Abstract
C19H18Br2O4, monoclinic, P21/n (no. 14), a = 14.323(3) Å, b = 7.6704(17) Å, c = 17.112(4) Å, β = 111.948(4)°, V = 1743.7(7) Å3, Z = 4, Rgt(F) = 0.0530, wRref(F2) = 0.1219, T = 296(2) K.
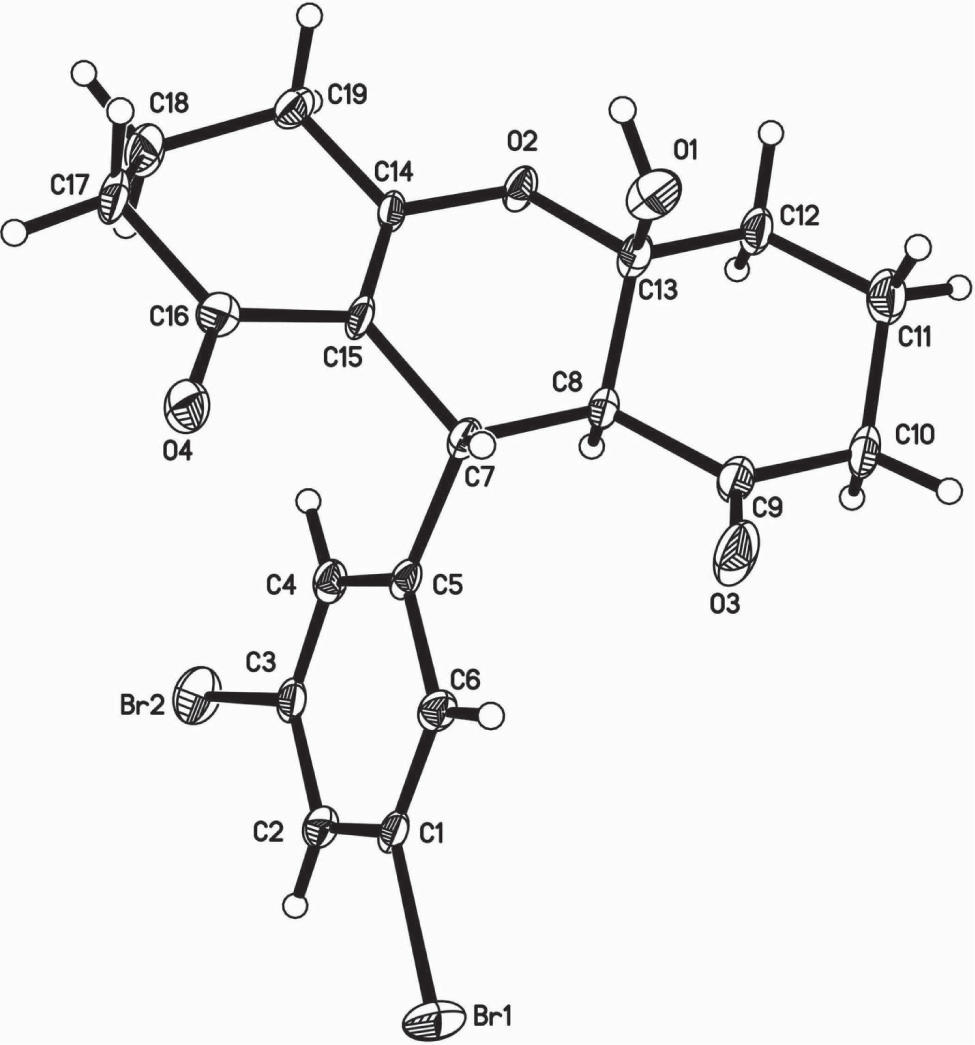
The crystal structure is shown in the figure. Tables 1 and 2 contain details on crystal structure and measurement conditions and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.29 × 0.25 × 0.21 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 46.7 cm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| 2θmax, completeness: | 50°, >95% |
| N(hkl)measured, N(hkl)unique, Rint: | 8516, 2915, 0.062 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 2027 |
| N(param)refined: | 226 |
| Programs: | Bruker programs [6], SHELX [7] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| Br1 | 1.00650(5) | 0.81925(8) | 0.06462(4) | 0.0470(2) |
| Br2 | 1.23178(5) | 0.21928(9) | 0.19884(4) | 0.0482(2) |
| O1 | 0.6541(3) | 0.0622(5) | 0.0879(2) | 0.0410(10) |
| H1A | 0.6417 | −0.0062 | 0.1196 | 0.061* |
| O2 | 0.7948(3) | −0.1207(4) | 0.1317(2) | 0.0285(9) |
| O3 | 0.7062(3) | 0.4095(5) | −0.0087(2) | 0.0522(12) |
| O4 | 0.9010(3) | 0.3606(5) | 0.3078(2) | 0.0379(10) |
| C1 | 1.0118(4) | 0.5872(6) | 0.1046(3) | 0.0271(13) |
| C2 | 1.1035(4) | 0.5026(7) | 0.1288(3) | 0.0292(13) |
| H2A | 1.1598 | 0.5547 | 0.1239 | 0.035* |
| C3 | 1.1068(4) | 0.3366(7) | 0.1606(3) | 0.0277(13) |
| C4 | 1.0249(4) | 0.2575(7) | 0.1664(3) | 0.0270(13) |
| H4A | 1.0304 | 0.1452 | 0.1882 | 0.032* |
| C5 | 0.9334(4) | 0.3432(6) | 0.1402(3) | 0.0222(12) |
| C6 | 0.9283(4) | 0.5132(6) | 0.1099(3) | 0.0267(13) |
| H6A | 0.8683 | 0.5753 | 0.0935 | 0.032* |
| C7 | 0.8390(4) | 0.2519(6) | 0.1398(3) | 0.0232(12) |
| H7A | 0.7913 | 0.3399 | 0.1437 | 0.028* |
| C8 | 0.7906(4) | 0.1511(7) | 0.0580(3) | 0.0270(13) |
| H8A | 0.8457 | 0.1084 | 0.0424 | 0.032* |
| C9 | 0.7230(4) | 0.2596(8) | −0.0158(3) | 0.0315(14) |
| C10 | 0.6782(5) | 0.1608(7) | −0.0964(3) | 0.0389(15) |
| H10A | 0.7309 | 0.1302 | −0.1166 | 0.047* |
| H10B | 0.6302 | 0.2346 | −0.1384 | 0.047* |
| C11 | 0.6258(5) | −0.0039(7) | −0.0856(3) | 0.0413(15) |
| H11A | 0.6054 | −0.0715 | −0.1372 | 0.050* |
| H11B | 0.5655 | 0.0274 | −0.0756 | 0.050* |
| C12 | 0.6933(5) | −0.1150(7) | −0.0127(3) | 0.0330(14) |
| H12A | 0.6554 | −0.2128 | −0.0039 | 0.040* |
| H12B | 0.7490 | −0.1606 | −0.0257 | 0.040* |
| C13 | 0.7326(4) | −0.0058(7) | 0.0654(3) | 0.0284(13) |
| C14 | 0.8474(4) | −0.0434(6) | 0.2061(3) | 0.0225(12) |
| C15 | 0.8636(4) | 0.1273(7) | 0.2143(3) | 0.0256(13) |
| C16 | 0.8988(4) | 0.2017(7) | 0.2986(3) | 0.0318(14) |
| C17 | 0.9251(5) | 0.0807(8) | 0.3723(3) | 0.0417(16) |
| H17A | 0.9784 | 0.1322 | 0.4201 | 0.050* |
| H17B | 0.8668 | 0.0658 | 0.3875 | 0.050* |
| C18 | 0.9586(5) | −0.0934(8) | 0.3538(4) | 0.0425(16) |
| H18A | 0.9692 | −0.1706 | 0.4012 | 0.051* |
| H18B | 1.0222 | −0.0808 | 0.3464 | 0.051* |
| C19 | 0.8818(4) | −0.1731(7) | 0.2753(3) | 0.0325(14) |
| H19A | 0.9113 | −0.2723 | 0.2579 | 0.039* |
| H19B | 0.8246 | −0.2144 | 0.2874 | 0.039* |
Source of material
The title compound was synthesized according to a reported procedure [1]. A mixture of 3,5-cyclohexanedione (20 mmol) and 3,5-dibromo-benzaldehyde (10 mmol) in ethanol (100 mL) was refluxed for 2–3 h and then cooled to room temperature. After filtering the precipitates, they were sequentially washed with ice-cooled water and ethanol and then dried under vacuum.
Experimental details
Hydrogen atoms were placed in calculated positions and were included in the refinement in the riding model approximation, with Uiso(H) set to 1.2Ueq(C) and 1.5Ueq(O).
Discussion
Bis-enone derivatives are widely available in nature. Some derivatives exhibit biological activities, such as anticoagulant, insecticidal, antihelminthic, hypnotic, and antifungal activities, phytoalexin production, and HIV protease inhibition [2], [3], [4]. Moreover, these compounds are gaining increasing interest because of their versatile activities through chemical modifications (different substituents at the phenyl moiety) [5]. Recognizing the considerable importance of the compounds, research focused on the synthesis of bis-enone derivatives.
In the crystal structure of the title compound, the central six-membered ring containing one oxygen atom is almost perpendicular to the dibromo-phenyl moiety. The mean planes of the two annulated six-membered rings are almost parallel to the aforementioned oxan-2-enyl moiety.
Funding source: National Natural Science Foundation of China
Award Identifier / Grant number: 81274112
Award Identifier / Grant number: 81373986
Award Identifier / Grant number: 81473372
Funding statement: This work was supported by grants from the National Natural Science Foundation of China (81274112; 81373986; 81473372) and Beijing Municipal Natural Science Foundation (7152106).
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (81274112; 81373986; 81473372) and Beijing Municipal Natural Science Foundation (7152106).
References
1 Hilgeroth, A.; Langner, A.: Plasma protein binding properties of dimeric 4-aryl-1,4-dihydropyridines as novel non peptidic HIV-1 protease inhibitors. Pharmazie 55 (2000) 542–543.Search in Google Scholar
2 Ghorbani-Choghamarani, A.; Azadi, G.: Synthesis, characterization, and application of Fe3O4-SA-PPCA as a novel nanomagnetic reusable catalyst for theefficient synthesis of 2,3-dihydroquinazolin-4(1H)-ones and polyhydroquinolines. RSC Adv. 5 (2015) 9752–9758.10.1039/C4RA15315DSearch in Google Scholar
3 Hilgeroth, A.; Heinemann, F. W.: Novel solid-state synthesis of dimeric 4-aryl-1,4-dihydropyridines. J. Heterocycl. Chem. 35 (1998) 359–364.10.1002/jhet.5570350217Search in Google Scholar
4 Hilgeroth, A.; Wiese, M.; Billich, A.: Synthesis and biological evaluation of the first N-alkyl cage dimeric 4-aryl-1,4-dihydropyridines as novel nonpeptidic HIV-1 protease inhibitors. J. Med. Chem. 42 (1999) 4729–4732.10.1021/jm991115kSearch in Google Scholar
5 Hilgeroth, A.; Billich, A.; Lilie, H.: Synthesis and biological evaluation of first N-alkyl syn dimeric 4-aryl-1,4-dihydropyridines as competitive HIV-1 protease inhibitors. Eur. J. Med. Chem. 36 (2001) 367–374.10.1016/S0223-5234(01)01228-4Search in Google Scholar
6 Bruker. APEX2, SAINT and SADABS. Brucker AXS Inc., Madison, Wisconsin, USA, 2009.Search in Google Scholar
7 Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Search in Google Scholar PubMed
©2016 Jing Li et al., published by De Gruyter.
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Articles in the same Issue
- Cover and Frontmatter
- The crystal structure of triphenylphosphineoxide – 2,5-dichloro-3,6-dihydroxycyclohexa-2,5-diene-1,4-dione (2/1), C42H32Cl2O6P2
- Crystal structure of poly-[diaqua-[bis(μ2-hydroxy)-bis(μ4-3,4,5,6-tetrachlorophthalato-κ3O,O′:O′; κ2O′′:O′′′)dilanthanum(III)], C8H3Cl4LaO6
- Crystal structure of 1,1′-(3,4-diphenylthieno[2,3-b]thiophene-2,5-diyl)bis[1-phenyl-methanone], C32H20O2S2
- Crystal structure of 4a-hydroxy-9-(3,5-dibromo-phenyl)-3,4,4a,5,6,7,9,9a-octahydro-2H-xanthene-1,8-dione, C19H18Br2O4
- Crystal structure of 5-hydroxy-4,6,9,10-tetramethyl-1-oxo-6-vinyldecahydro-3a,9-propanocyclopenta[8]annulen-8-yl 2-((2-methyl-1-(3-methylbenzamido)propan-2-yl)thio)acetate, C34H49NO5S
- Crystal structure of pyridinium bis(naphthalane-2,3-diolato-κ2O,O′)borate monohydrate, C25H20BNO5
- Crystal structure of 1,1′-((1E,1′E)-(((ethane-1,2-diylbis(oxy))bis(2,1-phenylene))bis(azanylylidene))bis(methanylylidene))bis(naphthalen-2-olato)nickel(II), C72H52N4O8Ni2
- The crystal structure of 3-(2-acetyl-4-butyramido-phenoxy)-2-hydroxy-N-isopropylpropan-1-aminium tetraphenylborate, C42H49BN2O4
- Crystal structure of 4-bromobenzyl (Z)-N′-(adamantan-1-yl)-4-phenylpiperazine-1-carbothioimidate, C28H34BrN3S
- Crystal structure of poly-[(μ6-benzene-1,2,4,5-tetracarboxylato)-(μ2-1,2-bis(imidazol-1-ylmethyl)benzene)dicobalt(II)], Co2C24H16N4O8
- Crystal structure of catena-(bis(μ2-1, 2-bis(imidazole-1-ylmethyl)benzene-κN:N′)-dichlororido-nickel(II)), C28H28Cl2N8Ni
- Crystal structure of (Z)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-hydroxy-3-(4-methoxyphenyl)prop-2-en-1-one, C15H16N2O3
- Crystal structure of (Z)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-hydroxy-3-phenylprop-2-en-1-one, C14H14N2O2
- Crystal structure of (E)-2-(4-hydroxy-3-methoxybenzylidene)-6-methoxy-3,4-dihydronaphthalen-1(2H)-one, C19H18O4
- Crystal structure of (Z)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-(4-ethoxyphenyl)-3-hydroxyprop-2-en-1-one, C16H18N2O3
- Crystal structure of (Z)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-hydroxy-3-(p-toly)prop-2-en-1-one, C15H16N2O2
- Crystal structure of 1-acetyl-3-(3-chlorophenyl)-5-(4-isopropylphenyl)-4,5-dihydro-(1H)-pyrazole, C20H21ClN2O
- The crystal structure of 1-methyl-2,4-dinitro-5-iodoimidazole, C4H3IN4O4
- The crystal structure of 4-chloro-3,5-dinitroaniline, C6H4ClN3O4
- Crystal structure of N,N-dimethyl-N′-(2-methyl-4-oxo-5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidin-3(4H)-yl)formimidamide, C14H18N4OS
- Crystal structure of tetrakis(μ2-acetato-κ2O:O′)-bis[μ3-4-chloro-2,6-bis((methylimino)methyl)phenolato-κ2N,O:O,N′]-(μ4-oxido)tetracopper(II), C28H32Cl2Cu4N4O11
- Crystal structure of catena-poly[diaqua-bis(μ2-ethane-1,2-diyl-bis(pyridine-3-carboxylate-κ2N:N′))copper(II)] dinitrate, C28H28CuN6O16
- Synthesis and crystal structure of catena-poly[(μ2-nicotinato-κ2O,O′: κ1N)-(nitrato-κ1O)-(bis(2-benzimidazol-ylmethyl)amine-κ3N,N′,N′′)lead(II)], C22H18N7O5Pb
- The twinned crystal structure of (4SR)-7-benzyl-2,4,8,8-tetramethyl-7,8-dihydroimidazo[5,1-c][1,2,4]triazine-3,6(2H,4H)-dione, C16H20N4O2
- Crystal structure of (Z)-3-hydroxy-3-(4-methoxyphenyl)-1-(pyridin-2-yl)prop-2-en-1-one, C15H13NO3
- Crystal structure of 2-amino-4-(2,3-dichlorophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H12Cl2N2O2
- Crystal structure of catena-poly[(μ2-butane-1,4-diyl-bis(pyridine-3-carboxylato-κN))silver(I)] tetrafluoroborate, C16H16AgN2O4BF4
- Crystal structure of poly[diaqua-(1,10-phenanthroline-κ2N,N′)-(μ2-2,5-dihydroxytere-phthalato)-bis(μ4-2,5-dihydroxyterephthalato)dicerium(III)], C24H16CeN2O10
- Crystal structure of 5,7,4′-trihydroxy-3,8,3′-trymethoxyflavone, C18H16O8
- Crystal structure of N-(3,4-dichlorobenzylidene)-4-methylaniline, C14H11Cl2N
- Crystal structure of 4-(3-Methoxy-phenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylic acid ethyl ester, C22H27NO4
- Crystal structure of 2-amino-4-(3-fluorophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H13FN2O2
- Crystal structure of 1,1,(3,4-dihydroxythieno[2,3-b] thiophene-2,5-diyl)bis(2-bromoethanone), C10H6Br2O4S2
- The crystal structure of N,N′-(4,4′-oxydibenzyl)-bisisonicotinamide 3.5 hydrate, C24H24N4O6
- Crystal structure of catena-poly[hexakis(μ2-chlorido)-hexakis(4-(1H-pyrazol-5-yl)pyridine-κN)tricadmium(II)], Cd3C48H42Cl6N18
- Crystal structure of 2-(4-(dimethylamino)phenyl)-1,3-dimethyl-1H-perimidin-3-ium iodide, C21H22I1N3
- Crystal structure of 4-(1,3-dimethyl-2,3-dihydro-1H-perimidin-2-yl)benzonitrile, C20H17N3
- Crystal structure of tetrakis(μ2-acetato-κ2O:O′)-bis(2,2′-sulfonyldipyrazine-κ1N)dicopper(II), C24H24Cu2N8O12S2
- Crystal structure of 1-(4-chlorophenyl)-6,8-diphenyl-1H-pyrazolo[4,3-c]quinoline, C28H18ClN3
- Crystal structure of methyl 3-((1-(2-(methoxycarbonyl)benzyl)-1H-1,2,3-triazol-4-yl)methoxy)-2-naphthoate, C24H21N3O5
- Crystal structure of (tris(2-pyridylmethyl)amine-κ4N,N′,N′′,N′′′′)-chloranilato-κO,O′-zinc(II) – methanol (1/1), C25H22Cl2N4O5Zn
- Crystal structure of 1,1-dimethyl-3-(4-methoxyphenyl)urea, C10H14N2O2
- Crystal structure of 4a-Hydroxy-9-(2-nitro-phenyl)-3,4,4a,5,6,7,9,9a-octahydro-2H-xanthene-1,8-dione, C19H19NO6
- Crystal structure of chlorido-(η6–1-isopropyl-4-methyl benzene)-(1-(pyridin-2-yl)-N-(p-tolyl)methanimine-κ2N,N′)ruthenium(II) hexafluorophosphate(V), C23H26ClF6N2PRu
- Crystal structure of phenyl(2-phenyl-2,3-dihydro-1H-perimidin-2-yl)methanone, C24H18N2O
- Crystal structure of (E)-3-methyl-4-((3-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)methylene)-1-phenyl-1H-pyrazol-5(4H)-one, C29H23N7O
- Crystal structure of 2-(4-(2-butyl-1,3-dioxo-2,3-dihydro-1H-benzo[de]isoquinolin-6-yl)piperazin-1-yl)-2-oxoethyldiethylcarbamodithioate, C27H34N4O3S2
- Crystal structure of poly-[diaqua-bis(μ-4,4′-bipyridine-κ2N:N′)cobalt(II)] bis(4-chlorobenzenesulfonate) – 4,4′-bipyridine – water (1/1/2), C42H40Cl2CoN6O10S2
- Crystal structure of (η6-benzene)-(N-(2,6-dimethylphenyl)-1-(pyridin-2-yl)methanimine-κ2N,N′)ruthenium(II) perchlorate monohydrate, C20H20Cl2N2O5Ru
- Crystal structure of 4,10,16,22-tetrahydroxy-6,12,18,24-tetramethoxy-2,8,14,20-tetraethylphenylresorcin[4]arene – ethyl acetate (1/1), C68H72O10
- Crystal structure of chlorido-(N-(2,5-dichlorophenyl)-1-(pyridin-2-yl)methanimine-κ2N,N′)(η6-1-isopropyl-4-methyl benzene) ruthenium (II) tetrafluoroborate, C22H22Cl3N2BF4Ru
- Crystal structure of 3-(5-methyl-1-p-tolyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazole-4-carbaldehyde, a rare Z′ = 3 structure, C20H17N5O
- Crystal structure of 5-(5-(4-chlorophenyl)-1-phenyl-1H-pyrazol-3-yl)-N-phenyl-1,3,4-thiadiazol-2-amine, C23H16ClN5S
- Crystal structure of 7-hydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one-N,N-dimethylformamide (1/1), C18H17NO5
- Crystal structure of halogen-bonded 2-chloro-1,10-phenanthroline—1,4-diiodotetrafluorobenzene (2/1), C30H14Cl2F4I2N4
- Crystal structure of 1-(4,4-dimethyl-2,6-dithioxo-1,3,5-triazinan-1-yl)-3-(diethylaminocarbonyl)thiourea, C11H20N6OS3
- Crystal structure of methyl 1-(4-fluorobenzyl)-3-phenyl-1H-pyrazole-5-carboxylate, C18H15FN2O2
- Crystal structure of 1,1-dimethyl-3-(4-methylphenyl)urea, C10H14N2O
- Crystal structure of yttrium gallium antimonide, Y5Ga1.24Sb2.77
- Crystal structure of 2-(bis(4-methoxyphenyl)amino)-2-oxoacetic acid, C16H15NO5
Articles in the same Issue
- Cover and Frontmatter
- The crystal structure of triphenylphosphineoxide – 2,5-dichloro-3,6-dihydroxycyclohexa-2,5-diene-1,4-dione (2/1), C42H32Cl2O6P2
- Crystal structure of poly-[diaqua-[bis(μ2-hydroxy)-bis(μ4-3,4,5,6-tetrachlorophthalato-κ3O,O′:O′; κ2O′′:O′′′)dilanthanum(III)], C8H3Cl4LaO6
- Crystal structure of 1,1′-(3,4-diphenylthieno[2,3-b]thiophene-2,5-diyl)bis[1-phenyl-methanone], C32H20O2S2
- Crystal structure of 4a-hydroxy-9-(3,5-dibromo-phenyl)-3,4,4a,5,6,7,9,9a-octahydro-2H-xanthene-1,8-dione, C19H18Br2O4
- Crystal structure of 5-hydroxy-4,6,9,10-tetramethyl-1-oxo-6-vinyldecahydro-3a,9-propanocyclopenta[8]annulen-8-yl 2-((2-methyl-1-(3-methylbenzamido)propan-2-yl)thio)acetate, C34H49NO5S
- Crystal structure of pyridinium bis(naphthalane-2,3-diolato-κ2O,O′)borate monohydrate, C25H20BNO5
- Crystal structure of 1,1′-((1E,1′E)-(((ethane-1,2-diylbis(oxy))bis(2,1-phenylene))bis(azanylylidene))bis(methanylylidene))bis(naphthalen-2-olato)nickel(II), C72H52N4O8Ni2
- The crystal structure of 3-(2-acetyl-4-butyramido-phenoxy)-2-hydroxy-N-isopropylpropan-1-aminium tetraphenylborate, C42H49BN2O4
- Crystal structure of 4-bromobenzyl (Z)-N′-(adamantan-1-yl)-4-phenylpiperazine-1-carbothioimidate, C28H34BrN3S
- Crystal structure of poly-[(μ6-benzene-1,2,4,5-tetracarboxylato)-(μ2-1,2-bis(imidazol-1-ylmethyl)benzene)dicobalt(II)], Co2C24H16N4O8
- Crystal structure of catena-(bis(μ2-1, 2-bis(imidazole-1-ylmethyl)benzene-κN:N′)-dichlororido-nickel(II)), C28H28Cl2N8Ni
- Crystal structure of (Z)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-hydroxy-3-(4-methoxyphenyl)prop-2-en-1-one, C15H16N2O3
- Crystal structure of (Z)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-hydroxy-3-phenylprop-2-en-1-one, C14H14N2O2
- Crystal structure of (E)-2-(4-hydroxy-3-methoxybenzylidene)-6-methoxy-3,4-dihydronaphthalen-1(2H)-one, C19H18O4
- Crystal structure of (Z)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-(4-ethoxyphenyl)-3-hydroxyprop-2-en-1-one, C16H18N2O3
- Crystal structure of (Z)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-hydroxy-3-(p-toly)prop-2-en-1-one, C15H16N2O2
- Crystal structure of 1-acetyl-3-(3-chlorophenyl)-5-(4-isopropylphenyl)-4,5-dihydro-(1H)-pyrazole, C20H21ClN2O
- The crystal structure of 1-methyl-2,4-dinitro-5-iodoimidazole, C4H3IN4O4
- The crystal structure of 4-chloro-3,5-dinitroaniline, C6H4ClN3O4
- Crystal structure of N,N-dimethyl-N′-(2-methyl-4-oxo-5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidin-3(4H)-yl)formimidamide, C14H18N4OS
- Crystal structure of tetrakis(μ2-acetato-κ2O:O′)-bis[μ3-4-chloro-2,6-bis((methylimino)methyl)phenolato-κ2N,O:O,N′]-(μ4-oxido)tetracopper(II), C28H32Cl2Cu4N4O11
- Crystal structure of catena-poly[diaqua-bis(μ2-ethane-1,2-diyl-bis(pyridine-3-carboxylate-κ2N:N′))copper(II)] dinitrate, C28H28CuN6O16
- Synthesis and crystal structure of catena-poly[(μ2-nicotinato-κ2O,O′: κ1N)-(nitrato-κ1O)-(bis(2-benzimidazol-ylmethyl)amine-κ3N,N′,N′′)lead(II)], C22H18N7O5Pb
- The twinned crystal structure of (4SR)-7-benzyl-2,4,8,8-tetramethyl-7,8-dihydroimidazo[5,1-c][1,2,4]triazine-3,6(2H,4H)-dione, C16H20N4O2
- Crystal structure of (Z)-3-hydroxy-3-(4-methoxyphenyl)-1-(pyridin-2-yl)prop-2-en-1-one, C15H13NO3
- Crystal structure of 2-amino-4-(2,3-dichlorophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H12Cl2N2O2
- Crystal structure of catena-poly[(μ2-butane-1,4-diyl-bis(pyridine-3-carboxylato-κN))silver(I)] tetrafluoroborate, C16H16AgN2O4BF4
- Crystal structure of poly[diaqua-(1,10-phenanthroline-κ2N,N′)-(μ2-2,5-dihydroxytere-phthalato)-bis(μ4-2,5-dihydroxyterephthalato)dicerium(III)], C24H16CeN2O10
- Crystal structure of 5,7,4′-trihydroxy-3,8,3′-trymethoxyflavone, C18H16O8
- Crystal structure of N-(3,4-dichlorobenzylidene)-4-methylaniline, C14H11Cl2N
- Crystal structure of 4-(3-Methoxy-phenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylic acid ethyl ester, C22H27NO4
- Crystal structure of 2-amino-4-(3-fluorophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H13FN2O2
- Crystal structure of 1,1,(3,4-dihydroxythieno[2,3-b] thiophene-2,5-diyl)bis(2-bromoethanone), C10H6Br2O4S2
- The crystal structure of N,N′-(4,4′-oxydibenzyl)-bisisonicotinamide 3.5 hydrate, C24H24N4O6
- Crystal structure of catena-poly[hexakis(μ2-chlorido)-hexakis(4-(1H-pyrazol-5-yl)pyridine-κN)tricadmium(II)], Cd3C48H42Cl6N18
- Crystal structure of 2-(4-(dimethylamino)phenyl)-1,3-dimethyl-1H-perimidin-3-ium iodide, C21H22I1N3
- Crystal structure of 4-(1,3-dimethyl-2,3-dihydro-1H-perimidin-2-yl)benzonitrile, C20H17N3
- Crystal structure of tetrakis(μ2-acetato-κ2O:O′)-bis(2,2′-sulfonyldipyrazine-κ1N)dicopper(II), C24H24Cu2N8O12S2
- Crystal structure of 1-(4-chlorophenyl)-6,8-diphenyl-1H-pyrazolo[4,3-c]quinoline, C28H18ClN3
- Crystal structure of methyl 3-((1-(2-(methoxycarbonyl)benzyl)-1H-1,2,3-triazol-4-yl)methoxy)-2-naphthoate, C24H21N3O5
- Crystal structure of (tris(2-pyridylmethyl)amine-κ4N,N′,N′′,N′′′′)-chloranilato-κO,O′-zinc(II) – methanol (1/1), C25H22Cl2N4O5Zn
- Crystal structure of 1,1-dimethyl-3-(4-methoxyphenyl)urea, C10H14N2O2
- Crystal structure of 4a-Hydroxy-9-(2-nitro-phenyl)-3,4,4a,5,6,7,9,9a-octahydro-2H-xanthene-1,8-dione, C19H19NO6
- Crystal structure of chlorido-(η6–1-isopropyl-4-methyl benzene)-(1-(pyridin-2-yl)-N-(p-tolyl)methanimine-κ2N,N′)ruthenium(II) hexafluorophosphate(V), C23H26ClF6N2PRu
- Crystal structure of phenyl(2-phenyl-2,3-dihydro-1H-perimidin-2-yl)methanone, C24H18N2O
- Crystal structure of (E)-3-methyl-4-((3-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)methylene)-1-phenyl-1H-pyrazol-5(4H)-one, C29H23N7O
- Crystal structure of 2-(4-(2-butyl-1,3-dioxo-2,3-dihydro-1H-benzo[de]isoquinolin-6-yl)piperazin-1-yl)-2-oxoethyldiethylcarbamodithioate, C27H34N4O3S2
- Crystal structure of poly-[diaqua-bis(μ-4,4′-bipyridine-κ2N:N′)cobalt(II)] bis(4-chlorobenzenesulfonate) – 4,4′-bipyridine – water (1/1/2), C42H40Cl2CoN6O10S2
- Crystal structure of (η6-benzene)-(N-(2,6-dimethylphenyl)-1-(pyridin-2-yl)methanimine-κ2N,N′)ruthenium(II) perchlorate monohydrate, C20H20Cl2N2O5Ru
- Crystal structure of 4,10,16,22-tetrahydroxy-6,12,18,24-tetramethoxy-2,8,14,20-tetraethylphenylresorcin[4]arene – ethyl acetate (1/1), C68H72O10
- Crystal structure of chlorido-(N-(2,5-dichlorophenyl)-1-(pyridin-2-yl)methanimine-κ2N,N′)(η6-1-isopropyl-4-methyl benzene) ruthenium (II) tetrafluoroborate, C22H22Cl3N2BF4Ru
- Crystal structure of 3-(5-methyl-1-p-tolyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazole-4-carbaldehyde, a rare Z′ = 3 structure, C20H17N5O
- Crystal structure of 5-(5-(4-chlorophenyl)-1-phenyl-1H-pyrazol-3-yl)-N-phenyl-1,3,4-thiadiazol-2-amine, C23H16ClN5S
- Crystal structure of 7-hydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one-N,N-dimethylformamide (1/1), C18H17NO5
- Crystal structure of halogen-bonded 2-chloro-1,10-phenanthroline—1,4-diiodotetrafluorobenzene (2/1), C30H14Cl2F4I2N4
- Crystal structure of 1-(4,4-dimethyl-2,6-dithioxo-1,3,5-triazinan-1-yl)-3-(diethylaminocarbonyl)thiourea, C11H20N6OS3
- Crystal structure of methyl 1-(4-fluorobenzyl)-3-phenyl-1H-pyrazole-5-carboxylate, C18H15FN2O2
- Crystal structure of 1,1-dimethyl-3-(4-methylphenyl)urea, C10H14N2O
- Crystal structure of yttrium gallium antimonide, Y5Ga1.24Sb2.77
- Crystal structure of 2-(bis(4-methoxyphenyl)amino)-2-oxoacetic acid, C16H15NO5

