Abstract
C14H18N8O8SnCl6, monoclinic, P21/n (no. 14), a = 8.1810(2) Å, b = 12.6195(3) Å, c = 11.3811(2) Å, β = 90.258(2)°, Z = 2, V = 1174.97(5) Å3, Rgt(F) = 0.0266, wRref = 0.0620, T = 290 K.
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.30 × 0.16 × 0.10 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 1.81 mm−1 |
| Diffractometer, scan mode: | Xcalibur EOS, ω |
| θmax, completeness: | 33.0°, 98% |
| N(hkl)measured, N(hkl)unique, Rint: | 17679, 4333, 0.025 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 3278 |
| N(param)refined: | 165 |
| Programs: | Diamond [1], CrysAlisPRO [2], SHELX [3, 4], ShelXle [5] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| Sn1 | 0.500000 | 0.500000 | 0.500000 | 0.02128 (5) |
| Cl1 | 0.43257 (7) | 0.48605 (6) | 0.70878 (5) | 0.04115 (14) |
| Cl2 | 0.51798 (9) | 0.30903 (4) | 0.48832 (6) | 0.04624 (17) |
| Cl3 | 0.21278 (6) | 0.49002 (5) | 0.44895 (5) | 0.03561 (12) |
| O1 | 0.52203 (19) | 0.04847 (13) | 0.64313 (15) | 0.0351 (4) |
| O2 | 0.63937 (19) | 0.31667 (13) | 0.90004 (14) | 0.0324 (3) |
| N1 | 0.5731 (2) | 0.19005 (14) | 0.76250 (16) | 0.0268 (3) |
| N2 | 0.7595 (2) | 0.13645 (13) | 0.61508 (15) | 0.0259 (3) |
| N3 | 0.9486 (2) | 0.35070 (15) | 0.75585 (16) | 0.0276 (4) |
| H3 | 0.953 (3) | 0.405 (2) | 0.800 (2) | 0.037 (7)* |
| N4 | 1.0039 (2) | 0.24896 (15) | 0.60733 (17) | 0.0296 (4) |
| H4 | 1.052 (4) | 0.227 (2) | 0.542 (3) | 0.054 (9)* |
| C1 | 0.6133 (3) | 0.12098 (16) | 0.67220 (19) | 0.0265 (4) |
| C2 | 0.8569 (2) | 0.21706 (16) | 0.65178 (18) | 0.0251 (4) |
| C3 | 0.8203 (2) | 0.28065 (16) | 0.74453 (17) | 0.0243 (4) |
| C4 | 0.6753 (2) | 0.26708 (16) | 0.81081 (18) | 0.0251 (4) |
| C5 | 1.0552 (3) | 0.33142 (18) | 0.6724 (2) | 0.0316 (4) |
| H5 | 1.151389 | 0.369097 | 0.660252 | 0.043 (7)* |
| C6 | 0.4111 (3) | 0.1765 (2) | 0.8148 (2) | 0.0389 (5) |
| H6A | 0.367669 | 0.244483 | 0.835927 | 0.064 (6)* |
| H6B | 0.419974 | 0.132962 | 0.883729 | 0.064 (6)* |
| H6C | 0.339429 | 0.143028 | 0.758983 | 0.064 (6)* |
| C7 | 0.7991 (3) | 0.0684 (2) | 0.5151 (2) | 0.0408 (6) |
| H7A | 0.707185 | 0.065270 | 0.462509 | 0.100 (8)* |
| H7B | 0.824267 | −0.001657 | 0.542783 | 0.100 (8)* |
| H7C | 0.891865 | 0.096789 | 0.474470 | 0.100 (8)* |
Source of material
All chemicals were obtained from commercial sources and used as purchased. The Raman spectra were measured using a Bruker MULTIRAM spectrometer (Nd: YAG-laser at 1064 nm; InGaAs detector) with an apodized resolution of 8 cm−1 in the region of 4000–70 cm−1. The title compound was synthesized by dissolving 0.18 g (1 mmol) theophylline 180.16 and 0.14 SnCl4 (0.5 mmol) in 1 mL concentrated hydrochloric acid. Short-time warming until both components were dissolved yielded a colourless solution. From the aforementioned colourless solution a large number of colourless block crystals grew upon slow cooling to room temperature within minutes.
Experimental details
A single crystal of the title compound was directly selected from the mother liquor and rapidly transferred to the Xcalibur four-circle diffractometer equipped with an EOS detector [2]. An absorption correction (Gaussian method) was applied [2]. The structure solution and the refinement were successfully carried out using the SHELX program system [3], [4], [5]. The pseudo-orthogonal unit cell (see the Figure and the Abstract) and the occupancy of special positions by the
Comment
Introduction
Theophylline is a well-known natural product and was first described by Kossel in 1888 [8], [9]. He was able to isolate theophylline from tea leaves, which origins the naming up to now. There is still a fundamental interest in this compound and the corresponding solid state phases [10], [11] and co-crystals [12], [13], [14], [15]. Nowadays theophylline is often used as pharmaceutical agent due to its effects on the respiratory system [16], [17], [18], [19]. Recently, theophylline was also used in SARS–CoV-2 therapy [20].
We have already shown that heterocyclic cations like some pyridinium derivatives [21], [22] and the derived N-protonated cations of naturally occurring bases like nicotine [23], [24], caffeine [25] and theophylline [26], [27] are excellent tectons to construct hydrogen bonded networks. A database check (Cambridge Structural database [28]) showed that not more than about 20 crystal structures have been deposited so far, that contain a theophyllinium cation. The
From the reaction of theophylline (systematic name: 1,3-dimethyl-3,7-dihydro-1H-purine-2,6-dione) with hydrochloric in the presence of one equivalent of SnCl4, colorless block crystals of the title compound were obtained.
Structural comments
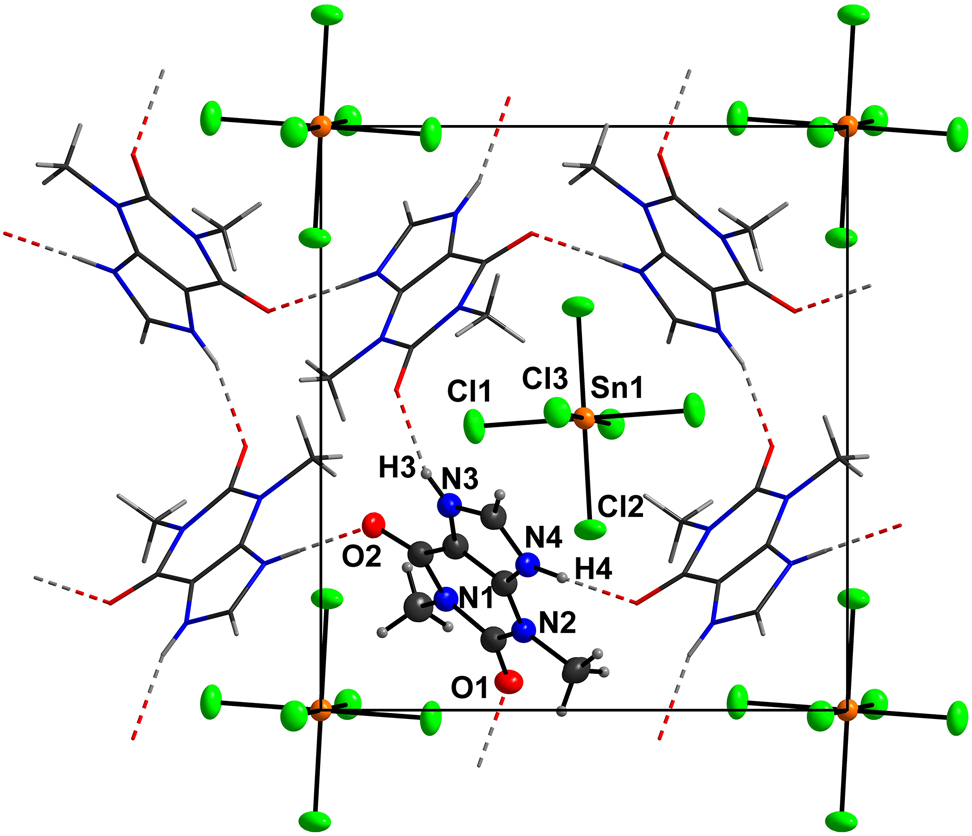
The asymmetric unit of the title compound consists of one N-protonated theophyllinium cation (TheoH) residing on a general position, and one half of a hexachloridostannate(IV) anion located on an inversion center. Bond lengths within the TheoH cation are all in the expected ranges [26], [27]. The same is true for the [SnCl6]2− anion [29], [30], [31], [32], [33], [34], [35], [36]. In detail, the Sn–Cl distances range from 2.4182(5)–2.4482(6) Å and the cis-angles range from 89.63(2)–90.37(2)°. Each theophyllinium cation is connected to four neighbouring cations by classical NH···O hydrogen bonds (see the Figure). These connections construct a layered, hydrogen bonded structure in the (101) plane. In detail, the N–H donor groups of TheoH are involved in unbifurcated classical hydrogen bond (see the Figure; N3···O1′ = 2.758(2) Å; ′ = 1.5 − x, 0.5 + y, 1.5 − z; N4···O2″ = 2.739(2) Å; ″ = 0.5 + x, 0.5 − y, −0.5 − z). Each mesh of the hydrogen-bonded net consists of four cations. It is obvious that larger and smaller meshes are present in this structure (see the Figure). Consequently, the [SnCl6]2− anions (Wyckoff site: 2a) are located beneath and above of the larger meshs, whereas two inversion symmetry related methyl groups of two TheoH cations fill the smaller meshes around the Wyckoff sites 2b and 2c.
The [SnCl6]2− anion as a weak to medium-strong hydrogen-bond acceptor is not involved in any strong hydrogen bonds in the title structure. This was bound to happen as all classsic hydrogen donors in the title struc-ture are involved in medium-strong unbifurcated NH···O hydrogen bonds. Nevertheless the chlorido ligand Cl2, which features the shortest Sn–Cl distance shows the longest Cl···H distance, which is in total accord with our expectations of a bulky anion interacting with neighbours via weak intermolecular forces. Thus we suppose that the shape and the group radius of the [SnCl6]2− determines or at least supports the formation of the hydrogen-bonded, two-dimensional network.
This suggestion is supported by the fact the in the case of another theophyllinium salt (
Raman spectroscopy
There are three very strong signals in the Raman spectrum at 312, 162, and 135 cm−1 respectively as well as one medium-strong signal at 239 cm−1. These four signals must be assigned to the SnCl62− anion and are similar to those for detected for (PCl4)2SnCl6 [40] and K2SnCl6 [41].
Conclusion and outlook
We have shown that the counter anion may influence the hydrogen-bonding scheme of the theophyllinium sub system. Also typical for this class of compounds is the pseudosymmetric arrangement [42], [43], [44], [45] of the anionic sub structure of the hexachloridostannate(IV) anions [39], [46]. We expect that further theophyllinium hexahalogeni-dometallate salts will be accessible using other metalchlorides as educts: M = Ir [39], Pb [46], Os [30], Pt [30].
Funding source: Ministry of Innovation, Science and Research of North-Rhine Westphalia
Funding source: the German Research Foundation (DFG)
Award Identifier / Grant number: INST 208/533-1
Funding source: Heinrich-Heine-Universität Düsseldorf
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: We gratefully acknowledge support by the Ministry of Innovation, Science and Research of North-Rhine Westphalia and the German Research Foundation (DFG) for financial support (Xcalibur diffractometer; INST 208/533-1, project no. 162659349). Finally, funding by the open access fund of the Heinrich-Heine-Universität Düsseldorf is also gratefully acknowledged.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Brandenburg, K. DIAMOND. Visual Crystal Structure Information System. Ver.4.5.2; Crystal Impact: Bonn, Germany, 2018.Suche in Google Scholar
2. Oxford Diffraction. CrysAlisPRO. (version 1.171.33.42); Oxford Diffraction Ltd.: Oxford, UK, 2009.Suche in Google Scholar
3. Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Suche in Google Scholar
4. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar
5. Hübschle, C. B., Sheldrick, G. M., Dittrich, B. ShelXle: a Qt graphical user interface for SHELXL. J. Appl. Crystallogr. 2011, 44, 1281–1284; https://doi.org/10.1107/s0021889811043202.Suche in Google Scholar PubMed PubMed Central
6. Reiss, G. J. A reinvestigation of Wilm’s salt, (NH4)4 [RhCl6]NO3 – structure, spectroscopy and thermal analysis -. Z. Kristallogr. - Cryst. Mater. 2002, 217, 550–556.10.1524/zkri.217.10.550.20794Suche in Google Scholar
7. Megen, M., Reiss, G. J. The pseudosymmetric structure of bis(pentane-1,5-diaminium) iodide tris(triiodide). Acta Crystallogr. E 2012, 68, o1331–o1332; https://doi.org/10.1107/s1600536812014420.Suche in Google Scholar PubMed PubMed Central
8. Kossel, A. Ueber eine neue Base aus dem Pflanzenreich. Ber. Dtsch. Chem. Ges. 1888, 21, 2164; https://doi.org/10.1002/cber.188802101422.Suche in Google Scholar
9. Kossel, A. Ueber das Theophyllin, einen neuen Bestandtheil des Thees. Z. Physiolog. Chem. 1889, 13, 298–308; https://doi.org/10.1515/bchm1.1889.13.3.298.Suche in Google Scholar
10. Fucke, K., McIntyre, G. J., Wilkinson, C., Henry, M., Howard, J. A., Steed, J. W. New insights into an old molecule: interaction energies of theophylline crystal forms. Cryst. Growth Des. 2012, 12, 1395–1401; https://doi.org/10.1021/cg201499s.Suche in Google Scholar
11. Matsuo, K., Matsuoka, M. Solid-state polymorphic transition of theophylline anhydrate and humidity effect. Cryst. Growth Des. 2007, 7, 411–415; https://doi.org/10.1021/cg060299i.Suche in Google Scholar
12. Darwish, S., Zeglinski, J., Krishna, G. R., Shaikh, R., Khraisheh, M., Walker, G. M., Croker, D. M. A new 1:1 drug-drug cocrystal of Theophylline and Aspirin: discovery, characterization, and construction of ternary phase diagrams. Cryst. Growth Des. 2018, 18, 7526–7532; https://doi.org/10.1021/acs.cgd.8b01330.Suche in Google Scholar
13. Wang, L., Luo, M., Li, J., Wang, J., Zhang, H., Deng, Z. Sweet Theophylline cocrystal with two tautomers of acesulfame. Cryst. Growth Des. 2015, 15, 2574–2578; https://doi.org/10.1021/acs.cgd.5b00207.Suche in Google Scholar
14. McTague, H., Rasmuson, A. C. Nucleation of the theophylline : salicylic acid 1:1 cocrystal. Cryst. Growth Des. 2021, 21, 2711–2719 https://doi.org/10.1021/acs.cgd.0c01594.Suche in Google Scholar PubMed PubMed Central
15. Lange, L., Sadowski, G. Polymorphs, hydrates, cocrystals, and cocrystal hydrates: thermodynamic modeling of theophylline systems. Cryst. Growth Des. 2016, 16, 4439–4449; https://doi.org/10.1021/acs.cgd.6b00554.Suche in Google Scholar
16. Persson, C. G. A. Overview of effects of theophylline. J. Allergy Clin. Immunol. 1986, 78, 780–787; https://doi.org/10.1016/0091-6749(86)90061-8.Suche in Google Scholar PubMed
17. Sofian, Z. M., Benaouda, F., Wang, J. T. W., Lu, Y., Barlow, D. J., Royall, P. G., Farag, D. B., Rahman, K. M., Al Jamal, K. T., Forbes, B., Jones, S. A. A Cyclodextrin stabilized spermine tagged drug triplex that targets Theophylline to the lungs selectively in respiratory emergency. Adv. Ther. 2020, 3, 2000153.10.1002/adtp.202000153Suche in Google Scholar PubMed PubMed Central
18. Benaouda, F., Jones, S. A., Chana, J., Dal Corno, B. M., Barlow, D. J., Hider, R. C., Page, C. P., Forbes, B. Ion-pairing with spermine targets Theophylline to the lungs via the polyamine transport system. Mol. Pharm. 2018, 15, 861–870; https://doi.org/10.1021/acs.molpharmaceut.7b00715.Suche in Google Scholar PubMed
19. Tanaka, R., Hattori, Y., Otsuka, M., Ashizawa, K. Application of spray freeze drying to theophylline-oxalic acid cocrystal engineering for inhaled dry powder technology. Drug Dev. Ind. Pharm. 2020, 46, 179–187; https://doi.org/10.1080/03639045.2020.1716367.Suche in Google Scholar PubMed
20. Zhou, C., Gao, C., Xie, Y., Xu, M. COVID-19 with spontaneous pneumomediastinum. Lancet Infect. Dis. 2020, 20, 510; https://doi.org/10.1016/s1473-3099(20)30156-0.Suche in Google Scholar PubMed PubMed Central
21. Reiss, G. J., Leske, P. B. The twinned crystal structure of bis(4-aminopyridin-1-ium) iodide triiodide, C20H28I8N8. Z. Kristallogr. N. Cryst. Struct. 2014, 229, 452–454, https://doi.org/10.1515/ncrs-2014-0193.Suche in Google Scholar
22. Reiss, G. J., van Megen, M. Two new polyiodides in the 4,4′-bipyridinium diiodide/iodine system. Z. Naturforsch. B Chem. Sci. 2012, 67, 5–10; https://doi.org/10.1515/znb-2012-0102.Suche in Google Scholar
23. Reiss, G. J. I5- polymers with a layered arrangement: synthesis, spectroscopy, and structure of a new polyiodide salt in the nicotine/HI/I2 system. Z. Naturforsch. B Chem. Sci. 2015, 70, 735–740; https://doi.org/10.1515/znb-2015-0092.Suche in Google Scholar
24. Reiss, G. J., Sergeeva, A. Hydrogen bonding versus packing effects in the crystal structure of 3-((1R, 2S)-1-methylpyrrolidin-1-ium-2-yl) pyridin-1-ium tetraiodidozincate(II). Z. Kristallogr. N. Cryst. Struct. 2020, 235, 959–962; https://doi.org/10.1515/ncrs-2020-0123.Suche in Google Scholar
25. Merkelbach, J., Majewski, M. A., Reiss, G. J. Crystal structure of caffeinium triiodide-caffeine (1/1), C16H21I3N8O4. Z. Kristallogr. N. Cryst. Struct. 2018, 233, 941–944; https://doi.org/10.1515/ncrs-2018-0125.Suche in Google Scholar
26. Reiss, G. J. A cyclic I102− anion in the layered crystal structure of theophyllinium pentaiodide, C7H9I5N4O2. Z. Kristallogr. N. Cryst. Struct. 2019, 234, 737–739; https://doi.org/10.1515/ncrs-2019-0082.Suche in Google Scholar
27. Wyshusek, M., Reiss, G. J., Frank, W. The triple salt 2(C7H9N4O2)[MoOCl4(H2O)] · 2(C7H9N4O2)Cl · (H17O8)Cl containing a C2 -symmetrical unbranched H+(H2O)8 Zundel type species in a framework composed of Theophyllinium, aquatetrachloridooxidomolybdate and chloride ions. Z. Anorg. Allg. Chem. 2021, 647, 575–581; https://doi.org/10.1002/zaac.202100007.Suche in Google Scholar
28. Groom, C. R., Bruno, I. J., Lightfoot, M. P., Ward, S. C. The Cambridge structural database. Acta Crystallogr. B 2016, 72, 171–179; https://doi.org/10.1107/s2052520616003954.Suche in Google Scholar
29. Megen, M., Prömper, S., Reiss, G. J. Bis(3-azaniumylpyridin-1-ium) hexachloridostannate(IV) dichloride. Acta Crystallogr. E 2013, 69, m217. https://doi.org/10.1107/s1600536813006806.Suche in Google Scholar PubMed PubMed Central
30. Dolling, B., Gillon, A. L., Orpen, A. G., Starbuck, J., Wang, X.-M. Homologous families of chloride-rich 4,4′-bipyridinium salt structures. Chem. Commun. 2001, 567–568; https://doi.org/10.1039/b009467f.Suche in Google Scholar
31. Szafrański, M. St åahl K. Phase transitions in layered diguanidinium hexachlorostannate(IV). Cryst. Growth Des. 2016, 16, 2157–2166.10.1021/acs.cgd.5b01830Suche in Google Scholar
32. Moussa, O. B., Chebbi, H., Arfaoui, Y., Falvello, L. R., Tomas, M., Zid, M. F. Structural study, vibrational and optical properties, Hirshfeld surface analysis and DFT investigation of a novel organic cation hexachloridostannate(IV), (C5H8N3)2[SnCl6]. J. Mol. Struct. 2019, 1195, 344–354; https://doi.org/10.1016/j.molstruc.2019.05.066.Suche in Google Scholar
33. Ferjani, H. Crystal structure, optical property and Hirshfeld surface analysis of bis[1-(prop-2-en-1-yl)-1H-imidazol-3-ium] hexachloridostannate(IV). Acta Crystallogr. E 2020, 76, 1624–1628; https://doi.org/10.1107/s2056989020012177.Suche in Google Scholar PubMed PubMed Central
34. Ghallab, R., Boutebdja, M., Denes, G., Merazig, H. Synthesis, crystal structure and Hirshfeld surface of bis(2-aminopyridinium) hexachloridostannate(IV). Acta Crystallogr. E 2020, 76, 1279–1283; https://doi.org/10.1107/s205698902000941x.Suche in Google Scholar PubMed PubMed Central
35. Manonmani, M., Balakrishnan, C., Dhanalakshmi, M., Ahamed, S. R., Vinitha, G., Sockalingam, R. M. Synthesis, structure, third-order nonlinear optical properties and Hirshfeld surface analysis of tetrakis(azepanium) hexachlorostannate(IV) dichloride and tetrakis(azepanium) hexabromostannate(IV) dibromide. J. Mol. Struct. 2021, 1227, 129515; https://doi.org/10.1016/j.molstruc.2020.129515.Suche in Google Scholar
36. Hajji, R., Fersi, M. A., Hajji, S., Hlel, F., Ben Ahmed, A. Hirshfeld surface analysis, vibrational spectra, optical, DFT studies and biological activities of (C7H12N2)2[SnCl6]Cl2.1.5H2O compound. Chem. Phys. Lett. 2019, 722, 160–172; https://doi.org/10.1016/j.cplett.2019.02.045.Suche in Google Scholar
37. Klösener, J., Wiesemann, M., Neumann, B., Stammler, H.-G., Hoge, B. Hypercoordinated fluoro(pentafluoroethyl)stannanes and -stannates. Eur. J. Inorg. Chem. 2018, 2018, 3960–3970; https://doi.org/10.1002/ejic.201800795.Suche in Google Scholar
38. Lee, S. M., Lo, K. M., Tiekink, E. R. T. Crystal structure of 2-(pyridin-2-ylamino)pyridinium chloride dibenzyldichlorostannane, [C10H10N3]Cl, C14H14Cl2Sn. Z. Kristallogr. N. Cryst. Struct. 2020, 235, 1515–1517; https://doi.org/10.1515/ncrs-2020-0375.Suche in Google Scholar
39. Reiss, G. J. The pseudosymmetric structure of bis(diisopropylammonium) hexachloroiridate(IV) and its relationship to potassium hexachloroiridate(III). Acta Crystallogr. E 2002, 58, m47–m50. and cited references; https://doi.org/10.1107/s0108767302095569.Suche in Google Scholar
40. Brockner, W., Demiray, A. F. Raman–Spektroskopische Untersuchungen an PCl5. IV. Das System PCl5–SnCl4. Z. Naturforsch. 1979, 34, 976–978; https://doi.org/10.1515/zna-1979-0808.Suche in Google Scholar
41. Pelzl, J., Engels, P., Florian, F. Raman spectroscopic study of the structural phase transitions in K2SnCl6. Phys. Status Solidi B 1977, 82, 145–148; https://doi.org/10.1002/pssb.2220820114.Suche in Google Scholar
42. Baggio, R. A simple graphical method to pinpoint local pseudosymmetries in Z′ >1 cases. Acta Crystallogr. C 2019, 75, 837–850; https://doi.org/10.1107/s2053229619007861.Suche in Google Scholar
43. Baggio R, R. Playing around with MP, a tool for the analysis of pseudosymmetry: recurrent appearance of local pseudo-space groups in the asymmetric unit of Z′ = 4 structures. Acta Crystallogr. C 2020, 76, 258–268; https://doi.org/10.1107/s2053229620001904.Suche in Google Scholar
44. Somov, N. V., Chuprunov, E. V. The translational and inversion pseudosymmetry of the atomic crystal structures of organic and organometallic compounds. Crystallogr. Rep. 2009, 54, 773–779; https://doi.org/10.1134/s1063774509050022.Suche in Google Scholar
45. Ruck, M. Kristallographische Konsequenzen von Pseudosymmetrie in Kristallstrukturen. Z. Kristallogr. - Cryst. Mater. 2000, 215, 148–156; https://doi.org/10.1524/zkri.2000.215.3.148.Suche in Google Scholar
46. Ouasri, A., Rhandour, A., Schloots, S., Reiss, G. J. The crystal structure of bis(diethylammonium) hexachloridoplumbate(IV) derived from powder diffraction data and its relation to structurally related hexachloridometallate salts. J. Chem. Crystallogr. 2017, 47, 173–181; https://doi.org/10.1007/s10870-017-0695-x.Suche in Google Scholar
© 2021 Guido J. Reiss and Maik Wyshusek, published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of [aqua-(4-iodopyridine-2,6-dicarboxylato-κ3 O,N,O′)-(1,10-phenanothroline-κ2 N,N′)copper(II)] dihydrate, C19H16O7N3CuI
- The crystal structure of tetrakis(1-isopropyl-1H-imidazolium) octamolybdate, C24H44Mo8N8O26
- Crystal structure of catena-poly[bis(µ2-3,5-bis(1-imidazolyl)pyridine-κ2 N:N′)-(µ2-3-nitrophthalato-k3 O,O′:O″)cadmium(II)] dihydrate, C30H25N11O8Cd
- The crystal structure of diaqua-bis(2-(3-(1H-pyrazol-4-yl)-1H-1,2,4-triazol-5-yl)pyridine-κ2 N:N′)-bis(3,5-dicarboxybenzoato-κ1 O)cobalt(II), C38H30CoN12O14
- Crystal structure of the nickel(II) complex aqua-(2,6-di(pyrazin-2-yl)-4,4′-bipyridine-κ3 N,N′,N′′)-(phthalato-κ2 O,O′)nickel(II) tetrahydrate, C26H26N6O9Ni
- The crystal structure of 1-[5-(2-fluorophenyl)-1-(pyridine-3-sulfonyl)-1H-pyrrol-3-yl]-N-methylmethanaminium 3-carboxyprop-2-enoate, C21H20FN3O6S
- The crystal structure of 1,2-bis(4-pyridyl)ethane - 4,4-dihydroxydiphenylmethane (1/1), C25H21N2O2
- Crystal structure of bis(2-((E)-5-chloro-2-hydroxybenzylidene)hydrazineyl)methaniminium trifluoroacetate dihydrate, C34H36Cl4N10O12
- Crystal structure of 1-cyclopropyl-7-ethoxy-6,8-difluoro-4-oxo-1,4-dihydro-quinoline-3-carboxylic acid, C15H13F2NO4
- Crystal structure of methyl 3-(1H-naphtho[1,8-de][1,3,2]diazaborinin-2(3H)-yl)benzoate, C18H15BN2O2
- Crystal structure of (E)-N′-(2-chloro-6-hydroxybenzylidene)-2-hydroxybenzohydrazide, C14H11ClN2O3
- Crystal structure of Al-rich fluorophlogopite, K1.0(Mg2.8Al0.2)(Si2.8Al1.2)O10F2
- The crystal structure of 4,5-diiodo-1,3-dimesityl-1H-1,2,3-triazol-3-ium hexafluoridoantimonate(V), C20H22F6I2N3Sb
- Crystal structure of tris(3-iodopyridin-1-ium) catena-poly[(hexachlorido-κ1 Cl)-(μ2-trichlorido-κ2 Cl:Cl)diantimony(III)], C15H15Cl9I3N3Sb2
- Crystal structure of methyl 2-(1H-naphtho[1,8-de][1.3.2]diazaborinin-2(3H-yl)benzoate C18H15BN2O2
- The crystal structure of 1,8-bis(4-methoxybenzoyl)naphthalene-2,7-diyl dibenzoate, C40H28O8
- Crystal structure of 2-bromo-1,3,6,8-tetramethylBOPHY (BOPHY = bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl)methylene)hydrazine), C14H15B2BrF4N4
- The crystal structure of (E)-3-chloro-2-(2-(2-fluorobenzylidene)hydrazinyl)pyridine, C12H9ClFN3
- Crystal structure of bis(µ2- 4-iodopyridine-2,6-dicarboxylato-κ3O:N:O′)-bis(4-iodopyridine-2,6-dicarboxylato-κ3O:N:O′)-bis(µ2-1-(4-pyridyl)piperazine-κ2N:N′)-hexa-aqua-tetra-copper(II), C46H46Cu4I4N10O22
- Crystal structure of poly[diaqua-(μ2-2,5-dihydroxyterephthalato-κ2O:O′)(μ2-bis(4-pyridylformyl)piperazine-κ2N:N′)cadmium(II)] dihydrate, C24H28CdN4O12
- Crystal structure of poly[aqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)-(μ3-2,3,5,6-tetrafluoroterephthalato-κ3O:O′:O′′)cadmium(II)], C17H14N4O5F4Cd
- Crystal structure of 6-(quinolin-8-yl)benzo[a]phenanthridin-5(6H)-one, C26H16N2O
- The crystal structure of aqua-bis(6-chloropicolinato-κ2N,O)copper(II), C12H8Cl2N2O5Cu
- Crystal structure of catena-poly[diaqua-bis(μ2-4,4′-bipyridyl-κ2N:N′) disilver(I)] 4-oxidopyridine-3-sulfonate trihydrate, C25H29Ag2N5O9S
- The crystal structure of 4-(3-bromophenyl)pyrimidin-2-amine, C10H8BrN3
- Crystal structure of 6-oxo-4-phenyl-1-propyl-1,6-dihydropyridine-3-carbonitrile, C15H14N2O
- Crystal structure of 4-(2,2-difluoroethyl)-2,4-dimethyl-6-(trifluoromethyl)isoquinoline-1,3(2H,4H)-dione, C14H12F5NO2
- Crystal structure of dibromido-(1-methyl-1H-imidazole-κ1N)-(3-(3-methyl-1H-imidazol-3-ium-1-yl)propanoato-κ1O)zinc(II), C11H16Br2N4O2Zn
- The crystal structure of 1,1′-(((2 (dimethylamino)ethyl)azanediyl)bis(methylene)) bis(naphthalen-2-olato-κ4 N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)- titanium(IV) ─ dichloromethane (2/1), C33H29N3O6Ti
- The layered crystal structure of bis(theophyllinium) hexachloridostannate (IV), C14H18N8O8SnCl6
- The crystal structre of 3-(1-ethenyl-1H-imidazol-3-ium-3-yl)propane-1-sulfonate, C8H12N2O3S
- Synthesis and crystal structure of di-tert-butyl 1″-acetyl-2,2″,9′-trioxo-4a′,9a′-dihydro-1′H,3′H,9′H-dispiro[indoline-3,2′-xanthene-4′,3″-indoline]-1,3′-dicarboxylate, C39H38N2O9
- The crystal structure of 4-chloro-2-(quinolin-8-yl)isoindoline-1,3-dione, C17H9ClN2O2
- The crystal structure of 1-fluoro-4-(p-tolylethynyl)benzene, C15H11F
- The crystal structure of bis[4-bromo-2-(1H-pyrazol-3-yl) phenolato-κ2N,O] copper(II), C18H12Br2CuN4O2
- The crystal structure of poly[(μ 3-imidazolato-κ 3 N:N:N′)(tetrahydrofuran- κ 1 O)lithium(I)], C7H11LiN2O
- Crystal structure of N′,N′′′-((1E,1′E)-(propane-2,2-diylbis(1H-pyrrole-5,2diyl))bis(methaneylylidene))di(nicotinohydrazide) pentahydrate, C25H24N8O2·5H2O
- Crystal structure of 3-(2-ethoxy-2-oxoethyl)-1-ethyl-1H-imidazol-3-ium hexafluoridophos-phate(V), C9H15F6N2O2P
- Crystal structure of (1,10-phenanthroline-κ2N,N′)-bis(3-thiophenecarboxylato-κ2O,O′)copper(II), C22H14N2O4S2Cu
- The crystal structure of 2-amino-3-carboxypyridin-1-ium iodide hemihydrate, C6H8IN2O2.5
- Crystal structure of (E)-7-methoxy-2-((6-methoxypyridin-2-yl)methylene)-tetralone, C18H17NO3
- The crystal structure of [μ-hydroxido-bis[(5,5′-dimethyl-2,2′-bipyridine-κ2N,N′)-tricarbonylrhenium(I)] bromide hemihydrate, C30H26N4O9Re2Br
- The crystal structure of 2,5-bis(3,5-dimethylphenyl)thiazolo[5,4-d]thiazole, C20H18N2S2
- The crystal structure of 5-benzoyl-1-[(E)-(4-fluorobenzylidene)amino]-4-phenylpyrimidin-2(1H)-one, C24H16FN3O2
- Crystal structure of monocarbonyl(N-nitroso-N-oxido-phenylamine-κ 2 O,O′)(tricyclohexylphosphine-κP)rhodium(I), C25H39N2O3PRh
- Crystal structure of poly[bis[μ3-1,3,5-tris[(1H-imidazol-1-yl)methyl]benzene-κ3N:N′:N″]nickel(II)] hexafluorosilicate, C36H36N12NiSiF6
- The crystal structure of 13-(pyrazole-1-yl-4-carbonitrile)-matrine, C19H25N5O
- Crystal structure of 3,5-bis((E)-4-methoxy-2-(trifluoromethyl)benzylidene)-1-methylpiperidin-4-one, C24H21F6NO3
- The crystal structure of N,N′-(Disulfanediyldi-2,1-phenylene)di(6′-methylpyridine)-2-carboxamide, C26H22N4O2S2
- Crystal structure of (E)-7-fluoro-2-(4-methoxy-2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C19H14F4O2
- Crystal structure of ethyl 1-(4-fluorophenyl)-4-phenyl-1H-pyrrole-3-carboxylate, C19H16FNO2
- The crystal structure of cis-diaqua-bis (N-butyl-N-(pyridin-2-yl)pyridin-2-amine-κ2N,N′)cobalt(II)] dichloride trihydrate, C28H44Cl2N6O5Co
- Crystal structure of (E)-7-methoxy-2-((6-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C18H17NO3
- Crystal structure of (E)-2-((3-fluoropyridin-4-yl)methylene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- The crystal structure of 6-bromohexanoic acid, C6H11BrO2
- The crystal structure of 4-chloro-thiophenol, C6H5ClS
- The crystal structure of 4-bromobenzyl chloride, C7H6BrCl
- The crystal structure of di-tert-butyl dicarbonate, C10H18O5
- The crystal structure of (2-(4-chlorophenyl)-5-methyl-1,3-dioxan-5-yl)methanol, C12H15ClO3
- The crystal structure of the co-crystal: 2-hydroxybenzoic acid – N′-(butan-2-ylidene)pyridine-4-carbohydrazide, C10H13N3O·C7H6O3
- Crystal structure and anti-inflammatory activity of (E)-7-fluoro-2-((5-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- Crystal structure of (E)-7-fluoro-2-((6-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- Crystal structure of 1,1′-(butane-1,4-diyl)bis(3-propyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C32H56F24N8P4
- The crystal structure of dichlorido-bis(3-methyl-3-imidazolium-1-ylpropionato-κ2)-cadmium(II), C14H20CdCl2N4O4
- Crystal structure of 1-(2-cyanobenzyl)-3-cyano-4-phenyl-4-(2-cyanobenzyl)-1,4-dihydropyridine monohydrate, C56H42N8O
- The crystal structure of 3-(carboxymethyl)-1-ethenyl-1H-imidazol-3-ium chloride, C7H9N2O2Cl
- The crystal structure of adamantylmethoxydiphenylsilane, C23H28OSi
- Redetermination of the crystal structure of (2E,4Z,13E,15Z)-3,5,14,16-tetramethyl-2,6,13,17-tetraazatricyclo[16.4.0.07,12]docosa-1(22),2,4,7,9,11,13,15,18,20-decaene, C22H24N4
- Crystal structure of (E)-7-hydroxy-2-((6-methoxypyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H15NO3
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2 N:N′)cobalt(II)] dinitrate, C18H28N10O8Co
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of [aqua-(4-iodopyridine-2,6-dicarboxylato-κ3 O,N,O′)-(1,10-phenanothroline-κ2 N,N′)copper(II)] dihydrate, C19H16O7N3CuI
- The crystal structure of tetrakis(1-isopropyl-1H-imidazolium) octamolybdate, C24H44Mo8N8O26
- Crystal structure of catena-poly[bis(µ2-3,5-bis(1-imidazolyl)pyridine-κ2 N:N′)-(µ2-3-nitrophthalato-k3 O,O′:O″)cadmium(II)] dihydrate, C30H25N11O8Cd
- The crystal structure of diaqua-bis(2-(3-(1H-pyrazol-4-yl)-1H-1,2,4-triazol-5-yl)pyridine-κ2 N:N′)-bis(3,5-dicarboxybenzoato-κ1 O)cobalt(II), C38H30CoN12O14
- Crystal structure of the nickel(II) complex aqua-(2,6-di(pyrazin-2-yl)-4,4′-bipyridine-κ3 N,N′,N′′)-(phthalato-κ2 O,O′)nickel(II) tetrahydrate, C26H26N6O9Ni
- The crystal structure of 1-[5-(2-fluorophenyl)-1-(pyridine-3-sulfonyl)-1H-pyrrol-3-yl]-N-methylmethanaminium 3-carboxyprop-2-enoate, C21H20FN3O6S
- The crystal structure of 1,2-bis(4-pyridyl)ethane - 4,4-dihydroxydiphenylmethane (1/1), C25H21N2O2
- Crystal structure of bis(2-((E)-5-chloro-2-hydroxybenzylidene)hydrazineyl)methaniminium trifluoroacetate dihydrate, C34H36Cl4N10O12
- Crystal structure of 1-cyclopropyl-7-ethoxy-6,8-difluoro-4-oxo-1,4-dihydro-quinoline-3-carboxylic acid, C15H13F2NO4
- Crystal structure of methyl 3-(1H-naphtho[1,8-de][1,3,2]diazaborinin-2(3H)-yl)benzoate, C18H15BN2O2
- Crystal structure of (E)-N′-(2-chloro-6-hydroxybenzylidene)-2-hydroxybenzohydrazide, C14H11ClN2O3
- Crystal structure of Al-rich fluorophlogopite, K1.0(Mg2.8Al0.2)(Si2.8Al1.2)O10F2
- The crystal structure of 4,5-diiodo-1,3-dimesityl-1H-1,2,3-triazol-3-ium hexafluoridoantimonate(V), C20H22F6I2N3Sb
- Crystal structure of tris(3-iodopyridin-1-ium) catena-poly[(hexachlorido-κ1 Cl)-(μ2-trichlorido-κ2 Cl:Cl)diantimony(III)], C15H15Cl9I3N3Sb2
- Crystal structure of methyl 2-(1H-naphtho[1,8-de][1.3.2]diazaborinin-2(3H-yl)benzoate C18H15BN2O2
- The crystal structure of 1,8-bis(4-methoxybenzoyl)naphthalene-2,7-diyl dibenzoate, C40H28O8
- Crystal structure of 2-bromo-1,3,6,8-tetramethylBOPHY (BOPHY = bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl)methylene)hydrazine), C14H15B2BrF4N4
- The crystal structure of (E)-3-chloro-2-(2-(2-fluorobenzylidene)hydrazinyl)pyridine, C12H9ClFN3
- Crystal structure of bis(µ2- 4-iodopyridine-2,6-dicarboxylato-κ3O:N:O′)-bis(4-iodopyridine-2,6-dicarboxylato-κ3O:N:O′)-bis(µ2-1-(4-pyridyl)piperazine-κ2N:N′)-hexa-aqua-tetra-copper(II), C46H46Cu4I4N10O22
- Crystal structure of poly[diaqua-(μ2-2,5-dihydroxyterephthalato-κ2O:O′)(μ2-bis(4-pyridylformyl)piperazine-κ2N:N′)cadmium(II)] dihydrate, C24H28CdN4O12
- Crystal structure of poly[aqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)-(μ3-2,3,5,6-tetrafluoroterephthalato-κ3O:O′:O′′)cadmium(II)], C17H14N4O5F4Cd
- Crystal structure of 6-(quinolin-8-yl)benzo[a]phenanthridin-5(6H)-one, C26H16N2O
- The crystal structure of aqua-bis(6-chloropicolinato-κ2N,O)copper(II), C12H8Cl2N2O5Cu
- Crystal structure of catena-poly[diaqua-bis(μ2-4,4′-bipyridyl-κ2N:N′) disilver(I)] 4-oxidopyridine-3-sulfonate trihydrate, C25H29Ag2N5O9S
- The crystal structure of 4-(3-bromophenyl)pyrimidin-2-amine, C10H8BrN3
- Crystal structure of 6-oxo-4-phenyl-1-propyl-1,6-dihydropyridine-3-carbonitrile, C15H14N2O
- Crystal structure of 4-(2,2-difluoroethyl)-2,4-dimethyl-6-(trifluoromethyl)isoquinoline-1,3(2H,4H)-dione, C14H12F5NO2
- Crystal structure of dibromido-(1-methyl-1H-imidazole-κ1N)-(3-(3-methyl-1H-imidazol-3-ium-1-yl)propanoato-κ1O)zinc(II), C11H16Br2N4O2Zn
- The crystal structure of 1,1′-(((2 (dimethylamino)ethyl)azanediyl)bis(methylene)) bis(naphthalen-2-olato-κ4 N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)- titanium(IV) ─ dichloromethane (2/1), C33H29N3O6Ti
- The layered crystal structure of bis(theophyllinium) hexachloridostannate (IV), C14H18N8O8SnCl6
- The crystal structre of 3-(1-ethenyl-1H-imidazol-3-ium-3-yl)propane-1-sulfonate, C8H12N2O3S
- Synthesis and crystal structure of di-tert-butyl 1″-acetyl-2,2″,9′-trioxo-4a′,9a′-dihydro-1′H,3′H,9′H-dispiro[indoline-3,2′-xanthene-4′,3″-indoline]-1,3′-dicarboxylate, C39H38N2O9
- The crystal structure of 4-chloro-2-(quinolin-8-yl)isoindoline-1,3-dione, C17H9ClN2O2
- The crystal structure of 1-fluoro-4-(p-tolylethynyl)benzene, C15H11F
- The crystal structure of bis[4-bromo-2-(1H-pyrazol-3-yl) phenolato-κ2N,O] copper(II), C18H12Br2CuN4O2
- The crystal structure of poly[(μ 3-imidazolato-κ 3 N:N:N′)(tetrahydrofuran- κ 1 O)lithium(I)], C7H11LiN2O
- Crystal structure of N′,N′′′-((1E,1′E)-(propane-2,2-diylbis(1H-pyrrole-5,2diyl))bis(methaneylylidene))di(nicotinohydrazide) pentahydrate, C25H24N8O2·5H2O
- Crystal structure of 3-(2-ethoxy-2-oxoethyl)-1-ethyl-1H-imidazol-3-ium hexafluoridophos-phate(V), C9H15F6N2O2P
- Crystal structure of (1,10-phenanthroline-κ2N,N′)-bis(3-thiophenecarboxylato-κ2O,O′)copper(II), C22H14N2O4S2Cu
- The crystal structure of 2-amino-3-carboxypyridin-1-ium iodide hemihydrate, C6H8IN2O2.5
- Crystal structure of (E)-7-methoxy-2-((6-methoxypyridin-2-yl)methylene)-tetralone, C18H17NO3
- The crystal structure of [μ-hydroxido-bis[(5,5′-dimethyl-2,2′-bipyridine-κ2N,N′)-tricarbonylrhenium(I)] bromide hemihydrate, C30H26N4O9Re2Br
- The crystal structure of 2,5-bis(3,5-dimethylphenyl)thiazolo[5,4-d]thiazole, C20H18N2S2
- The crystal structure of 5-benzoyl-1-[(E)-(4-fluorobenzylidene)amino]-4-phenylpyrimidin-2(1H)-one, C24H16FN3O2
- Crystal structure of monocarbonyl(N-nitroso-N-oxido-phenylamine-κ 2 O,O′)(tricyclohexylphosphine-κP)rhodium(I), C25H39N2O3PRh
- Crystal structure of poly[bis[μ3-1,3,5-tris[(1H-imidazol-1-yl)methyl]benzene-κ3N:N′:N″]nickel(II)] hexafluorosilicate, C36H36N12NiSiF6
- The crystal structure of 13-(pyrazole-1-yl-4-carbonitrile)-matrine, C19H25N5O
- Crystal structure of 3,5-bis((E)-4-methoxy-2-(trifluoromethyl)benzylidene)-1-methylpiperidin-4-one, C24H21F6NO3
- The crystal structure of N,N′-(Disulfanediyldi-2,1-phenylene)di(6′-methylpyridine)-2-carboxamide, C26H22N4O2S2
- Crystal structure of (E)-7-fluoro-2-(4-methoxy-2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C19H14F4O2
- Crystal structure of ethyl 1-(4-fluorophenyl)-4-phenyl-1H-pyrrole-3-carboxylate, C19H16FNO2
- The crystal structure of cis-diaqua-bis (N-butyl-N-(pyridin-2-yl)pyridin-2-amine-κ2N,N′)cobalt(II)] dichloride trihydrate, C28H44Cl2N6O5Co
- Crystal structure of (E)-7-methoxy-2-((6-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C18H17NO3
- Crystal structure of (E)-2-((3-fluoropyridin-4-yl)methylene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- The crystal structure of 6-bromohexanoic acid, C6H11BrO2
- The crystal structure of 4-chloro-thiophenol, C6H5ClS
- The crystal structure of 4-bromobenzyl chloride, C7H6BrCl
- The crystal structure of di-tert-butyl dicarbonate, C10H18O5
- The crystal structure of (2-(4-chlorophenyl)-5-methyl-1,3-dioxan-5-yl)methanol, C12H15ClO3
- The crystal structure of the co-crystal: 2-hydroxybenzoic acid – N′-(butan-2-ylidene)pyridine-4-carbohydrazide, C10H13N3O·C7H6O3
- Crystal structure and anti-inflammatory activity of (E)-7-fluoro-2-((5-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- Crystal structure of (E)-7-fluoro-2-((6-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- Crystal structure of 1,1′-(butane-1,4-diyl)bis(3-propyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C32H56F24N8P4
- The crystal structure of dichlorido-bis(3-methyl-3-imidazolium-1-ylpropionato-κ2)-cadmium(II), C14H20CdCl2N4O4
- Crystal structure of 1-(2-cyanobenzyl)-3-cyano-4-phenyl-4-(2-cyanobenzyl)-1,4-dihydropyridine monohydrate, C56H42N8O
- The crystal structure of 3-(carboxymethyl)-1-ethenyl-1H-imidazol-3-ium chloride, C7H9N2O2Cl
- The crystal structure of adamantylmethoxydiphenylsilane, C23H28OSi
- Redetermination of the crystal structure of (2E,4Z,13E,15Z)-3,5,14,16-tetramethyl-2,6,13,17-tetraazatricyclo[16.4.0.07,12]docosa-1(22),2,4,7,9,11,13,15,18,20-decaene, C22H24N4
- Crystal structure of (E)-7-hydroxy-2-((6-methoxypyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H15NO3
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2 N:N′)cobalt(II)] dinitrate, C18H28N10O8Co

