Abstract
C23H26ClF6N2PRu, triclinic, P1̅ (no. 2), a = 8.9950(6) Å, b = 11.1669(8) Å, c = 12.4128(9) Å, α = 80.520(2)°, β = 87.5360(10)°, γ = 86.3200(10)°, V = 1226.59(15) Å3, Z = 2, Rgt(F) = 0.0267, wRref(F2) = 0.0622, T = 173(2) K.
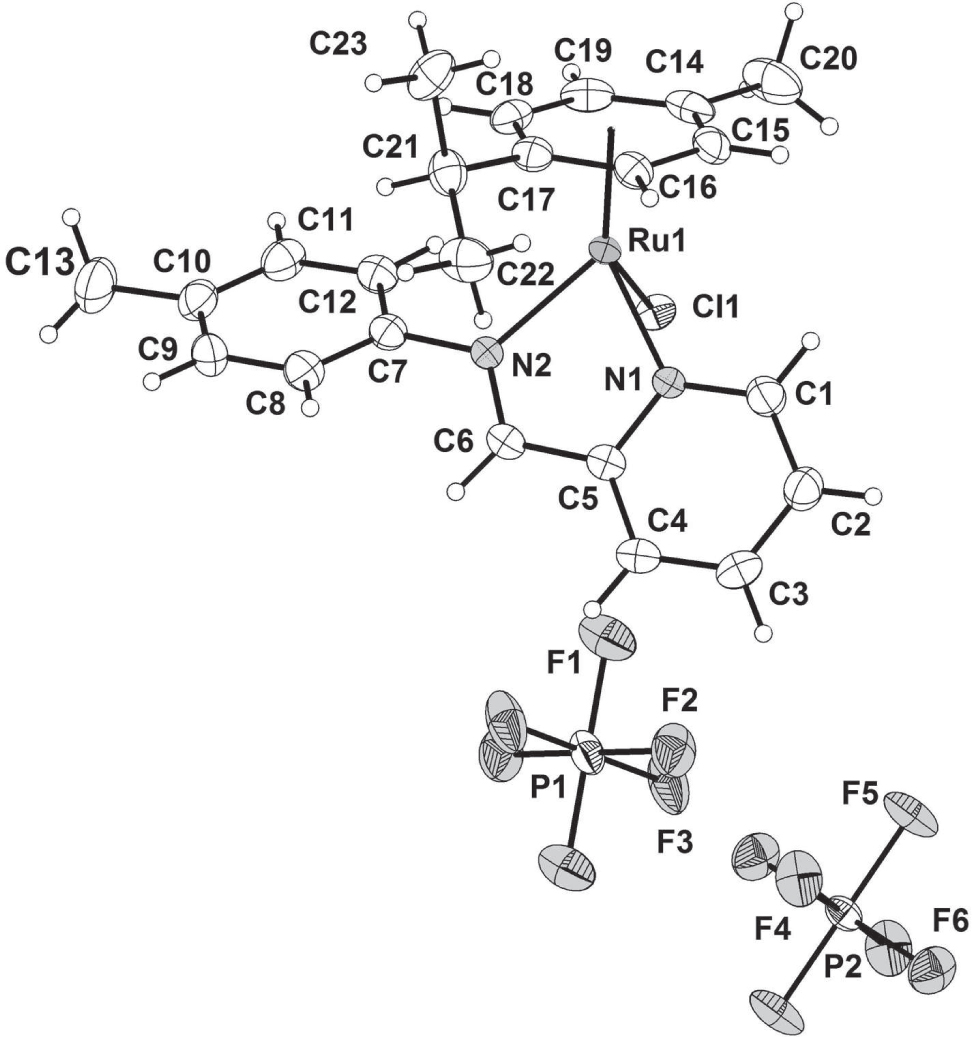
The title crystal structure is shown in the figure. Tables 1 and 2 contain details on crystal structure and measurement conditions and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Yellow block |
| Size: | 0.18 × 0.13 × 0.12 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 8.7 cm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| 2θmax, completeness: | 56.8°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 31876, 6121, 0.042 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 5409 |
| N(param)refined: | 314 |
| Programs: | Bruker programs [11], SHELX [12] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1 | 0.6025(2) | 0.52363(19) | 0.65711(18) | 0.0284(4) |
| H1 | 0.7034 | 0.543 | 0.6419 | 0.034* |
| C2 | 0.5595(2) | 0.41164(19) | 0.64094(18) | 0.0304(5) |
| H2 | 0.6296 | 0.3558 | 0.6135 | 0.036* |
| C3 | 0.4138(2) | 0.38115(19) | 0.66484(18) | 0.0296(5) |
| H3 | 0.3829 | 0.3039 | 0.6554 | 0.036* |
| C4 | 0.3141(2) | 0.46535(19) | 0.70286(17) | 0.0266(4) |
| H4 | 0.2136 | 0.4464 | 0.7208 | 0.032* |
| C5 | 0.3625(2) | 0.57688(17) | 0.71424(16) | 0.0211(4) |
| C6 | 0.2654(2) | 0.67694(18) | 0.74080(16) | 0.0231(4) |
| H6 | 0.1621 | 0.6675 | 0.7567 | 0.028* |
| C7 | 0.2267(2) | 0.88000(18) | 0.76569(16) | 0.0226(4) |
| C8 | 0.1139(2) | 0.8632(2) | 0.84537(18) | 0.0285(4) |
| H8 | 0.1031 | 0.7856 | 0.8887 | 0.034* |
| C9 | 0.0165(2) | 0.9612(2) | 0.86144(19) | 0.0328(5) |
| H9 | −0.0609 | 0.9495 | 0.916 | 0.039* |
| C10 | 0.0300(2) | 1.0759(2) | 0.79935(19) | 0.0319(5) |
| C11 | 0.1461(2) | 1.0908(2) | 0.72200(18) | 0.0304(5) |
| H11 | 0.158 | 1.1688 | 0.6796 | 0.036* |
| C12 | 0.2453(2) | 0.99492(18) | 0.70497(17) | 0.0255(4) |
| H12 | 0.3251 | 1.0075 | 0.6524 | 0.031* |
| C13 | −0.0788(3) | 1.1806(2) | 0.8156(2) | 0.0492(7) |
| H13A | −0.1061 | 1.2266 | 0.7443 | 0.074* |
| H13B | −0.0324 | 1.2341 | 0.8579 | 0.074* |
| H13C | −0.1684 | 1.1491 | 0.8552 | 0.074* |
| C14 | 0.7773(2) | 0.8593(2) | 0.67413(17) | 0.0293(5) |
| C15 | 0.7926(2) | 0.7466(2) | 0.74044(17) | 0.0268(4) |
| H15 | 0.8613 | 0.6859 | 0.7192 | 0.032* |
| C16 | 0.7067(2) | 0.72056(19) | 0.83990(16) | 0.0235(4) |
| H16 | 0.7166 | 0.6419 | 0.8828 | 0.028* |
| C17 | 0.6073(2) | 0.80976(18) | 0.87537(16) | 0.0236(4) |
| C18 | 0.5902(2) | 0.92438(18) | 0.80519(17) | 0.0257(4) |
| H18 | 0.5222 | 0.9856 | 0.8264 | 0.031* |
| C19 | 0.6709(2) | 0.94879(19) | 0.70600(18) | 0.0300(5) |
| H19 | 0.6551 | 1.0249 | 0.6597 | 0.036* |
| C20 | 0.8668(3) | 0.8864(3) | 0.5692(2) | 0.0466(7) |
| H20A | 0.9617 | 0.9184 | 0.5835 | 0.07* |
| H20B | 0.8111 | 0.947 | 0.5176 | 0.07* |
| H20C | 0.8862 | 0.8117 | 0.5379 | 0.07* |
| C21 | 0.5222(2) | 0.7893(2) | 0.98378(17) | 0.0291(4) |
| H21 | 0.4293 | 0.8438 | 0.9778 | 0.035* |
| C22 | 0.4784(3) | 0.6589(2) | 1.01948(19) | 0.0391(6) |
| H22A | 0.4244 | 0.6332 | 0.9611 | 0.059* |
| H22B | 0.4143 | 0.6542 | 1.0857 | 0.059* |
| H22C | 0.5683 | 0.6054 | 1.0346 | 0.059* |
| C23 | 0.6200(3) | 0.8277(2) | 1.06876(19) | 0.0379(5) |
| H23A | 0.7119 | 0.7753 | 1.0756 | 0.057* |
| H23B | 0.5658 | 0.8199 | 1.1395 | 0.057* |
| H23C | 0.6448 | 0.9125 | 1.0455 | 0.057* |
| N1 | 0.50673(17) | 0.60562(14) | 0.69351(13) | 0.0209(3) |
| N2 | 0.32296(16) | 0.77905(14) | 0.74233(13) | 0.0205(3) |
| F1 | 0.12870(16) | 0.59118(16) | 0.49576(15) | 0.0605(5) |
| F2 | 0.12009(16) | 0.38912(15) | 0.53006(13) | 0.0527(4) |
| F3 | 0.02962(16) | 0.49340(18) | 0.37312(12) | 0.0594(5) |
| F4 | 0.02056(16) | 0.47202(14) | 0.12879(11) | 0.0455(4) |
| F5 | 0.16799(14) | 0.53898(14) | −0.01701(13) | 0.0496(4) |
| F6 | 0.05565(16) | 0.36324(12) | −0.00774(12) | 0.0446(3) |
| P1 | 0 | 0.5 | 0.5 | 0.03507(19) |
| P2 | 0 | 0.5 | 0 | 0.02441(16) |
| Cl1 | 0.49348(6) | 0.82821(5) | 0.52082(4) | 0.02720(11) |
| Ru1 | 0.55275(2) | 0.78196(2) | 0.70978(2) | 0.01841(5) |
Source of material
To a suspension of [(η6-p-cymene)Ru(μ-Cl)Cl]2 (0.2 mmol) in methanol (20 mL) was added (1-(pyridin-2-yl)-N-(p-tolyl)methanimine (0.42 mmol). The mixture was stirred at room temperature for 3 hours followed by the reduction in the volume of the solvent in vacuo to about 10 mL before adding NH4PF6 (0.42 mmol). The mixture was then cooled in an ice bath while stirring for 2 hours leading to a precipitate which was collected by filtration. The filtrate was washed with diethyl ether and dried in vacuo. Crystals of the title compound were obtained by the liquid diffusion method, in which the solutions of the compounds in acetone were layered with hexane and left undisturbed for two days.
Orange powder. Yield 80%. m.p. 188 °C (decomp). 1H-NMR (400 MHz, DMSO-d6). δ = 9.56 (d, JHH = 5.44 Hz, 1H, Py), 8.88 (s, 1H, CH = N), 8.30 (t, JHHH = 7.56 Hz, 2H, Py), 7.87 (s, 1H, Py), 7.71 (d, JHH = 8.16 Hz, 2H, Ar), 7.44 (d, JHH = 8.16 Hz, 2H, Ar), 6.09 (d, JHH = 6.20 Hz, 1H, p-cymene), 5.75 (d, JHH = 6.21 Hz, 1H, Ar, p-cymene), 5.62 (q, 2H, Ar, p-cymene), 2.67 (sep, 1H, CH(Me)2), 2.44 (s, 3H, CH3), 2.16 (s, 3H, CH3), 0.98 (d, JHH = 6.84 Hz, 6H, CH-Me2 (p-cymene)). 13C-NMR (400 MHz, DMSO-d6). δ 167.02 (CH = N), 155.91 (Py), 154.56 (Py), 149.42 (Py), 139.88 (Py), 139.62 (Py), 129.87 (Ar), 128.74 (Ar), 122.40 (Ar), 104.93 (Ar), 103.56 (Ar), 86.50 (Ar, p-cymene), 86.24; (Ar, p-cymene), 85.07 (Ar, p-cymene), 84.75 (Ar, p-cymene), 30.45 (CH), 21.68 (CH3), 21.54 (CH3), 20.78 (CH3), 18.26 (CH3); IR (solid state): (−CH = N) 1608 cm−1, (P-F) 833 cm−1. MS (ESI, m/z): 467.08 [M+].
Experimental details
All hydrogen atoms were placed in idealised positions and refined in riding models with Uiso assigned the values of 1.2Ueq those of their parent atoms. The distances of C-H were constrained to 0.95 Å for all aromatic hydrogens.
Discussion
Half-sandwich ruthenium(II) arene complexes have found application as catalyst precursors for a wide range of chemical transformations, and are used in biological applications [1], [2]. Among the half-sandwich complexes, arene ruthenium complexes represent one of the most sought after organometallic compounds due to their potential applications in catalytic and biological applications [3]. The coordinated arene moieties are relatively inert towards substitution and act as spectator ligand [4]. These stabilize and protect the metal centre and thereby prevent rapid oxidation of the Ru(II) to Ru(III) [4]. The title compound is part of our continuing studies of half-sandwich ruthenium(II) complexes [5], [6], [7], [8].
The asymmetric unit of the title structure contains one cationic ruthenium complex in general position and two half [PF6]− anions located at two inversion centers of the triclinic space group P1̅. The cationic ruthenium(II) complex possess a piano stool geometry where the chelating ligand and the chloride occupy the positions of the three legs of a piano-stool and the arene ring occupies the remaining coordination sites as the seat of the stool [3]. The Ru-N bond lengths of the complex are 2.0606(12) and 2.0804(13) Å. These distances are comparable to those reported for other arene ruthenium complexes with N,N′-donor ligands [5], [6], [7]. The N-Ru-N bond angles was derived to be 76.52(6)°. The N-Ru-Cl bond angles are 84.04(4) and 84.05(5)°. These values are close to those reported for related compounds [5], [6], [7], [8], [9], [10]. All C—C, C—N and P—F bond lengths and angles are in the expected ranges.
Acknowledgement
We wish to extend our sincere thanks to the NRF, THRIP (Grant No. Tp 1208035643) and UKZN (URF) for financial support. Gichumbi, M. Joel thanks Prof. E.N. Njoka for his support.
References
1 Gloria, G.; Gupta, G.; Rao Anna, N.; Das, B.; Rao, K. M.: Synthesis and characterization of mono- and binuclear iminopyridyl and salicylaldimine Ru(II)–arene complexes. Polyhedron 53 (2013) 56–61.10.1016/j.poly.2013.01.014Search in Google Scholar
2 Thangavel, S.; Rajamanikandan, R.; Friedrich, H. B.; Ilanchelian, M.; Omondi, B.: Binding interaction, conformational change, and molecular docking study of N-(pyridin-2-ylmethylene)aniline derivatives and carbazole Ru(II) complexes with human serum albumins. Polyhedron 107 (2016) 124–135.10.1016/j.poly.2016.01.017Search in Google Scholar
3 Kumar, P.; Gupta, R.: Half-sandwich arene ruthenium complexes: synthetic strategies and relevance in catalysis. Chem. Soc. Rev. 43 (2014) 707–733.10.1039/C3CS60189GSearch in Google Scholar
4 Therrien, B.: Functionalised η6-arene ruthenium complexes. Coord. Chem. Rev. 253 (2009) 493–519.10.1016/j.ccr.2008.04.014Search in Google Scholar
5 Gichumbi, J. M.; Friedrich, H. B.; Omondi, B.: Solvato-polymorph of [(η6-C6H6)RuCl(L)]PF6 (L = (2,6-dimethyl-phenyl-pyridin-2-yl methylene amine). J. Mol. Struct. 1113 (2016) 55–59.10.1016/j.molstruc.2016.02.040Search in Google Scholar
6 Gichumbi, J. M.; Friedrich, H. B.; Omondi, B.: Synthesis and characterization of piano-stool ruthenium complexes with N,N′-pyridine imine bidentate ligands and their application in styrene oxidation. J. Organomet. Chem. 808 (2016) 87–96.10.1016/j.jorganchem.2016.02.015Search in Google Scholar
7 Gichumbi, J. M.; Friedrich, H. B.; Omondi, B.: Application of arene ruthenium(II) complexes with pyridine-2-carboxaldimine ligands in the transfer hydrogenation of ketones. J. Mol. Cat. A: Chem. Chem. 416 (2016) 29–38.10.1016/j.molcata.2016.02.012Search in Google Scholar
8 Nyawade, E. A.; Friedrich, H. B.; Omondi, B.; Chenia, H. Y.; Singh, M.; Gorle, S.: Synthesis and characterization of new α,α′-diaminoalkane-bridged dicarbonyl (η5-cyclopentadienyl)ruthenium(II) complex salts: antibacterial activity tests of η5-cyclopentadienyl dicarbonyl ruthenium(II) amine complexes. J. Organomet. Chem. 799–800 (2015) 138–146.10.1016/j.jorganchem.2015.09.007Search in Google Scholar
9 Chow, M.; Licona, C.; Yuan Qian Wong, D.; Pastorin, G.; Gaiddon, C.; Ang, W. H.: Discovery and investigation of anticancer ruthenium–arene Schiff-Base complexes via water-promoted combinatorial three-component assembly. J. Med. Chem. 57 (2014) 6043–6442.10.1021/jm500455pSearch in Google Scholar
10 Mandal, S. K.; Chakravorty, A. R.: Arene ruthenium complexes of N,N′- and N,O-donor Schiff base ligands: an x-ray structure of [(η6-p-cymene)RuCl(C5H4N-2-CH-NC6H4-p-Me)Cl⋅C6H6⋅H2O. Polyhedron. 11 (1992) 823–827.10.1016/S0277-5387(00)86017-9Search in Google Scholar
11 Bruker-AXS, APEX2, SADABS, SMART, Madison, Wisconsin, USA, (2009).Search in Google Scholar
12 Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Search in Google Scholar PubMed
©2017 Joel M. Gichumbi et al., published by De Gruyter.
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Articles in the same Issue
- Cover and Frontmatter
- The crystal structure of triphenylphosphineoxide – 2,5-dichloro-3,6-dihydroxycyclohexa-2,5-diene-1,4-dione (2/1), C42H32Cl2O6P2
- Crystal structure of poly-[diaqua-[bis(μ2-hydroxy)-bis(μ4-3,4,5,6-tetrachlorophthalato-κ3O,O′:O′; κ2O′′:O′′′)dilanthanum(III)], C8H3Cl4LaO6
- Crystal structure of 1,1′-(3,4-diphenylthieno[2,3-b]thiophene-2,5-diyl)bis[1-phenyl-methanone], C32H20O2S2
- Crystal structure of 4a-hydroxy-9-(3,5-dibromo-phenyl)-3,4,4a,5,6,7,9,9a-octahydro-2H-xanthene-1,8-dione, C19H18Br2O4
- Crystal structure of 5-hydroxy-4,6,9,10-tetramethyl-1-oxo-6-vinyldecahydro-3a,9-propanocyclopenta[8]annulen-8-yl 2-((2-methyl-1-(3-methylbenzamido)propan-2-yl)thio)acetate, C34H49NO5S
- Crystal structure of pyridinium bis(naphthalane-2,3-diolato-κ2O,O′)borate monohydrate, C25H20BNO5
- Crystal structure of 1,1′-((1E,1′E)-(((ethane-1,2-diylbis(oxy))bis(2,1-phenylene))bis(azanylylidene))bis(methanylylidene))bis(naphthalen-2-olato)nickel(II), C72H52N4O8Ni2
- The crystal structure of 3-(2-acetyl-4-butyramido-phenoxy)-2-hydroxy-N-isopropylpropan-1-aminium tetraphenylborate, C42H49BN2O4
- Crystal structure of 4-bromobenzyl (Z)-N′-(adamantan-1-yl)-4-phenylpiperazine-1-carbothioimidate, C28H34BrN3S
- Crystal structure of poly-[(μ6-benzene-1,2,4,5-tetracarboxylato)-(μ2-1,2-bis(imidazol-1-ylmethyl)benzene)dicobalt(II)], Co2C24H16N4O8
- Crystal structure of catena-(bis(μ2-1, 2-bis(imidazole-1-ylmethyl)benzene-κN:N′)-dichlororido-nickel(II)), C28H28Cl2N8Ni
- Crystal structure of (Z)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-hydroxy-3-(4-methoxyphenyl)prop-2-en-1-one, C15H16N2O3
- Crystal structure of (Z)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-hydroxy-3-phenylprop-2-en-1-one, C14H14N2O2
- Crystal structure of (E)-2-(4-hydroxy-3-methoxybenzylidene)-6-methoxy-3,4-dihydronaphthalen-1(2H)-one, C19H18O4
- Crystal structure of (Z)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-(4-ethoxyphenyl)-3-hydroxyprop-2-en-1-one, C16H18N2O3
- Crystal structure of (Z)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-hydroxy-3-(p-toly)prop-2-en-1-one, C15H16N2O2
- Crystal structure of 1-acetyl-3-(3-chlorophenyl)-5-(4-isopropylphenyl)-4,5-dihydro-(1H)-pyrazole, C20H21ClN2O
- The crystal structure of 1-methyl-2,4-dinitro-5-iodoimidazole, C4H3IN4O4
- The crystal structure of 4-chloro-3,5-dinitroaniline, C6H4ClN3O4
- Crystal structure of N,N-dimethyl-N′-(2-methyl-4-oxo-5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidin-3(4H)-yl)formimidamide, C14H18N4OS
- Crystal structure of tetrakis(μ2-acetato-κ2O:O′)-bis[μ3-4-chloro-2,6-bis((methylimino)methyl)phenolato-κ2N,O:O,N′]-(μ4-oxido)tetracopper(II), C28H32Cl2Cu4N4O11
- Crystal structure of catena-poly[diaqua-bis(μ2-ethane-1,2-diyl-bis(pyridine-3-carboxylate-κ2N:N′))copper(II)] dinitrate, C28H28CuN6O16
- Synthesis and crystal structure of catena-poly[(μ2-nicotinato-κ2O,O′: κ1N)-(nitrato-κ1O)-(bis(2-benzimidazol-ylmethyl)amine-κ3N,N′,N′′)lead(II)], C22H18N7O5Pb
- The twinned crystal structure of (4SR)-7-benzyl-2,4,8,8-tetramethyl-7,8-dihydroimidazo[5,1-c][1,2,4]triazine-3,6(2H,4H)-dione, C16H20N4O2
- Crystal structure of (Z)-3-hydroxy-3-(4-methoxyphenyl)-1-(pyridin-2-yl)prop-2-en-1-one, C15H13NO3
- Crystal structure of 2-amino-4-(2,3-dichlorophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H12Cl2N2O2
- Crystal structure of catena-poly[(μ2-butane-1,4-diyl-bis(pyridine-3-carboxylato-κN))silver(I)] tetrafluoroborate, C16H16AgN2O4BF4
- Crystal structure of poly[diaqua-(1,10-phenanthroline-κ2N,N′)-(μ2-2,5-dihydroxytere-phthalato)-bis(μ4-2,5-dihydroxyterephthalato)dicerium(III)], C24H16CeN2O10
- Crystal structure of 5,7,4′-trihydroxy-3,8,3′-trymethoxyflavone, C18H16O8
- Crystal structure of N-(3,4-dichlorobenzylidene)-4-methylaniline, C14H11Cl2N
- Crystal structure of 4-(3-Methoxy-phenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylic acid ethyl ester, C22H27NO4
- Crystal structure of 2-amino-4-(3-fluorophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H13FN2O2
- Crystal structure of 1,1,(3,4-dihydroxythieno[2,3-b] thiophene-2,5-diyl)bis(2-bromoethanone), C10H6Br2O4S2
- The crystal structure of N,N′-(4,4′-oxydibenzyl)-bisisonicotinamide 3.5 hydrate, C24H24N4O6
- Crystal structure of catena-poly[hexakis(μ2-chlorido)-hexakis(4-(1H-pyrazol-5-yl)pyridine-κN)tricadmium(II)], Cd3C48H42Cl6N18
- Crystal structure of 2-(4-(dimethylamino)phenyl)-1,3-dimethyl-1H-perimidin-3-ium iodide, C21H22I1N3
- Crystal structure of 4-(1,3-dimethyl-2,3-dihydro-1H-perimidin-2-yl)benzonitrile, C20H17N3
- Crystal structure of tetrakis(μ2-acetato-κ2O:O′)-bis(2,2′-sulfonyldipyrazine-κ1N)dicopper(II), C24H24Cu2N8O12S2
- Crystal structure of 1-(4-chlorophenyl)-6,8-diphenyl-1H-pyrazolo[4,3-c]quinoline, C28H18ClN3
- Crystal structure of methyl 3-((1-(2-(methoxycarbonyl)benzyl)-1H-1,2,3-triazol-4-yl)methoxy)-2-naphthoate, C24H21N3O5
- Crystal structure of (tris(2-pyridylmethyl)amine-κ4N,N′,N′′,N′′′′)-chloranilato-κO,O′-zinc(II) – methanol (1/1), C25H22Cl2N4O5Zn
- Crystal structure of 1,1-dimethyl-3-(4-methoxyphenyl)urea, C10H14N2O2
- Crystal structure of 4a-Hydroxy-9-(2-nitro-phenyl)-3,4,4a,5,6,7,9,9a-octahydro-2H-xanthene-1,8-dione, C19H19NO6
- Crystal structure of chlorido-(η6–1-isopropyl-4-methyl benzene)-(1-(pyridin-2-yl)-N-(p-tolyl)methanimine-κ2N,N′)ruthenium(II) hexafluorophosphate(V), C23H26ClF6N2PRu
- Crystal structure of phenyl(2-phenyl-2,3-dihydro-1H-perimidin-2-yl)methanone, C24H18N2O
- Crystal structure of (E)-3-methyl-4-((3-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)methylene)-1-phenyl-1H-pyrazol-5(4H)-one, C29H23N7O
- Crystal structure of 2-(4-(2-butyl-1,3-dioxo-2,3-dihydro-1H-benzo[de]isoquinolin-6-yl)piperazin-1-yl)-2-oxoethyldiethylcarbamodithioate, C27H34N4O3S2
- Crystal structure of poly-[diaqua-bis(μ-4,4′-bipyridine-κ2N:N′)cobalt(II)] bis(4-chlorobenzenesulfonate) – 4,4′-bipyridine – water (1/1/2), C42H40Cl2CoN6O10S2
- Crystal structure of (η6-benzene)-(N-(2,6-dimethylphenyl)-1-(pyridin-2-yl)methanimine-κ2N,N′)ruthenium(II) perchlorate monohydrate, C20H20Cl2N2O5Ru
- Crystal structure of 4,10,16,22-tetrahydroxy-6,12,18,24-tetramethoxy-2,8,14,20-tetraethylphenylresorcin[4]arene – ethyl acetate (1/1), C68H72O10
- Crystal structure of chlorido-(N-(2,5-dichlorophenyl)-1-(pyridin-2-yl)methanimine-κ2N,N′)(η6-1-isopropyl-4-methyl benzene) ruthenium (II) tetrafluoroborate, C22H22Cl3N2BF4Ru
- Crystal structure of 3-(5-methyl-1-p-tolyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazole-4-carbaldehyde, a rare Z′ = 3 structure, C20H17N5O
- Crystal structure of 5-(5-(4-chlorophenyl)-1-phenyl-1H-pyrazol-3-yl)-N-phenyl-1,3,4-thiadiazol-2-amine, C23H16ClN5S
- Crystal structure of 7-hydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one-N,N-dimethylformamide (1/1), C18H17NO5
- Crystal structure of halogen-bonded 2-chloro-1,10-phenanthroline—1,4-diiodotetrafluorobenzene (2/1), C30H14Cl2F4I2N4
- Crystal structure of 1-(4,4-dimethyl-2,6-dithioxo-1,3,5-triazinan-1-yl)-3-(diethylaminocarbonyl)thiourea, C11H20N6OS3
- Crystal structure of methyl 1-(4-fluorobenzyl)-3-phenyl-1H-pyrazole-5-carboxylate, C18H15FN2O2
- Crystal structure of 1,1-dimethyl-3-(4-methylphenyl)urea, C10H14N2O
- Crystal structure of yttrium gallium antimonide, Y5Ga1.24Sb2.77
- Crystal structure of 2-(bis(4-methoxyphenyl)amino)-2-oxoacetic acid, C16H15NO5
Articles in the same Issue
- Cover and Frontmatter
- The crystal structure of triphenylphosphineoxide – 2,5-dichloro-3,6-dihydroxycyclohexa-2,5-diene-1,4-dione (2/1), C42H32Cl2O6P2
- Crystal structure of poly-[diaqua-[bis(μ2-hydroxy)-bis(μ4-3,4,5,6-tetrachlorophthalato-κ3O,O′:O′; κ2O′′:O′′′)dilanthanum(III)], C8H3Cl4LaO6
- Crystal structure of 1,1′-(3,4-diphenylthieno[2,3-b]thiophene-2,5-diyl)bis[1-phenyl-methanone], C32H20O2S2
- Crystal structure of 4a-hydroxy-9-(3,5-dibromo-phenyl)-3,4,4a,5,6,7,9,9a-octahydro-2H-xanthene-1,8-dione, C19H18Br2O4
- Crystal structure of 5-hydroxy-4,6,9,10-tetramethyl-1-oxo-6-vinyldecahydro-3a,9-propanocyclopenta[8]annulen-8-yl 2-((2-methyl-1-(3-methylbenzamido)propan-2-yl)thio)acetate, C34H49NO5S
- Crystal structure of pyridinium bis(naphthalane-2,3-diolato-κ2O,O′)borate monohydrate, C25H20BNO5
- Crystal structure of 1,1′-((1E,1′E)-(((ethane-1,2-diylbis(oxy))bis(2,1-phenylene))bis(azanylylidene))bis(methanylylidene))bis(naphthalen-2-olato)nickel(II), C72H52N4O8Ni2
- The crystal structure of 3-(2-acetyl-4-butyramido-phenoxy)-2-hydroxy-N-isopropylpropan-1-aminium tetraphenylborate, C42H49BN2O4
- Crystal structure of 4-bromobenzyl (Z)-N′-(adamantan-1-yl)-4-phenylpiperazine-1-carbothioimidate, C28H34BrN3S
- Crystal structure of poly-[(μ6-benzene-1,2,4,5-tetracarboxylato)-(μ2-1,2-bis(imidazol-1-ylmethyl)benzene)dicobalt(II)], Co2C24H16N4O8
- Crystal structure of catena-(bis(μ2-1, 2-bis(imidazole-1-ylmethyl)benzene-κN:N′)-dichlororido-nickel(II)), C28H28Cl2N8Ni
- Crystal structure of (Z)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-hydroxy-3-(4-methoxyphenyl)prop-2-en-1-one, C15H16N2O3
- Crystal structure of (Z)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-hydroxy-3-phenylprop-2-en-1-one, C14H14N2O2
- Crystal structure of (E)-2-(4-hydroxy-3-methoxybenzylidene)-6-methoxy-3,4-dihydronaphthalen-1(2H)-one, C19H18O4
- Crystal structure of (Z)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-(4-ethoxyphenyl)-3-hydroxyprop-2-en-1-one, C16H18N2O3
- Crystal structure of (Z)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-hydroxy-3-(p-toly)prop-2-en-1-one, C15H16N2O2
- Crystal structure of 1-acetyl-3-(3-chlorophenyl)-5-(4-isopropylphenyl)-4,5-dihydro-(1H)-pyrazole, C20H21ClN2O
- The crystal structure of 1-methyl-2,4-dinitro-5-iodoimidazole, C4H3IN4O4
- The crystal structure of 4-chloro-3,5-dinitroaniline, C6H4ClN3O4
- Crystal structure of N,N-dimethyl-N′-(2-methyl-4-oxo-5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidin-3(4H)-yl)formimidamide, C14H18N4OS
- Crystal structure of tetrakis(μ2-acetato-κ2O:O′)-bis[μ3-4-chloro-2,6-bis((methylimino)methyl)phenolato-κ2N,O:O,N′]-(μ4-oxido)tetracopper(II), C28H32Cl2Cu4N4O11
- Crystal structure of catena-poly[diaqua-bis(μ2-ethane-1,2-diyl-bis(pyridine-3-carboxylate-κ2N:N′))copper(II)] dinitrate, C28H28CuN6O16
- Synthesis and crystal structure of catena-poly[(μ2-nicotinato-κ2O,O′: κ1N)-(nitrato-κ1O)-(bis(2-benzimidazol-ylmethyl)amine-κ3N,N′,N′′)lead(II)], C22H18N7O5Pb
- The twinned crystal structure of (4SR)-7-benzyl-2,4,8,8-tetramethyl-7,8-dihydroimidazo[5,1-c][1,2,4]triazine-3,6(2H,4H)-dione, C16H20N4O2
- Crystal structure of (Z)-3-hydroxy-3-(4-methoxyphenyl)-1-(pyridin-2-yl)prop-2-en-1-one, C15H13NO3
- Crystal structure of 2-amino-4-(2,3-dichlorophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H12Cl2N2O2
- Crystal structure of catena-poly[(μ2-butane-1,4-diyl-bis(pyridine-3-carboxylato-κN))silver(I)] tetrafluoroborate, C16H16AgN2O4BF4
- Crystal structure of poly[diaqua-(1,10-phenanthroline-κ2N,N′)-(μ2-2,5-dihydroxytere-phthalato)-bis(μ4-2,5-dihydroxyterephthalato)dicerium(III)], C24H16CeN2O10
- Crystal structure of 5,7,4′-trihydroxy-3,8,3′-trymethoxyflavone, C18H16O8
- Crystal structure of N-(3,4-dichlorobenzylidene)-4-methylaniline, C14H11Cl2N
- Crystal structure of 4-(3-Methoxy-phenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylic acid ethyl ester, C22H27NO4
- Crystal structure of 2-amino-4-(3-fluorophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H13FN2O2
- Crystal structure of 1,1,(3,4-dihydroxythieno[2,3-b] thiophene-2,5-diyl)bis(2-bromoethanone), C10H6Br2O4S2
- The crystal structure of N,N′-(4,4′-oxydibenzyl)-bisisonicotinamide 3.5 hydrate, C24H24N4O6
- Crystal structure of catena-poly[hexakis(μ2-chlorido)-hexakis(4-(1H-pyrazol-5-yl)pyridine-κN)tricadmium(II)], Cd3C48H42Cl6N18
- Crystal structure of 2-(4-(dimethylamino)phenyl)-1,3-dimethyl-1H-perimidin-3-ium iodide, C21H22I1N3
- Crystal structure of 4-(1,3-dimethyl-2,3-dihydro-1H-perimidin-2-yl)benzonitrile, C20H17N3
- Crystal structure of tetrakis(μ2-acetato-κ2O:O′)-bis(2,2′-sulfonyldipyrazine-κ1N)dicopper(II), C24H24Cu2N8O12S2
- Crystal structure of 1-(4-chlorophenyl)-6,8-diphenyl-1H-pyrazolo[4,3-c]quinoline, C28H18ClN3
- Crystal structure of methyl 3-((1-(2-(methoxycarbonyl)benzyl)-1H-1,2,3-triazol-4-yl)methoxy)-2-naphthoate, C24H21N3O5
- Crystal structure of (tris(2-pyridylmethyl)amine-κ4N,N′,N′′,N′′′′)-chloranilato-κO,O′-zinc(II) – methanol (1/1), C25H22Cl2N4O5Zn
- Crystal structure of 1,1-dimethyl-3-(4-methoxyphenyl)urea, C10H14N2O2
- Crystal structure of 4a-Hydroxy-9-(2-nitro-phenyl)-3,4,4a,5,6,7,9,9a-octahydro-2H-xanthene-1,8-dione, C19H19NO6
- Crystal structure of chlorido-(η6–1-isopropyl-4-methyl benzene)-(1-(pyridin-2-yl)-N-(p-tolyl)methanimine-κ2N,N′)ruthenium(II) hexafluorophosphate(V), C23H26ClF6N2PRu
- Crystal structure of phenyl(2-phenyl-2,3-dihydro-1H-perimidin-2-yl)methanone, C24H18N2O
- Crystal structure of (E)-3-methyl-4-((3-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)methylene)-1-phenyl-1H-pyrazol-5(4H)-one, C29H23N7O
- Crystal structure of 2-(4-(2-butyl-1,3-dioxo-2,3-dihydro-1H-benzo[de]isoquinolin-6-yl)piperazin-1-yl)-2-oxoethyldiethylcarbamodithioate, C27H34N4O3S2
- Crystal structure of poly-[diaqua-bis(μ-4,4′-bipyridine-κ2N:N′)cobalt(II)] bis(4-chlorobenzenesulfonate) – 4,4′-bipyridine – water (1/1/2), C42H40Cl2CoN6O10S2
- Crystal structure of (η6-benzene)-(N-(2,6-dimethylphenyl)-1-(pyridin-2-yl)methanimine-κ2N,N′)ruthenium(II) perchlorate monohydrate, C20H20Cl2N2O5Ru
- Crystal structure of 4,10,16,22-tetrahydroxy-6,12,18,24-tetramethoxy-2,8,14,20-tetraethylphenylresorcin[4]arene – ethyl acetate (1/1), C68H72O10
- Crystal structure of chlorido-(N-(2,5-dichlorophenyl)-1-(pyridin-2-yl)methanimine-κ2N,N′)(η6-1-isopropyl-4-methyl benzene) ruthenium (II) tetrafluoroborate, C22H22Cl3N2BF4Ru
- Crystal structure of 3-(5-methyl-1-p-tolyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazole-4-carbaldehyde, a rare Z′ = 3 structure, C20H17N5O
- Crystal structure of 5-(5-(4-chlorophenyl)-1-phenyl-1H-pyrazol-3-yl)-N-phenyl-1,3,4-thiadiazol-2-amine, C23H16ClN5S
- Crystal structure of 7-hydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one-N,N-dimethylformamide (1/1), C18H17NO5
- Crystal structure of halogen-bonded 2-chloro-1,10-phenanthroline—1,4-diiodotetrafluorobenzene (2/1), C30H14Cl2F4I2N4
- Crystal structure of 1-(4,4-dimethyl-2,6-dithioxo-1,3,5-triazinan-1-yl)-3-(diethylaminocarbonyl)thiourea, C11H20N6OS3
- Crystal structure of methyl 1-(4-fluorobenzyl)-3-phenyl-1H-pyrazole-5-carboxylate, C18H15FN2O2
- Crystal structure of 1,1-dimethyl-3-(4-methylphenyl)urea, C10H14N2O
- Crystal structure of yttrium gallium antimonide, Y5Ga1.24Sb2.77
- Crystal structure of 2-(bis(4-methoxyphenyl)amino)-2-oxoacetic acid, C16H15NO5

