Abstract
C16H15NO5, monoclinic, P21/n (no. 14), a = 6.7689(5) Å, b = 45.219(3) Å, c = 10.1102(6) Å, β = 101.360(7)°, V = 3033.9(4) Å3, T = 298(2) K.
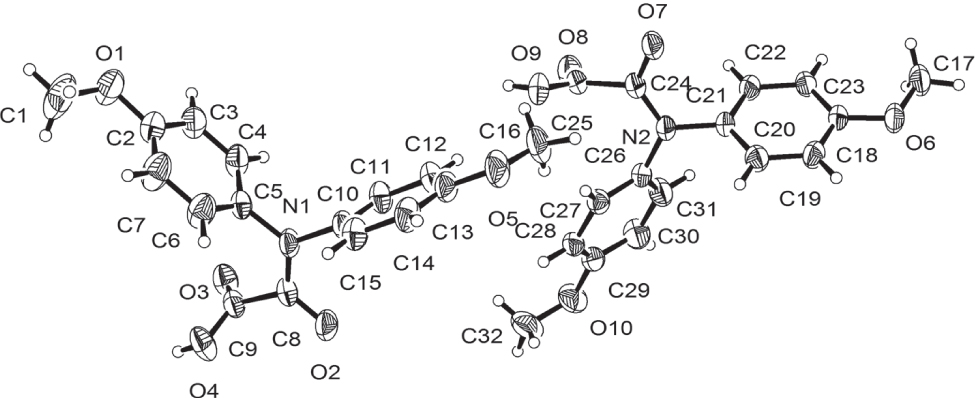
Tables 1 and 2 contain details of the measurement method and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colorless block |
| Size: | 0.30 × 0.22 × 0.17 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.10 mm−1 |
| Diffractometer, scan mode: | Atlas, ω |
| 2θmax, completeness: | 58°, 97.3% |
| N(hkl)measured, N(hkl)unique, Rint: | 15585, 6846, 0.353 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 3929 |
| N(param)refined: | 403 |
| Programs: | CrysAlisPRO [18], SHELX [19], WinGX [20], CHEMDRAW Ultra [21] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1 | −0.3411(7) | 0.26406(10) | −0.1012(4) | 0.1293(17) |
| H1A | −0.4716 | 0.2549 | −0.1178 | 0.194* |
| H1B | −0.3171 | 0.2728 | −0.1831 | 0.194* |
| H1C | −0.3359 | 0.2791 | −0.0336 | 0.194* |
| C2 | −0.2196(4) | 0.22459(6) | 0.0489(3) | 0.0588(7) |
| C3 | −0.0928(4) | 0.20057(6) | 0.0757(3) | 0.0633(7) |
| H3 | 0.0066 | 0.1975 | 0.0254 | 0.076* |
| C4 | −0.1137(4) | 0.18125(6) | 0.1767(3) | 0.0610(7) |
| H4 | −0.0282 | 0.1650 | 0.1943 | 0.073* |
| C5 | −0.2587(4) | 0.18557(5) | 0.2517(2) | 0.0477(6) |
| C6 | −0.3827(5) | 0.20947(7) | 0.2264(3) | 0.0724(9) |
| H6 | −0.4807 | 0.2126 | 0.2777 | 0.087* |
| C7 | −0.3637(5) | 0.22914(7) | 0.1245(3) | 0.0789(9) |
| H7 | −0.4489 | 0.2454 | 0.1076 | 0.095* |
| C8 | −0.3962(4) | 0.14275(6) | 0.3527(2) | 0.0507(6) |
| C9 | −0.5151(4) | 0.13514(6) | 0.2117(3) | 0.0504(6) |
| C10 | −0.1313(4) | 0.17106(5) | 0.4890(2) | 0.0496(6) |
| C11 | 0.0482(4) | 0.15607(6) | 0.5151(2) | 0.0576(7) |
| H11 | 0.0779 | 0.1424 | 0.4531 | 0.069* |
| C12 | 0.1853(4) | 0.16125(6) | 0.6335(3) | 0.0629(7) |
| H12 | 0.3077 | 0.1512 | 0.6510 | 0.075* |
| C13 | 0.1395(4) | 0.18131(6) | 0.7255(3) | 0.0596(7) |
| C14 | −0.0412(5) | 0.19644(6) | 0.6983(3) | 0.0633(7) |
| H14 | −0.0713 | 0.2101 | 0.7601 | 0.076* |
| C15 | −0.1772(4) | 0.19128(6) | 0.5798(3) | 0.0595(7) |
| H15 | −0.2992 | 0.2014 | 0.5616 | 0.071* |
| C16 | 0.4445(5) | 0.17124(8) | 0.8805(3) | 0.0927(11) |
| H16A | 0.4118 | 0.1506 | 0.8745 | 0.139* |
| H16B | 0.5101 | 0.1759 | 0.9711 | 0.139* |
| H16C | 0.5328 | 0.1758 | 0.8198 | 0.139* |
| C17 | 1.5746(4) | 0.04762(7) | 1.5043(3) | 0.0717(8) |
| H17A | 1.5792 | 0.0303 | 1.4501 | 0.108* |
| H17B | 1.6307 | 0.0431 | 1.5969 | 0.108* |
| H17C | 1.6512 | 0.0632 | 1.4738 | 0.108* |
| C18 | 1.2664(4) | 0.06381(5) | 1.3672(2) | 0.0468(6) |
Source of material
A solution of oxalyl chloride (1 mole equivalent) in dichloromethane (DCM) was added dropwise to a solution of bis(4-methoxyphenyl)amine (1 mole equivalent) in DCM in the presence of triethylamine at room temperature. The mixture was stirred for 1.5 h and water was added. The organic layer was separated, dried over anhydrous magnesium sulfate and evaporated under reduced pressure to give the title compound in 46% yield. The low yield could be a result of half of the amine acting as a base to abstract hydrogen chloride evolved from the reaction. To investigate this issue the reaction was repeated with two equivalents of bis(4-methoxyphenyl)amine and no triethylamine. Following aqueous work-up, the crude product was obtained in 75% yield based on oxalyl chloride. Crystallization using acetonitrile gave the title compound as colorless crystals, Mp. 122–123 °C. The NMR spectra recorded at room temperature showed two sets of signals for the two aryl rings, confirming restricted rotation about the C—N bond. The barriers to free rotation in such compounds are already known to be substantial [1], [2], [3]. 1H NMR (400 MHz, DMSO-d6): δ 7.04, 6.96 (2 d, J = 8.5 Hz, 4 H, H-2/H-6), 6.86, 6.69 (2 d, J = 8.5 Hz, 4 H, H-3/H-5), 3.82, 3.71 (2 s, 6 H, OMe); 13C NMR (100 MHz, DMSO-d6): δ 164.4 (s, CO2H), 159.2 (s, C = O), 158.3 (s, C-4), 134.0, 132.8 (2 s, C-1), 129.8, 127.7 (2 d, C-2/C-6), 114.8 (2 d, C-3/C-5), 56.0, 55.8 (2 q, OMe); ES+−MS: m/z (%) 302 (MH+, 100), 288 (12), 256 (21), 228 (12); HRMS (ES+): calculated for C16H16NO5 (MH+): 302.1028; found: 302.1028. IR (FT): νmax 3300, 1740, 1713, 1665, 1500, 1366, 1167 cm−1.
Experimental details
Non-hydrogen atoms were refined with anisotropic displacement parameters. All hydrogen atoms were placed in calculated positions and refined using a ring model. Methyl C—H bonds were fixed at 0.96 Å and displacement parameters were 1.5 times Ueq(C). The methyl groups were allowed to spin about the C—C bond. Aromatic C—H distances were set to 0.93 Å and their U(iso) parameters were set to 1.2 times Ueq(C). Hydroxyl O—H distances were set to 0.82 Å and their U(iso) set to 1.5 times Ueq(O). Crystal data, data collection and structure refinement details are summarized in Table 1.
Comment
Aryl oxamic acid derivatives have various interesting applications [4], [5], [6], [7], [8]. In addition, aryl oxamic acids can be used as intermediates for the synthesis of various classes of compounds including heterocycles [9], [10], [11], [12]. Oxamates can be synthesized by the use of various synthetic procedures [13], [14], [15], [16], [17].
In the title crystal structure, the asymmetric unit consists of two independent molecules of C16H15NO5. The oxoacetic acid fragments of the molecule are involved in intermolecular hydrogen bonding, of the type O—H⋯O, with the following geometric parameters: O4⋯O7 = 2.673(2) Å, O—H⋯O = 171.0°; and O9⋯O2 = 2.734(2) Å, O—H⋯O = 169.9° forming chains along [101].
These hydrogen bonds can be classified as medium strong. Bond lengths and angles in both crystallographically independent molecules are in the expected ranges.
Acknowledgement
We thank the EPSRC for the grant which supplied the MS instrumentation used in this study. M. Alamri thanks the Saudi Cultural Bureau, London for a scholarship and G. A. El-Hiti extends his appreciation to the Deanship of Scientific Research at King Saud University for its funding for this research through the research group project RGP-239.
References
1 Hobson, R. F.; Reeves, L. W.: Hindered rotation about the N-C bond in some vinylogous amides. J. Magn. Reson. 10 (1973) 243–252.10.1016/0022-2364(73)90247-3Suche in Google Scholar
2 Smith, B. D.; Goodenough-Lashua, D. M.; D’Souza, C. J. E.; Norton, K. J.; Schmidt, L. M.; Tung, J. C.: Substituent effects on the barrier to carbamate C–N rotation. Tetrahedron Lett. 45 (2004) 2747–2749.10.1016/j.tetlet.2004.02.037Suche in Google Scholar
3 Krishnan, V. V.; Thompson, W. B.; Goto, J. J.; Maitra, K.; Maitra, S.: Modulations in restricted amide rotation by steric induced conformational trapping. Chem. Phys. Lett. 523 (2012) 124–127.10.1016/j.cplett.2011.11.058Suche in Google Scholar PubMed PubMed Central
4 Maiore, L.; Aragoni, M. C.; Carcangiu, G.; Cocco, O.; Isaia, F.; Lippolis, V.; Meloni, P.; Murru, A.; Slawin, A. M. Z.; Tuveri, E.; Woollins, J. D.; Arca, M.: Oxamate salts as novel agents for the restoration of marble and limestone substrates: case study of ammonium N-phenyloxamate. New J. Chem. 40 (2016) 2768–2774.10.1039/C5NJ02505BSuche in Google Scholar
5 Miskimins, W. K.; Ahn, H. J.; Kim, J. Y.; Ryu, S.; Jung, Y.-S.; Choi, J. Y.: Synergistic anti-cancer effect of phenformin and oxamate. PLOS One 9 (2014) e85576, doi: 10.1371/journal.pone.0085576.10.1371/journal.pone.0085576Suche in Google Scholar PubMed PubMed Central
6 Choi, S.-R.; Beeler, A. B.; Pradhan, A.; Watkins, E. B.; Rimoldi, J. M.; Tekwani, B.; Avery, M. A.: Generation of oxamic acid libraries: antimalarials and inhibitors of plasmodium falciparum lactate dehydrogenase. J. Comb. Chem. 9 (2007) 292–300.10.1021/cc060110nSuche in Google Scholar PubMed
7 Hargrave, K. D.; Hess, F. K.; Oliver, J. T.: N-(4-Substituted-thiazolyl)oxamic acid derivatives, new series of potent, orally active antiallergy agents. J. Med. Chem. 26 (1983) 1158–1163.10.1021/jm00362a014Suche in Google Scholar PubMed
8 Klaubert, D. H.; Sellstedt, J. H.; Guinosso, C. J.; Capetola, R. J.; Bell, S. C.: N-(Aminophenyl)oxamic acids and esters as potent, orally active antiallergy agents. J. Med. Chem. 24 (1981) 742–748.10.1021/jm00138a020Suche in Google Scholar PubMed
9 Wang, H.; Guo, L.-N.; Wang, S.; Duan, X.-H.: Decarboxylative alkynylation of a-keto acids and oxamic acids in aqueous media. Org. Lett. 17 (2015) 3054–3057.10.1021/acs.orglett.5b01336Suche in Google Scholar PubMed
10 Loloiu, G.; Maior, O.: Isatin chemistry. Synthesis of N-methyl-2, 3-dioxo-2,3-dihydropyrrolo(2, 3-b) phenoxathiin. Rev. Roum. Chim. 42 (1997) 67–69.10.1002/chin.199745166Suche in Google Scholar
11 Molina, P.; Vilaplana, M. J.; Andreu, P. L.; Moller, J.: Oxamic acid derivatives in heterocyclic synthesis: preparation of 1,2,4-triazolo[1,5-a]pyrazine derivatives. J. Heterocycl. Chem. 24 (1984) 1281–1284.10.1002/chin.198815243Suche in Google Scholar
12 Downs, J. R.; Pastine, S. J.; Schady, D. A.; Greer, H. A.; Kelley, W.; Embree, M. C.; Townsend, J. D.; Beam, C. F.: Preparation of 1H-pyrazole-5-carboxamides from dilithiated C(α),N-phenylhydrazones and lithiated ethyl oxanilates or lithiated ethyl oxamate. J. Heterocycl. Chem. 38 (2001) 691–694.10.1002/jhet.5570380325Suche in Google Scholar
13 Gadge, S. T.; Kusumawati, E. N.; Harada, K.; Sasaki, T.; Nishio-Hamane, D.; Bhanage, B. M.: Synthesis of oxamate and urea by oxidative single and double carbonylation of amines using immobilized palladium metal-containing ionic liquid@SBA-15. J. Mol. Catal. A: Chem. 400 (2015) 170–178.10.1016/j.molcata.2015.01.009Suche in Google Scholar
14 Gadge, S. T.; Bhanage, B. M.: Pd/C-Catalyzed synthesis of oxamates by oxidative cross double carbonylation of amines and alcohols under Co-catalyst, base, dehydrating agent, and ligand-free conditions. J. Org. Chem. 78 (2013) 6793–6797.10.1021/jo401038eSuche in Google Scholar PubMed
15 Lisnard, L.; Chamoreau, L.-M.; Li, Y.; Journaux, Y.: Solvothermal synthesis of oxamate-based helicate: temperature dependence of the hydrogen bond structuring in the solid. Cryst. Growth Des. 12 (2012) 4955–4962.10.1021/cg300877rSuche in Google Scholar
16 Yang, G.; Zhang, H.; Huang, Y.; Chen, Z.: Synthesis of methyl N-aryl oxamate using soluble polymer support. Synth. Commun. 36 (2006) 611–619.10.1080/00397910500408357Suche in Google Scholar
17 Lesimple, P.; Bigg, D. C. H.: An improved procedure for the preparation of alkyl N-(4-aryl-2-thiazolyl)oxamates. Synthesis 1991(9) (1991) 763–764.10.1055/s-1991-26569Suche in Google Scholar
18 Agilent. CrysAlisPRO. Agilent Technologies, Yarnton, England, 2014.Suche in Google Scholar
19 Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Suche in Google Scholar PubMed
20 Farrugia, L. J.: WinGX and ORTEP for Windows: an update. J. Appl. Crystallogr. 45 (2012) 849–854.10.1107/S0021889812029111Suche in Google Scholar
21 Cambridge Soft. CHEMDRAW Ultra. Cambridge Soft Corporation, Cambridge, MA, USA, 2001.Suche in Google Scholar
©2017 Gamal A. El-Hiti et al., published by De Gruyter.
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Artikel in diesem Heft
- Cover and Frontmatter
- The crystal structure of triphenylphosphineoxide – 2,5-dichloro-3,6-dihydroxycyclohexa-2,5-diene-1,4-dione (2/1), C42H32Cl2O6P2
- Crystal structure of poly-[diaqua-[bis(μ2-hydroxy)-bis(μ4-3,4,5,6-tetrachlorophthalato-κ3O,O′:O′; κ2O′′:O′′′)dilanthanum(III)], C8H3Cl4LaO6
- Crystal structure of 1,1′-(3,4-diphenylthieno[2,3-b]thiophene-2,5-diyl)bis[1-phenyl-methanone], C32H20O2S2
- Crystal structure of 4a-hydroxy-9-(3,5-dibromo-phenyl)-3,4,4a,5,6,7,9,9a-octahydro-2H-xanthene-1,8-dione, C19H18Br2O4
- Crystal structure of 5-hydroxy-4,6,9,10-tetramethyl-1-oxo-6-vinyldecahydro-3a,9-propanocyclopenta[8]annulen-8-yl 2-((2-methyl-1-(3-methylbenzamido)propan-2-yl)thio)acetate, C34H49NO5S
- Crystal structure of pyridinium bis(naphthalane-2,3-diolato-κ2O,O′)borate monohydrate, C25H20BNO5
- Crystal structure of 1,1′-((1E,1′E)-(((ethane-1,2-diylbis(oxy))bis(2,1-phenylene))bis(azanylylidene))bis(methanylylidene))bis(naphthalen-2-olato)nickel(II), C72H52N4O8Ni2
- The crystal structure of 3-(2-acetyl-4-butyramido-phenoxy)-2-hydroxy-N-isopropylpropan-1-aminium tetraphenylborate, C42H49BN2O4
- Crystal structure of 4-bromobenzyl (Z)-N′-(adamantan-1-yl)-4-phenylpiperazine-1-carbothioimidate, C28H34BrN3S
- Crystal structure of poly-[(μ6-benzene-1,2,4,5-tetracarboxylato)-(μ2-1,2-bis(imidazol-1-ylmethyl)benzene)dicobalt(II)], Co2C24H16N4O8
- Crystal structure of catena-(bis(μ2-1, 2-bis(imidazole-1-ylmethyl)benzene-κN:N′)-dichlororido-nickel(II)), C28H28Cl2N8Ni
- Crystal structure of (Z)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-hydroxy-3-(4-methoxyphenyl)prop-2-en-1-one, C15H16N2O3
- Crystal structure of (Z)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-hydroxy-3-phenylprop-2-en-1-one, C14H14N2O2
- Crystal structure of (E)-2-(4-hydroxy-3-methoxybenzylidene)-6-methoxy-3,4-dihydronaphthalen-1(2H)-one, C19H18O4
- Crystal structure of (Z)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-(4-ethoxyphenyl)-3-hydroxyprop-2-en-1-one, C16H18N2O3
- Crystal structure of (Z)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-hydroxy-3-(p-toly)prop-2-en-1-one, C15H16N2O2
- Crystal structure of 1-acetyl-3-(3-chlorophenyl)-5-(4-isopropylphenyl)-4,5-dihydro-(1H)-pyrazole, C20H21ClN2O
- The crystal structure of 1-methyl-2,4-dinitro-5-iodoimidazole, C4H3IN4O4
- The crystal structure of 4-chloro-3,5-dinitroaniline, C6H4ClN3O4
- Crystal structure of N,N-dimethyl-N′-(2-methyl-4-oxo-5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidin-3(4H)-yl)formimidamide, C14H18N4OS
- Crystal structure of tetrakis(μ2-acetato-κ2O:O′)-bis[μ3-4-chloro-2,6-bis((methylimino)methyl)phenolato-κ2N,O:O,N′]-(μ4-oxido)tetracopper(II), C28H32Cl2Cu4N4O11
- Crystal structure of catena-poly[diaqua-bis(μ2-ethane-1,2-diyl-bis(pyridine-3-carboxylate-κ2N:N′))copper(II)] dinitrate, C28H28CuN6O16
- Synthesis and crystal structure of catena-poly[(μ2-nicotinato-κ2O,O′: κ1N)-(nitrato-κ1O)-(bis(2-benzimidazol-ylmethyl)amine-κ3N,N′,N′′)lead(II)], C22H18N7O5Pb
- The twinned crystal structure of (4SR)-7-benzyl-2,4,8,8-tetramethyl-7,8-dihydroimidazo[5,1-c][1,2,4]triazine-3,6(2H,4H)-dione, C16H20N4O2
- Crystal structure of (Z)-3-hydroxy-3-(4-methoxyphenyl)-1-(pyridin-2-yl)prop-2-en-1-one, C15H13NO3
- Crystal structure of 2-amino-4-(2,3-dichlorophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H12Cl2N2O2
- Crystal structure of catena-poly[(μ2-butane-1,4-diyl-bis(pyridine-3-carboxylato-κN))silver(I)] tetrafluoroborate, C16H16AgN2O4BF4
- Crystal structure of poly[diaqua-(1,10-phenanthroline-κ2N,N′)-(μ2-2,5-dihydroxytere-phthalato)-bis(μ4-2,5-dihydroxyterephthalato)dicerium(III)], C24H16CeN2O10
- Crystal structure of 5,7,4′-trihydroxy-3,8,3′-trymethoxyflavone, C18H16O8
- Crystal structure of N-(3,4-dichlorobenzylidene)-4-methylaniline, C14H11Cl2N
- Crystal structure of 4-(3-Methoxy-phenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylic acid ethyl ester, C22H27NO4
- Crystal structure of 2-amino-4-(3-fluorophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H13FN2O2
- Crystal structure of 1,1,(3,4-dihydroxythieno[2,3-b] thiophene-2,5-diyl)bis(2-bromoethanone), C10H6Br2O4S2
- The crystal structure of N,N′-(4,4′-oxydibenzyl)-bisisonicotinamide 3.5 hydrate, C24H24N4O6
- Crystal structure of catena-poly[hexakis(μ2-chlorido)-hexakis(4-(1H-pyrazol-5-yl)pyridine-κN)tricadmium(II)], Cd3C48H42Cl6N18
- Crystal structure of 2-(4-(dimethylamino)phenyl)-1,3-dimethyl-1H-perimidin-3-ium iodide, C21H22I1N3
- Crystal structure of 4-(1,3-dimethyl-2,3-dihydro-1H-perimidin-2-yl)benzonitrile, C20H17N3
- Crystal structure of tetrakis(μ2-acetato-κ2O:O′)-bis(2,2′-sulfonyldipyrazine-κ1N)dicopper(II), C24H24Cu2N8O12S2
- Crystal structure of 1-(4-chlorophenyl)-6,8-diphenyl-1H-pyrazolo[4,3-c]quinoline, C28H18ClN3
- Crystal structure of methyl 3-((1-(2-(methoxycarbonyl)benzyl)-1H-1,2,3-triazol-4-yl)methoxy)-2-naphthoate, C24H21N3O5
- Crystal structure of (tris(2-pyridylmethyl)amine-κ4N,N′,N′′,N′′′′)-chloranilato-κO,O′-zinc(II) – methanol (1/1), C25H22Cl2N4O5Zn
- Crystal structure of 1,1-dimethyl-3-(4-methoxyphenyl)urea, C10H14N2O2
- Crystal structure of 4a-Hydroxy-9-(2-nitro-phenyl)-3,4,4a,5,6,7,9,9a-octahydro-2H-xanthene-1,8-dione, C19H19NO6
- Crystal structure of chlorido-(η6–1-isopropyl-4-methyl benzene)-(1-(pyridin-2-yl)-N-(p-tolyl)methanimine-κ2N,N′)ruthenium(II) hexafluorophosphate(V), C23H26ClF6N2PRu
- Crystal structure of phenyl(2-phenyl-2,3-dihydro-1H-perimidin-2-yl)methanone, C24H18N2O
- Crystal structure of (E)-3-methyl-4-((3-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)methylene)-1-phenyl-1H-pyrazol-5(4H)-one, C29H23N7O
- Crystal structure of 2-(4-(2-butyl-1,3-dioxo-2,3-dihydro-1H-benzo[de]isoquinolin-6-yl)piperazin-1-yl)-2-oxoethyldiethylcarbamodithioate, C27H34N4O3S2
- Crystal structure of poly-[diaqua-bis(μ-4,4′-bipyridine-κ2N:N′)cobalt(II)] bis(4-chlorobenzenesulfonate) – 4,4′-bipyridine – water (1/1/2), C42H40Cl2CoN6O10S2
- Crystal structure of (η6-benzene)-(N-(2,6-dimethylphenyl)-1-(pyridin-2-yl)methanimine-κ2N,N′)ruthenium(II) perchlorate monohydrate, C20H20Cl2N2O5Ru
- Crystal structure of 4,10,16,22-tetrahydroxy-6,12,18,24-tetramethoxy-2,8,14,20-tetraethylphenylresorcin[4]arene – ethyl acetate (1/1), C68H72O10
- Crystal structure of chlorido-(N-(2,5-dichlorophenyl)-1-(pyridin-2-yl)methanimine-κ2N,N′)(η6-1-isopropyl-4-methyl benzene) ruthenium (II) tetrafluoroborate, C22H22Cl3N2BF4Ru
- Crystal structure of 3-(5-methyl-1-p-tolyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazole-4-carbaldehyde, a rare Z′ = 3 structure, C20H17N5O
- Crystal structure of 5-(5-(4-chlorophenyl)-1-phenyl-1H-pyrazol-3-yl)-N-phenyl-1,3,4-thiadiazol-2-amine, C23H16ClN5S
- Crystal structure of 7-hydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one-N,N-dimethylformamide (1/1), C18H17NO5
- Crystal structure of halogen-bonded 2-chloro-1,10-phenanthroline—1,4-diiodotetrafluorobenzene (2/1), C30H14Cl2F4I2N4
- Crystal structure of 1-(4,4-dimethyl-2,6-dithioxo-1,3,5-triazinan-1-yl)-3-(diethylaminocarbonyl)thiourea, C11H20N6OS3
- Crystal structure of methyl 1-(4-fluorobenzyl)-3-phenyl-1H-pyrazole-5-carboxylate, C18H15FN2O2
- Crystal structure of 1,1-dimethyl-3-(4-methylphenyl)urea, C10H14N2O
- Crystal structure of yttrium gallium antimonide, Y5Ga1.24Sb2.77
- Crystal structure of 2-(bis(4-methoxyphenyl)amino)-2-oxoacetic acid, C16H15NO5
Artikel in diesem Heft
- Cover and Frontmatter
- The crystal structure of triphenylphosphineoxide – 2,5-dichloro-3,6-dihydroxycyclohexa-2,5-diene-1,4-dione (2/1), C42H32Cl2O6P2
- Crystal structure of poly-[diaqua-[bis(μ2-hydroxy)-bis(μ4-3,4,5,6-tetrachlorophthalato-κ3O,O′:O′; κ2O′′:O′′′)dilanthanum(III)], C8H3Cl4LaO6
- Crystal structure of 1,1′-(3,4-diphenylthieno[2,3-b]thiophene-2,5-diyl)bis[1-phenyl-methanone], C32H20O2S2
- Crystal structure of 4a-hydroxy-9-(3,5-dibromo-phenyl)-3,4,4a,5,6,7,9,9a-octahydro-2H-xanthene-1,8-dione, C19H18Br2O4
- Crystal structure of 5-hydroxy-4,6,9,10-tetramethyl-1-oxo-6-vinyldecahydro-3a,9-propanocyclopenta[8]annulen-8-yl 2-((2-methyl-1-(3-methylbenzamido)propan-2-yl)thio)acetate, C34H49NO5S
- Crystal structure of pyridinium bis(naphthalane-2,3-diolato-κ2O,O′)borate monohydrate, C25H20BNO5
- Crystal structure of 1,1′-((1E,1′E)-(((ethane-1,2-diylbis(oxy))bis(2,1-phenylene))bis(azanylylidene))bis(methanylylidene))bis(naphthalen-2-olato)nickel(II), C72H52N4O8Ni2
- The crystal structure of 3-(2-acetyl-4-butyramido-phenoxy)-2-hydroxy-N-isopropylpropan-1-aminium tetraphenylborate, C42H49BN2O4
- Crystal structure of 4-bromobenzyl (Z)-N′-(adamantan-1-yl)-4-phenylpiperazine-1-carbothioimidate, C28H34BrN3S
- Crystal structure of poly-[(μ6-benzene-1,2,4,5-tetracarboxylato)-(μ2-1,2-bis(imidazol-1-ylmethyl)benzene)dicobalt(II)], Co2C24H16N4O8
- Crystal structure of catena-(bis(μ2-1, 2-bis(imidazole-1-ylmethyl)benzene-κN:N′)-dichlororido-nickel(II)), C28H28Cl2N8Ni
- Crystal structure of (Z)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-hydroxy-3-(4-methoxyphenyl)prop-2-en-1-one, C15H16N2O3
- Crystal structure of (Z)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-hydroxy-3-phenylprop-2-en-1-one, C14H14N2O2
- Crystal structure of (E)-2-(4-hydroxy-3-methoxybenzylidene)-6-methoxy-3,4-dihydronaphthalen-1(2H)-one, C19H18O4
- Crystal structure of (Z)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-(4-ethoxyphenyl)-3-hydroxyprop-2-en-1-one, C16H18N2O3
- Crystal structure of (Z)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-hydroxy-3-(p-toly)prop-2-en-1-one, C15H16N2O2
- Crystal structure of 1-acetyl-3-(3-chlorophenyl)-5-(4-isopropylphenyl)-4,5-dihydro-(1H)-pyrazole, C20H21ClN2O
- The crystal structure of 1-methyl-2,4-dinitro-5-iodoimidazole, C4H3IN4O4
- The crystal structure of 4-chloro-3,5-dinitroaniline, C6H4ClN3O4
- Crystal structure of N,N-dimethyl-N′-(2-methyl-4-oxo-5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidin-3(4H)-yl)formimidamide, C14H18N4OS
- Crystal structure of tetrakis(μ2-acetato-κ2O:O′)-bis[μ3-4-chloro-2,6-bis((methylimino)methyl)phenolato-κ2N,O:O,N′]-(μ4-oxido)tetracopper(II), C28H32Cl2Cu4N4O11
- Crystal structure of catena-poly[diaqua-bis(μ2-ethane-1,2-diyl-bis(pyridine-3-carboxylate-κ2N:N′))copper(II)] dinitrate, C28H28CuN6O16
- Synthesis and crystal structure of catena-poly[(μ2-nicotinato-κ2O,O′: κ1N)-(nitrato-κ1O)-(bis(2-benzimidazol-ylmethyl)amine-κ3N,N′,N′′)lead(II)], C22H18N7O5Pb
- The twinned crystal structure of (4SR)-7-benzyl-2,4,8,8-tetramethyl-7,8-dihydroimidazo[5,1-c][1,2,4]triazine-3,6(2H,4H)-dione, C16H20N4O2
- Crystal structure of (Z)-3-hydroxy-3-(4-methoxyphenyl)-1-(pyridin-2-yl)prop-2-en-1-one, C15H13NO3
- Crystal structure of 2-amino-4-(2,3-dichlorophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H12Cl2N2O2
- Crystal structure of catena-poly[(μ2-butane-1,4-diyl-bis(pyridine-3-carboxylato-κN))silver(I)] tetrafluoroborate, C16H16AgN2O4BF4
- Crystal structure of poly[diaqua-(1,10-phenanthroline-κ2N,N′)-(μ2-2,5-dihydroxytere-phthalato)-bis(μ4-2,5-dihydroxyterephthalato)dicerium(III)], C24H16CeN2O10
- Crystal structure of 5,7,4′-trihydroxy-3,8,3′-trymethoxyflavone, C18H16O8
- Crystal structure of N-(3,4-dichlorobenzylidene)-4-methylaniline, C14H11Cl2N
- Crystal structure of 4-(3-Methoxy-phenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylic acid ethyl ester, C22H27NO4
- Crystal structure of 2-amino-4-(3-fluorophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H13FN2O2
- Crystal structure of 1,1,(3,4-dihydroxythieno[2,3-b] thiophene-2,5-diyl)bis(2-bromoethanone), C10H6Br2O4S2
- The crystal structure of N,N′-(4,4′-oxydibenzyl)-bisisonicotinamide 3.5 hydrate, C24H24N4O6
- Crystal structure of catena-poly[hexakis(μ2-chlorido)-hexakis(4-(1H-pyrazol-5-yl)pyridine-κN)tricadmium(II)], Cd3C48H42Cl6N18
- Crystal structure of 2-(4-(dimethylamino)phenyl)-1,3-dimethyl-1H-perimidin-3-ium iodide, C21H22I1N3
- Crystal structure of 4-(1,3-dimethyl-2,3-dihydro-1H-perimidin-2-yl)benzonitrile, C20H17N3
- Crystal structure of tetrakis(μ2-acetato-κ2O:O′)-bis(2,2′-sulfonyldipyrazine-κ1N)dicopper(II), C24H24Cu2N8O12S2
- Crystal structure of 1-(4-chlorophenyl)-6,8-diphenyl-1H-pyrazolo[4,3-c]quinoline, C28H18ClN3
- Crystal structure of methyl 3-((1-(2-(methoxycarbonyl)benzyl)-1H-1,2,3-triazol-4-yl)methoxy)-2-naphthoate, C24H21N3O5
- Crystal structure of (tris(2-pyridylmethyl)amine-κ4N,N′,N′′,N′′′′)-chloranilato-κO,O′-zinc(II) – methanol (1/1), C25H22Cl2N4O5Zn
- Crystal structure of 1,1-dimethyl-3-(4-methoxyphenyl)urea, C10H14N2O2
- Crystal structure of 4a-Hydroxy-9-(2-nitro-phenyl)-3,4,4a,5,6,7,9,9a-octahydro-2H-xanthene-1,8-dione, C19H19NO6
- Crystal structure of chlorido-(η6–1-isopropyl-4-methyl benzene)-(1-(pyridin-2-yl)-N-(p-tolyl)methanimine-κ2N,N′)ruthenium(II) hexafluorophosphate(V), C23H26ClF6N2PRu
- Crystal structure of phenyl(2-phenyl-2,3-dihydro-1H-perimidin-2-yl)methanone, C24H18N2O
- Crystal structure of (E)-3-methyl-4-((3-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)methylene)-1-phenyl-1H-pyrazol-5(4H)-one, C29H23N7O
- Crystal structure of 2-(4-(2-butyl-1,3-dioxo-2,3-dihydro-1H-benzo[de]isoquinolin-6-yl)piperazin-1-yl)-2-oxoethyldiethylcarbamodithioate, C27H34N4O3S2
- Crystal structure of poly-[diaqua-bis(μ-4,4′-bipyridine-κ2N:N′)cobalt(II)] bis(4-chlorobenzenesulfonate) – 4,4′-bipyridine – water (1/1/2), C42H40Cl2CoN6O10S2
- Crystal structure of (η6-benzene)-(N-(2,6-dimethylphenyl)-1-(pyridin-2-yl)methanimine-κ2N,N′)ruthenium(II) perchlorate monohydrate, C20H20Cl2N2O5Ru
- Crystal structure of 4,10,16,22-tetrahydroxy-6,12,18,24-tetramethoxy-2,8,14,20-tetraethylphenylresorcin[4]arene – ethyl acetate (1/1), C68H72O10
- Crystal structure of chlorido-(N-(2,5-dichlorophenyl)-1-(pyridin-2-yl)methanimine-κ2N,N′)(η6-1-isopropyl-4-methyl benzene) ruthenium (II) tetrafluoroborate, C22H22Cl3N2BF4Ru
- Crystal structure of 3-(5-methyl-1-p-tolyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazole-4-carbaldehyde, a rare Z′ = 3 structure, C20H17N5O
- Crystal structure of 5-(5-(4-chlorophenyl)-1-phenyl-1H-pyrazol-3-yl)-N-phenyl-1,3,4-thiadiazol-2-amine, C23H16ClN5S
- Crystal structure of 7-hydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one-N,N-dimethylformamide (1/1), C18H17NO5
- Crystal structure of halogen-bonded 2-chloro-1,10-phenanthroline—1,4-diiodotetrafluorobenzene (2/1), C30H14Cl2F4I2N4
- Crystal structure of 1-(4,4-dimethyl-2,6-dithioxo-1,3,5-triazinan-1-yl)-3-(diethylaminocarbonyl)thiourea, C11H20N6OS3
- Crystal structure of methyl 1-(4-fluorobenzyl)-3-phenyl-1H-pyrazole-5-carboxylate, C18H15FN2O2
- Crystal structure of 1,1-dimethyl-3-(4-methylphenyl)urea, C10H14N2O
- Crystal structure of yttrium gallium antimonide, Y5Ga1.24Sb2.77
- Crystal structure of 2-(bis(4-methoxyphenyl)amino)-2-oxoacetic acid, C16H15NO5

