indoline-3,2′-xanthene-4′,3″-indoline]-1,3′-dicarboxylate, C39H38N2O9
- The crystal structure of 4-chloro-2-(quinolin-8-yl)isoindoline-1,3-dione, C17H9ClN2O2
- The crystal structure of 1-fluoro-4-(p-tolylethynyl)benzene, C15H11F
- The crystal structure of bis[4-bromo-2-(1H-pyrazol-3-yl) phenolato-κ2N,O] copper(II), C18H12Br2CuN4O2
- The crystal structure of poly[(μ 3-imidazolato-κ 3 N:N:N′)(tetrahydrofuran- κ 1 O)lithium(I)], C7H11LiN2O
- Crystal structure of N′,N′′′-((1E,1′E)-(propane-2,2-diylbis(1H-pyrrole-5,2diyl))bis(methaneylylidene))di(nicotinohydrazide) pentahydrate, C25H24N8O2·5H2O
- Crystal structure of 3-(2-ethoxy-2-oxoethyl)-1-ethyl-1H-imidazol-3-ium hexafluoridophos-phate(V), C9H15F6N2O2P
- Crystal structure of (1,10-phenanthroline-κ2N,N′)-bis(3-thiophenecarboxylato-κ2O,O′)copper(II), C22H14N2O4S2Cu
- The crystal structure of 2-amino-3-carboxypyridin-1-ium iodide hemihydrate, C6H8IN2O2.5
- Crystal structure of (E)-7-methoxy-2-((6-methoxypyridin-2-yl)methylene)-tetralone, C18H17NO3
- The crystal structure of [μ-hydroxido-bis[(5,5′-dimethyl-2,2′-bipyridine-κ2N,N′)-tricarbonylrhenium(I)] bromide hemihydrate, C30H26N4O9Re2Br
- The crystal structure of 2,5-bis(3,5-dimethylphenyl)thiazolo[5,4-d]thiazole, C20H18N2S2
- The crystal structure of 5-benzoyl-1-[(E)-(4-fluorobenzylidene)amino]-4-phenylpyrimidin-2(1H)-one, C24H16FN3O2
- Crystal structure of monocarbonyl(N-nitroso-N-oxido-phenylamine-κ 2 O,O′)(tricyclohexylphosphine-κP)rhodium(I), C25H39N2O3PRh
- Crystal structure of poly[bis[μ3-1,3,5-tris[(1H-imidazol-1-yl)methyl]benzene-κ3N:N′:N″]nickel(II)] hexafluorosilicate, C36H36N12NiSiF6
- The crystal structure of 13-(pyrazole-1-yl-4-carbonitrile)-matrine, C19H25N5O
- Crystal structure of 3,5-bis((E)-4-methoxy-2-(trifluoromethyl)benzylidene)-1-methylpiperidin-4-one, C24H21F6NO3
- The crystal structure of N,N′-(Disulfanediyldi-2,1-phenylene)di(6′-methylpyridine)-2-carboxamide, C26H22N4O2S2
- Crystal structure of (E)-7-fluoro-2-(4-methoxy-2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C19H14F4O2
- Crystal structure of ethyl 1-(4-fluorophenyl)-4-phenyl-1H-pyrrole-3-carboxylate, C19H16FNO2
- The crystal structure of cis-diaqua-bis (N-butyl-N-(pyridin-2-yl)pyridin-2-amine-κ2N,N′)cobalt(II)] dichloride trihydrate, C28H44Cl2N6O5Co
- Crystal structure of (E)-7-methoxy-2-((6-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C18H17NO3
- Crystal structure of (E)-2-((3-fluoropyridin-4-yl)methylene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- The crystal structure of 6-bromohexanoic acid, C6H11BrO2
- The crystal structure of 4-chloro-thiophenol, C6H5ClS
- The crystal structure of 4-bromobenzyl chloride, C7H6BrCl
- The crystal structure of di-tert-butyl dicarbonate, C10H18O5
- The crystal structure of (2-(4-chlorophenyl)-5-methyl-1,3-dioxan-5-yl)methanol, C12H15ClO3
- The crystal structure of the co-crystal: 2-hydroxybenzoic acid – N′-(butan-2-ylidene)pyridine-4-carbohydrazide, C10H13N3O·C7H6O3
- Crystal structure and anti-inflammatory activity of (E)-7-fluoro-2-((5-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- Crystal structure of (E)-7-fluoro-2-((6-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- Crystal structure of 1,1′-(butane-1,4-diyl)bis(3-propyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C32H56F24N8P4
- The crystal structure of dichlorido-bis(3-methyl-3-imidazolium-1-ylpropionato-κ2)-cadmium(II), C14H20CdCl2N4O4
- Crystal structure of 1-(2-cyanobenzyl)-3-cyano-4-phenyl-4-(2-cyanobenzyl)-1,4-dihydropyridine monohydrate, C56H42N8O
- The crystal structure of 3-(carboxymethyl)-1-ethenyl-1H-imidazol-3-ium chloride, C7H9N2O2Cl
- The crystal structure of adamantylmethoxydiphenylsilane, C23H28OSi
- Redetermination of the crystal structure of (2E,4Z,13E,15Z)-3,5,14,16-tetramethyl-2,6,13,17-tetraazatricyclo[16.4.0.07,12]docosa-1(22),2,4,7,9,11,13,15,18,20-decaene, C22H24N4
- Crystal structure of (E)-7-hydroxy-2-((6-methoxypyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H15NO3
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2 N:N′)cobalt(II)] dinitrate, C18H28N10O8Co

