Abstract
C11H20N6OS3, monoclinic, P21/c (no. 14), a = 10.0196(17) Å, b = 13.416(2) Å, c = 23.441(5) Å, β = 100.658(5)°, V = 1752.7(5) Å3, Z = 4, Rgt(F) = 0.0558, wRref(F2) = 0.1462, T = 303(2) K.
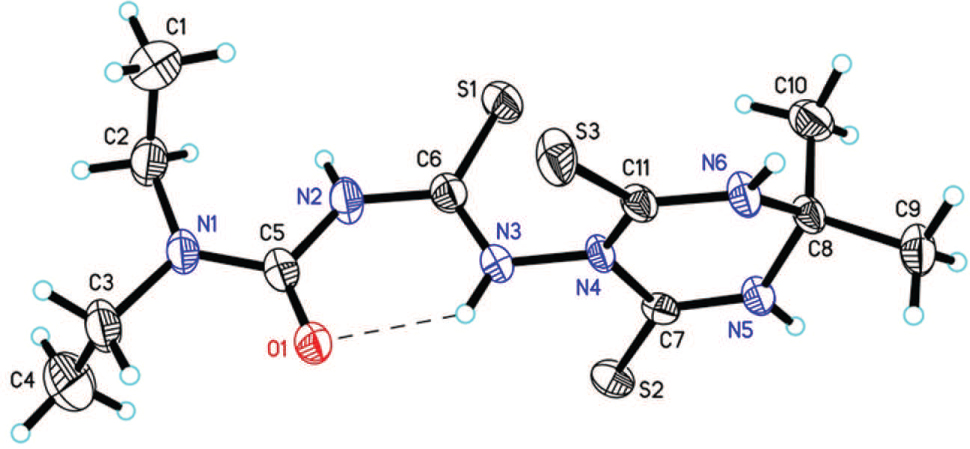
The crystal structure is shown in the figure. Tables 1 and 2 contain details on crystal structure and measurement conditions and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.60 × 0.39 × 0.16 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 4.3 cm−1 |
| Diffractometer, scan mode: | Bruker SMART, ω−scans |
| 2θmax, completeness: | 52°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 49159, 3452, 0.119 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 2162 |
| N(param)refined: | 210 |
| Programs: | Bruker programs [9], SHELX [10], PLATON [11] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| S1 | 0.09032(11) | 0.12038(8) | 0.59108(9) | 0.0640(3) |
| S2 | 0.35778(11) | 0.06218(8) | 0.39340(7) | 0.0529(3) |
| S3 | 0.44664(13) | 0.10000(8) | 0.79953(7) | 0.0647(4) |
| O1 | 0.3125(3) | −0.17590(18) | 0.64806(19) | 0.0494(6) |
| N1 | 0.1076(3) | −0.2347(2) | 0.6740(3) | 0.0547(9) |
| N2 | 0.1288(3) | −0.0707(2) | 0.6277(3) | 0.0544(9) |
| N3 | 0.3178(3) | 0.0167(2) | 0.6034(2) | 0.0426(7) |
| N4 | 0.3827(3) | 0.10545(19) | 0.59415(19) | 0.0387(7) |
| N5 | 0.4713(3) | 0.2186(2) | 0.4953(2) | 0.0426(7) |
| N6 | 0.5076(3) | 0.2364(2) | 0.6735(2) | 0.0443(8) |
| C1 | −0.0329(6) | −0.1572(4) | 0.7885(5) | 0.1051(19) |
| H1A | 0.0181 | −0.1927 | 0.8457 | 0.158* |
| H1B | −0.1251 | −0.1497 | 0.7979 | 0.158* |
| H1C | 0.0067 | −0.0926 | 0.7837 | 0.158* |
| C2 | −0.0305(4) | −0.2149(3) | 0.6909(4) | 0.0695(13) |
| H2A | −0.0773 | −0.2779 | 0.6939 | 0.083* |
| H2B | −0.0794 | −0.1775 | 0.6331 | 0.083* |
| C3 | 0.1565(5) | −0.3372(3) | 0.6890(4) | 0.0714(13) |
| H3A | 0.1323 | −0.3637 | 0.7512 | 0.086* |
| H3B | 0.2547 | −0.3377 | 0.6973 | 0.086* |
| C4 | 0.0971(7) | −0.4030(4) | 0.5997(5) | 0.119(2) |
| H4A | 0.0005 | −0.4073 | 0.5949 | 0.178* |
| H4B | 0.1361 | −0.4685 | 0.6100 | 0.178* |
| H4C | 0.1170 | −0.3752 | 0.5375 | 0.178* |
| C5 | 0.1911(4) | −0.1634(3) | 0.6497(3) | 0.0455(9) |
| C6 | 0.1863(4) | 0.0188(3) | 0.6077(3) | 0.0459(9) |
| C7 | 0.4065(3) | 0.1329(2) | 0.4963(2) | 0.0376(8) |
| C8 | 0.4898(4) | 0.2929(2) | 0.5775(2) | 0.0409(8) |
| C9 | 0.6180(4) | 0.3513(3) | 0.5755(3) | 0.0577(11) |
| H9A | 0.6932 | 0.3062 | 0.5796 | 0.087* |
| H9B | 0.6344 | 0.3962 | 0.6328 | 0.087* |
| H9C | 0.6080 | 0.3887 | 0.5129 | 0.087* |
| C10 | 0.3635(5) | 0.3587(3) | 0.5676(3) | 0.0642(12) |
| H10A | 0.3516 | 0.3948 | 0.5041 | 0.096* |
| H10B | 0.3743 | 0.4049 | 0.6237 | 0.096* |
| H10C | 0.2853 | 0.3177 | 0.5687 | 0.096* |
| C11 | 0.4464(4) | 0.1516(2) | 0.6858(2) | 0.0407(8) |
| H2 | 0.0408(12) | −0.065(3) | 0.620(4) | 0.086(16)* |
| H3 | 0.366(3) | −0.0375(17) | 0.614(3) | 0.061(12)* |
| H5 | 0.487(4) | 0.239(3) | 0.4371(16) | 0.072(14)* |
| H6 | 0.550(3) | 0.265(3) | 0.7288(18) | 0.065(13)* |
Source of material
A mixture of diethylcarbanoyl chloride (1.36 g, 0.01 mol) and ammonium thiocyanate (0.76 g, 0.01 mol) in acetone (40 mL) was heated under reflux for 3 h [1]. The mixture was cooled to room temperature and filtered off. A solution of thiosemicarbazide (0.91 g, 0.01 mol) in acetone (20 mL) was added to the filtrate and the mixture was heated under reflux for 2 h. The solid obtained on cooling was filtered off to give title compound in 83% yield (Mp.: 220–221 °C) as colourless crystals.
Experimental details
Carbon-bound H atoms were placed in calculated positions (C—H 0.95 Å) and were included in the refinement in the riding model approximation, with Uiso(H) set to 1.2Ueq(C). The H atoms of the methyl group were allowed to rotate with a fixed angle around the C—C bond to best fit the experimental electron density (HFIX 137 in the SHELX [10]), with Uiso(H) set to 1.5Ueq(C).
Discussion
Various 1,3,5-triazine derivatives have been synthesised and show interesting properties [2], [3], [4], [5], [6], [7]. The molecular structure of the title compound indicates that acetone has participated in the reaction and subsequently, the cyclization with the amine group of thiosemicarbazide resulted in the formation of 1,3,5-triazinyl moiety. The S1/O1/N1/N2/N3/C3/C5/C6 fragment is planar with a maximum deviation of 0.051(4) Å for N1 atom from the least square plane (cf. the figure). This fragment forms a dihedral angle of 88.32(14)° with the six-membered heterocyclic ring (N4/N5/N6/C7/C8/C11). The bond lengths and bond angles are in normal ranges and comparable to those reported in literature for 4,6-bis(nitroimino)-1,3,5-triazinan-2-one [8]. There is an intramolecular hydrogen bond (N3—H3⋯O1) to form the six membered N3/H3/O1/C5/N2/C6 ring. In the crystal molecules are linked by N3—H3⋯S2, N5—H5⋯S3 and N6—H6⋯O1 intermolecular hydrogen bonds to form one-dimensional chains along the c axis.
Acknowledgement
The authors would like to thank Universiti Kebangsaan Malaysia for research grants (GUP-2015-020 and FRGS/2/2013/ST01/UKM/01/2) and to Diyala and Al-Nahrain Universities for continued support. Also, the authors extend their appreciation to the Deanship of Scientific Research at King Saud University for its funding for this research through the research group project RGP-239.
References
1 Spurlock, L. A.; Newallis, P. E.: The reactions of carbamoyl chlorides with thiocyanate Ion. J. Org. Chem. 33 (1968) 2073–2076.10.1021/jo01269a078Search in Google Scholar
2 Pin, J.-M.; Sbirrazzuoli, N.; Sacarescu, L.; Mija, A.: Star-epoxy mesogen with 1,3,5-triazine core: a model of A4B3 fractal polymerization in a liquid crystalline thermoset media. Polym. Chem. 7 (2016) 1221–1225.10.1039/C5PY01884FSearch in Google Scholar
3 Balaha, M. F.; El-Hamamsy, M. H.; Sharaf El-Din, N. A.; El-Mahdy, N. A.: Synthesis, evaluation and docking study of 1, 3, 5-triazine derivatives as cytotoxic agents against lung cancer. J. Appl. Pharm. Sci. 6 (2016) 28–45.10.7324/JAPS.2016.60405Search in Google Scholar
4 Banerjee, R.; Pace, N. J.; Brown, D. R.; Weerapana, E.: 1,3,5-Triazine as a modular scaffold for covalent inhibitors with streamlined target identification. J. Am. Chem. Soc. 135 (2013) 2497–2500.10.1021/ja400427eSearch in Google Scholar PubMed
5 Blotny, G.: Recent applications of 2,4,6-trichloro-1,3,5-triazine and its derivatives in organic synthesis. Tetrahedron 62 (2006) 9507–9522.10.1016/j.tet.2006.07.039Search in Google Scholar
6 Wang, Q.; Liu, G.; Shao, R.; Huang, R.: Synthesis and antivirus activity of 1,3,5-triazine derivatives. Heteroat. Chem. 14 (2003) 542–545.10.1002/hc.10189Search in Google Scholar
7 Oh, B.-T.; Just, C. L.; Alvarez, P. J. J.: Hexahydro-1,3,5-trinitro-1,3,5-triazine mineralization by zerovalent iron and mixed anaerobic cultures. Environ. Sci. Technol. 35 (2001) 4341–4346.10.1021/es010852eSearch in Google Scholar PubMed
8 Simöes, P. N.; Pedroso, L. M.; Matos Beja, A. M.: Ramos Silva, M.; MacLean, E.; Portugal, A. A.: Crystal and molecular structure of 4,6-bis(nitroimino)-1,3,5-triazinan-2-one: theoretical and x-ray studies. J. Phys. Chem. A111 (2007) 150–158.10.1021/jp064473pSearch in Google Scholar PubMed
9 Bruker: SMART, SAINT and SADABS. Bruker AXS Inc., Madison, Wisconsin, USA (2009).Search in Google Scholar
10 Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Search in Google Scholar PubMed
11 Spek, A. L.: Structure validation in chemical crystallography. Acta Crystallogr. D65 (2009) 148–155.10.1107/S090744490804362XSearch in Google Scholar PubMed PubMed Central
©2017 Nasry Jassim Hussien et al., published by De Gruyter.
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Articles in the same Issue
- Cover and Frontmatter
- The crystal structure of triphenylphosphineoxide – 2,5-dichloro-3,6-dihydroxycyclohexa-2,5-diene-1,4-dione (2/1), C42H32Cl2O6P2
- Crystal structure of poly-[diaqua-[bis(μ2-hydroxy)-bis(μ4-3,4,5,6-tetrachlorophthalato-κ3O,O′:O′; κ2O′′:O′′′)dilanthanum(III)], C8H3Cl4LaO6
- Crystal structure of 1,1′-(3,4-diphenylthieno[2,3-b]thiophene-2,5-diyl)bis[1-phenyl-methanone], C32H20O2S2
- Crystal structure of 4a-hydroxy-9-(3,5-dibromo-phenyl)-3,4,4a,5,6,7,9,9a-octahydro-2H-xanthene-1,8-dione, C19H18Br2O4
- Crystal structure of 5-hydroxy-4,6,9,10-tetramethyl-1-oxo-6-vinyldecahydro-3a,9-propanocyclopenta[8]annulen-8-yl 2-((2-methyl-1-(3-methylbenzamido)propan-2-yl)thio)acetate, C34H49NO5S
- Crystal structure of pyridinium bis(naphthalane-2,3-diolato-κ2O,O′)borate monohydrate, C25H20BNO5
- Crystal structure of 1,1′-((1E,1′E)-(((ethane-1,2-diylbis(oxy))bis(2,1-phenylene))bis(azanylylidene))bis(methanylylidene))bis(naphthalen-2-olato)nickel(II), C72H52N4O8Ni2
- The crystal structure of 3-(2-acetyl-4-butyramido-phenoxy)-2-hydroxy-N-isopropylpropan-1-aminium tetraphenylborate, C42H49BN2O4
- Crystal structure of 4-bromobenzyl (Z)-N′-(adamantan-1-yl)-4-phenylpiperazine-1-carbothioimidate, C28H34BrN3S
- Crystal structure of poly-[(μ6-benzene-1,2,4,5-tetracarboxylato)-(μ2-1,2-bis(imidazol-1-ylmethyl)benzene)dicobalt(II)], Co2C24H16N4O8
- Crystal structure of catena-(bis(μ2-1, 2-bis(imidazole-1-ylmethyl)benzene-κN:N′)-dichlororido-nickel(II)), C28H28Cl2N8Ni
- Crystal structure of (Z)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-hydroxy-3-(4-methoxyphenyl)prop-2-en-1-one, C15H16N2O3
- Crystal structure of (Z)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-hydroxy-3-phenylprop-2-en-1-one, C14H14N2O2
- Crystal structure of (E)-2-(4-hydroxy-3-methoxybenzylidene)-6-methoxy-3,4-dihydronaphthalen-1(2H)-one, C19H18O4
- Crystal structure of (Z)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-(4-ethoxyphenyl)-3-hydroxyprop-2-en-1-one, C16H18N2O3
- Crystal structure of (Z)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-hydroxy-3-(p-toly)prop-2-en-1-one, C15H16N2O2
- Crystal structure of 1-acetyl-3-(3-chlorophenyl)-5-(4-isopropylphenyl)-4,5-dihydro-(1H)-pyrazole, C20H21ClN2O
- The crystal structure of 1-methyl-2,4-dinitro-5-iodoimidazole, C4H3IN4O4
- The crystal structure of 4-chloro-3,5-dinitroaniline, C6H4ClN3O4
- Crystal structure of N,N-dimethyl-N′-(2-methyl-4-oxo-5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidin-3(4H)-yl)formimidamide, C14H18N4OS
- Crystal structure of tetrakis(μ2-acetato-κ2O:O′)-bis[μ3-4-chloro-2,6-bis((methylimino)methyl)phenolato-κ2N,O:O,N′]-(μ4-oxido)tetracopper(II), C28H32Cl2Cu4N4O11
- Crystal structure of catena-poly[diaqua-bis(μ2-ethane-1,2-diyl-bis(pyridine-3-carboxylate-κ2N:N′))copper(II)] dinitrate, C28H28CuN6O16
- Synthesis and crystal structure of catena-poly[(μ2-nicotinato-κ2O,O′: κ1N)-(nitrato-κ1O)-(bis(2-benzimidazol-ylmethyl)amine-κ3N,N′,N′′)lead(II)], C22H18N7O5Pb
- The twinned crystal structure of (4SR)-7-benzyl-2,4,8,8-tetramethyl-7,8-dihydroimidazo[5,1-c][1,2,4]triazine-3,6(2H,4H)-dione, C16H20N4O2
- Crystal structure of (Z)-3-hydroxy-3-(4-methoxyphenyl)-1-(pyridin-2-yl)prop-2-en-1-one, C15H13NO3
- Crystal structure of 2-amino-4-(2,3-dichlorophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H12Cl2N2O2
- Crystal structure of catena-poly[(μ2-butane-1,4-diyl-bis(pyridine-3-carboxylato-κN))silver(I)] tetrafluoroborate, C16H16AgN2O4BF4
- Crystal structure of poly[diaqua-(1,10-phenanthroline-κ2N,N′)-(μ2-2,5-dihydroxytere-phthalato)-bis(μ4-2,5-dihydroxyterephthalato)dicerium(III)], C24H16CeN2O10
- Crystal structure of 5,7,4′-trihydroxy-3,8,3′-trymethoxyflavone, C18H16O8
- Crystal structure of N-(3,4-dichlorobenzylidene)-4-methylaniline, C14H11Cl2N
- Crystal structure of 4-(3-Methoxy-phenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylic acid ethyl ester, C22H27NO4
- Crystal structure of 2-amino-4-(3-fluorophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H13FN2O2
- Crystal structure of 1,1,(3,4-dihydroxythieno[2,3-b] thiophene-2,5-diyl)bis(2-bromoethanone), C10H6Br2O4S2
- The crystal structure of N,N′-(4,4′-oxydibenzyl)-bisisonicotinamide 3.5 hydrate, C24H24N4O6
- Crystal structure of catena-poly[hexakis(μ2-chlorido)-hexakis(4-(1H-pyrazol-5-yl)pyridine-κN)tricadmium(II)], Cd3C48H42Cl6N18
- Crystal structure of 2-(4-(dimethylamino)phenyl)-1,3-dimethyl-1H-perimidin-3-ium iodide, C21H22I1N3
- Crystal structure of 4-(1,3-dimethyl-2,3-dihydro-1H-perimidin-2-yl)benzonitrile, C20H17N3
- Crystal structure of tetrakis(μ2-acetato-κ2O:O′)-bis(2,2′-sulfonyldipyrazine-κ1N)dicopper(II), C24H24Cu2N8O12S2
- Crystal structure of 1-(4-chlorophenyl)-6,8-diphenyl-1H-pyrazolo[4,3-c]quinoline, C28H18ClN3
- Crystal structure of methyl 3-((1-(2-(methoxycarbonyl)benzyl)-1H-1,2,3-triazol-4-yl)methoxy)-2-naphthoate, C24H21N3O5
- Crystal structure of (tris(2-pyridylmethyl)amine-κ4N,N′,N′′,N′′′′)-chloranilato-κO,O′-zinc(II) – methanol (1/1), C25H22Cl2N4O5Zn
- Crystal structure of 1,1-dimethyl-3-(4-methoxyphenyl)urea, C10H14N2O2
- Crystal structure of 4a-Hydroxy-9-(2-nitro-phenyl)-3,4,4a,5,6,7,9,9a-octahydro-2H-xanthene-1,8-dione, C19H19NO6
- Crystal structure of chlorido-(η6–1-isopropyl-4-methyl benzene)-(1-(pyridin-2-yl)-N-(p-tolyl)methanimine-κ2N,N′)ruthenium(II) hexafluorophosphate(V), C23H26ClF6N2PRu
- Crystal structure of phenyl(2-phenyl-2,3-dihydro-1H-perimidin-2-yl)methanone, C24H18N2O
- Crystal structure of (E)-3-methyl-4-((3-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)methylene)-1-phenyl-1H-pyrazol-5(4H)-one, C29H23N7O
- Crystal structure of 2-(4-(2-butyl-1,3-dioxo-2,3-dihydro-1H-benzo[de]isoquinolin-6-yl)piperazin-1-yl)-2-oxoethyldiethylcarbamodithioate, C27H34N4O3S2
- Crystal structure of poly-[diaqua-bis(μ-4,4′-bipyridine-κ2N:N′)cobalt(II)] bis(4-chlorobenzenesulfonate) – 4,4′-bipyridine – water (1/1/2), C42H40Cl2CoN6O10S2
- Crystal structure of (η6-benzene)-(N-(2,6-dimethylphenyl)-1-(pyridin-2-yl)methanimine-κ2N,N′)ruthenium(II) perchlorate monohydrate, C20H20Cl2N2O5Ru
- Crystal structure of 4,10,16,22-tetrahydroxy-6,12,18,24-tetramethoxy-2,8,14,20-tetraethylphenylresorcin[4]arene – ethyl acetate (1/1), C68H72O10
- Crystal structure of chlorido-(N-(2,5-dichlorophenyl)-1-(pyridin-2-yl)methanimine-κ2N,N′)(η6-1-isopropyl-4-methyl benzene) ruthenium (II) tetrafluoroborate, C22H22Cl3N2BF4Ru
- Crystal structure of 3-(5-methyl-1-p-tolyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazole-4-carbaldehyde, a rare Z′ = 3 structure, C20H17N5O
- Crystal structure of 5-(5-(4-chlorophenyl)-1-phenyl-1H-pyrazol-3-yl)-N-phenyl-1,3,4-thiadiazol-2-amine, C23H16ClN5S
- Crystal structure of 7-hydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one-N,N-dimethylformamide (1/1), C18H17NO5
- Crystal structure of halogen-bonded 2-chloro-1,10-phenanthroline—1,4-diiodotetrafluorobenzene (2/1), C30H14Cl2F4I2N4
- Crystal structure of 1-(4,4-dimethyl-2,6-dithioxo-1,3,5-triazinan-1-yl)-3-(diethylaminocarbonyl)thiourea, C11H20N6OS3
- Crystal structure of methyl 1-(4-fluorobenzyl)-3-phenyl-1H-pyrazole-5-carboxylate, C18H15FN2O2
- Crystal structure of 1,1-dimethyl-3-(4-methylphenyl)urea, C10H14N2O
- Crystal structure of yttrium gallium antimonide, Y5Ga1.24Sb2.77
- Crystal structure of 2-(bis(4-methoxyphenyl)amino)-2-oxoacetic acid, C16H15NO5
Articles in the same Issue
- Cover and Frontmatter
- The crystal structure of triphenylphosphineoxide – 2,5-dichloro-3,6-dihydroxycyclohexa-2,5-diene-1,4-dione (2/1), C42H32Cl2O6P2
- Crystal structure of poly-[diaqua-[bis(μ2-hydroxy)-bis(μ4-3,4,5,6-tetrachlorophthalato-κ3O,O′:O′; κ2O′′:O′′′)dilanthanum(III)], C8H3Cl4LaO6
- Crystal structure of 1,1′-(3,4-diphenylthieno[2,3-b]thiophene-2,5-diyl)bis[1-phenyl-methanone], C32H20O2S2
- Crystal structure of 4a-hydroxy-9-(3,5-dibromo-phenyl)-3,4,4a,5,6,7,9,9a-octahydro-2H-xanthene-1,8-dione, C19H18Br2O4
- Crystal structure of 5-hydroxy-4,6,9,10-tetramethyl-1-oxo-6-vinyldecahydro-3a,9-propanocyclopenta[8]annulen-8-yl 2-((2-methyl-1-(3-methylbenzamido)propan-2-yl)thio)acetate, C34H49NO5S
- Crystal structure of pyridinium bis(naphthalane-2,3-diolato-κ2O,O′)borate monohydrate, C25H20BNO5
- Crystal structure of 1,1′-((1E,1′E)-(((ethane-1,2-diylbis(oxy))bis(2,1-phenylene))bis(azanylylidene))bis(methanylylidene))bis(naphthalen-2-olato)nickel(II), C72H52N4O8Ni2
- The crystal structure of 3-(2-acetyl-4-butyramido-phenoxy)-2-hydroxy-N-isopropylpropan-1-aminium tetraphenylborate, C42H49BN2O4
- Crystal structure of 4-bromobenzyl (Z)-N′-(adamantan-1-yl)-4-phenylpiperazine-1-carbothioimidate, C28H34BrN3S
- Crystal structure of poly-[(μ6-benzene-1,2,4,5-tetracarboxylato)-(μ2-1,2-bis(imidazol-1-ylmethyl)benzene)dicobalt(II)], Co2C24H16N4O8
- Crystal structure of catena-(bis(μ2-1, 2-bis(imidazole-1-ylmethyl)benzene-κN:N′)-dichlororido-nickel(II)), C28H28Cl2N8Ni
- Crystal structure of (Z)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-hydroxy-3-(4-methoxyphenyl)prop-2-en-1-one, C15H16N2O3
- Crystal structure of (Z)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-hydroxy-3-phenylprop-2-en-1-one, C14H14N2O2
- Crystal structure of (E)-2-(4-hydroxy-3-methoxybenzylidene)-6-methoxy-3,4-dihydronaphthalen-1(2H)-one, C19H18O4
- Crystal structure of (Z)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-(4-ethoxyphenyl)-3-hydroxyprop-2-en-1-one, C16H18N2O3
- Crystal structure of (Z)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-hydroxy-3-(p-toly)prop-2-en-1-one, C15H16N2O2
- Crystal structure of 1-acetyl-3-(3-chlorophenyl)-5-(4-isopropylphenyl)-4,5-dihydro-(1H)-pyrazole, C20H21ClN2O
- The crystal structure of 1-methyl-2,4-dinitro-5-iodoimidazole, C4H3IN4O4
- The crystal structure of 4-chloro-3,5-dinitroaniline, C6H4ClN3O4
- Crystal structure of N,N-dimethyl-N′-(2-methyl-4-oxo-5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidin-3(4H)-yl)formimidamide, C14H18N4OS
- Crystal structure of tetrakis(μ2-acetato-κ2O:O′)-bis[μ3-4-chloro-2,6-bis((methylimino)methyl)phenolato-κ2N,O:O,N′]-(μ4-oxido)tetracopper(II), C28H32Cl2Cu4N4O11
- Crystal structure of catena-poly[diaqua-bis(μ2-ethane-1,2-diyl-bis(pyridine-3-carboxylate-κ2N:N′))copper(II)] dinitrate, C28H28CuN6O16
- Synthesis and crystal structure of catena-poly[(μ2-nicotinato-κ2O,O′: κ1N)-(nitrato-κ1O)-(bis(2-benzimidazol-ylmethyl)amine-κ3N,N′,N′′)lead(II)], C22H18N7O5Pb
- The twinned crystal structure of (4SR)-7-benzyl-2,4,8,8-tetramethyl-7,8-dihydroimidazo[5,1-c][1,2,4]triazine-3,6(2H,4H)-dione, C16H20N4O2
- Crystal structure of (Z)-3-hydroxy-3-(4-methoxyphenyl)-1-(pyridin-2-yl)prop-2-en-1-one, C15H13NO3
- Crystal structure of 2-amino-4-(2,3-dichlorophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H12Cl2N2O2
- Crystal structure of catena-poly[(μ2-butane-1,4-diyl-bis(pyridine-3-carboxylato-κN))silver(I)] tetrafluoroborate, C16H16AgN2O4BF4
- Crystal structure of poly[diaqua-(1,10-phenanthroline-κ2N,N′)-(μ2-2,5-dihydroxytere-phthalato)-bis(μ4-2,5-dihydroxyterephthalato)dicerium(III)], C24H16CeN2O10
- Crystal structure of 5,7,4′-trihydroxy-3,8,3′-trymethoxyflavone, C18H16O8
- Crystal structure of N-(3,4-dichlorobenzylidene)-4-methylaniline, C14H11Cl2N
- Crystal structure of 4-(3-Methoxy-phenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylic acid ethyl ester, C22H27NO4
- Crystal structure of 2-amino-4-(3-fluorophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H13FN2O2
- Crystal structure of 1,1,(3,4-dihydroxythieno[2,3-b] thiophene-2,5-diyl)bis(2-bromoethanone), C10H6Br2O4S2
- The crystal structure of N,N′-(4,4′-oxydibenzyl)-bisisonicotinamide 3.5 hydrate, C24H24N4O6
- Crystal structure of catena-poly[hexakis(μ2-chlorido)-hexakis(4-(1H-pyrazol-5-yl)pyridine-κN)tricadmium(II)], Cd3C48H42Cl6N18
- Crystal structure of 2-(4-(dimethylamino)phenyl)-1,3-dimethyl-1H-perimidin-3-ium iodide, C21H22I1N3
- Crystal structure of 4-(1,3-dimethyl-2,3-dihydro-1H-perimidin-2-yl)benzonitrile, C20H17N3
- Crystal structure of tetrakis(μ2-acetato-κ2O:O′)-bis(2,2′-sulfonyldipyrazine-κ1N)dicopper(II), C24H24Cu2N8O12S2
- Crystal structure of 1-(4-chlorophenyl)-6,8-diphenyl-1H-pyrazolo[4,3-c]quinoline, C28H18ClN3
- Crystal structure of methyl 3-((1-(2-(methoxycarbonyl)benzyl)-1H-1,2,3-triazol-4-yl)methoxy)-2-naphthoate, C24H21N3O5
- Crystal structure of (tris(2-pyridylmethyl)amine-κ4N,N′,N′′,N′′′′)-chloranilato-κO,O′-zinc(II) – methanol (1/1), C25H22Cl2N4O5Zn
- Crystal structure of 1,1-dimethyl-3-(4-methoxyphenyl)urea, C10H14N2O2
- Crystal structure of 4a-Hydroxy-9-(2-nitro-phenyl)-3,4,4a,5,6,7,9,9a-octahydro-2H-xanthene-1,8-dione, C19H19NO6
- Crystal structure of chlorido-(η6–1-isopropyl-4-methyl benzene)-(1-(pyridin-2-yl)-N-(p-tolyl)methanimine-κ2N,N′)ruthenium(II) hexafluorophosphate(V), C23H26ClF6N2PRu
- Crystal structure of phenyl(2-phenyl-2,3-dihydro-1H-perimidin-2-yl)methanone, C24H18N2O
- Crystal structure of (E)-3-methyl-4-((3-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)methylene)-1-phenyl-1H-pyrazol-5(4H)-one, C29H23N7O
- Crystal structure of 2-(4-(2-butyl-1,3-dioxo-2,3-dihydro-1H-benzo[de]isoquinolin-6-yl)piperazin-1-yl)-2-oxoethyldiethylcarbamodithioate, C27H34N4O3S2
- Crystal structure of poly-[diaqua-bis(μ-4,4′-bipyridine-κ2N:N′)cobalt(II)] bis(4-chlorobenzenesulfonate) – 4,4′-bipyridine – water (1/1/2), C42H40Cl2CoN6O10S2
- Crystal structure of (η6-benzene)-(N-(2,6-dimethylphenyl)-1-(pyridin-2-yl)methanimine-κ2N,N′)ruthenium(II) perchlorate monohydrate, C20H20Cl2N2O5Ru
- Crystal structure of 4,10,16,22-tetrahydroxy-6,12,18,24-tetramethoxy-2,8,14,20-tetraethylphenylresorcin[4]arene – ethyl acetate (1/1), C68H72O10
- Crystal structure of chlorido-(N-(2,5-dichlorophenyl)-1-(pyridin-2-yl)methanimine-κ2N,N′)(η6-1-isopropyl-4-methyl benzene) ruthenium (II) tetrafluoroborate, C22H22Cl3N2BF4Ru
- Crystal structure of 3-(5-methyl-1-p-tolyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazole-4-carbaldehyde, a rare Z′ = 3 structure, C20H17N5O
- Crystal structure of 5-(5-(4-chlorophenyl)-1-phenyl-1H-pyrazol-3-yl)-N-phenyl-1,3,4-thiadiazol-2-amine, C23H16ClN5S
- Crystal structure of 7-hydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one-N,N-dimethylformamide (1/1), C18H17NO5
- Crystal structure of halogen-bonded 2-chloro-1,10-phenanthroline—1,4-diiodotetrafluorobenzene (2/1), C30H14Cl2F4I2N4
- Crystal structure of 1-(4,4-dimethyl-2,6-dithioxo-1,3,5-triazinan-1-yl)-3-(diethylaminocarbonyl)thiourea, C11H20N6OS3
- Crystal structure of methyl 1-(4-fluorobenzyl)-3-phenyl-1H-pyrazole-5-carboxylate, C18H15FN2O2
- Crystal structure of 1,1-dimethyl-3-(4-methylphenyl)urea, C10H14N2O
- Crystal structure of yttrium gallium antimonide, Y5Ga1.24Sb2.77
- Crystal structure of 2-(bis(4-methoxyphenyl)amino)-2-oxoacetic acid, C16H15NO5

