Abstract
C13H6Fe2O6S2, monoclinic, P21/c (no. 14), a = 10.0181(3) Å, b = 6.4719(2) Å, c = 24.3922(6) Å, β = 94.630(1)°, V = 1576.34(8) Å3, Z = 4, Rgt(F) = 0.0245, wRref(F2) = 0.0566, T = 150(2) K.
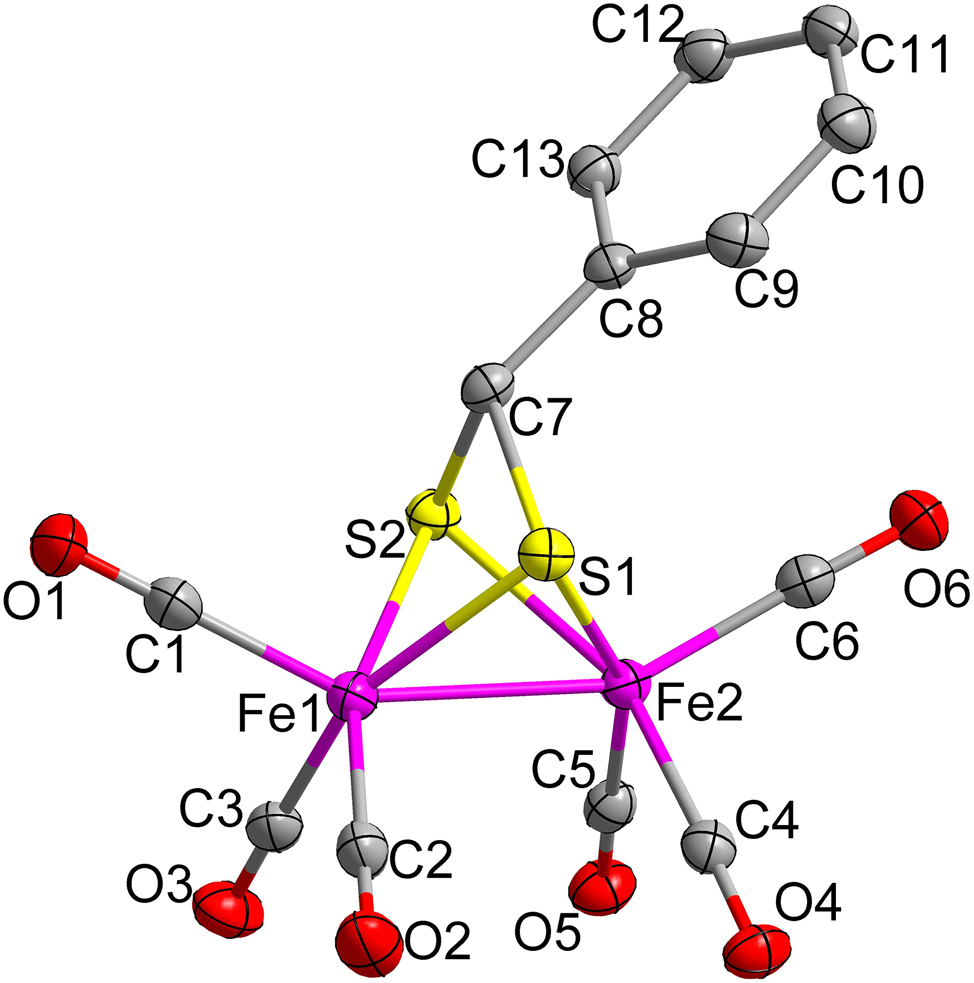
The molecular structure is shown in the figure. Table 1 contains the crystallographic data and the list of the atoms including atomic coordinates and displacement parameters can be found in the cif-file attached to this article.
Data collection and handling.
| Crystal: | Red block |
| Size: | 0.30 × 0.20 × 0.20 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 2.13 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω scans |
| θmax, completeness: | 26.4°, 100 % |
| N(hkl)measured, N(hkl)unique, Rint: | 28896, 3216, 0.042 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 2594 |
| N(param)refined: | 208 |
| Programs: | Bruker, 1 SHELX, 2 , 3 Olex2 4 |
1 Source of material
A mixture of 1-phenylmethanedithiol (0.156 g, 1.0 mmol) and triiron dodecacarbonyl (0.504 g, 1.0 mmol) was refluxed in THF (10 mL) under stirring for 2 h. After removal of the solvent under reduced pressure using a rotary evaporator, the residue was purified by preparative TLC with petroleum ether as the eluent. The main red band afforded the title complex as a red solid in 38 % yield. Single crystals were obtained by slow crystallization from a cold hexane solution.
2 Experimental details
The structure was solved by Direct Methods with the SHELXS program. Hydrogen atoms were positioned geometrically (C–H = 0.93–0.98 Å). Their Uiso values were set to 1.2Ueq or 1.5Ueq of the parent atoms.
3 Comment
The natural [FeFe]-hydrogenase exhibits exceptional catalytic efficiency in the reversible interconversion between protons and hydrogen gas, with turnover frequencies exceeding 10−4s−1, far surpassing most synthetic catalysts. 5 This remarkable capability establishes it as a paradigmatic model for investigating biological hydrogen production and hydrogen energy utilization mechanisms. Fe2(μ–ER)2(CO)6 complexes (E = S, Se, Te), which structurally mimic the [2Fe] H subcluster of the native enzyme active site, serve as pivotal synthetic platforms to decipher structure-function relationships in hydrogenase-inspired catalysis. 6 , 7 , 8 , 9 , 10 , 11 , 12 Given these considerations, the investigation of such model compounds represents a strategic entry point for simulating the enzymatic active center. Herein, we report a biomimetic compound C13H6Fe2O6S2 derived from 1-phenylmethanedithiol. The title complex features a butterfly-shaped [Fe2S2] cluster core coordinated with six terminal carbonyl ligands and a bridging phenylmethanedithiolate. The Fe1–Fe2 distance of 2.4881(4) Å is notably shorter than those reported for the natural [FeFe]-hydrogenases (2.55–2.60 Å). 13 The average Fe–C bond length (1.803 Å) and Fe–S bond distance (2.267 Å) fall within the normal range and align closely with values observed in analogous synthetic species. 14 , 15 , 16 , 17 , 18 , 19
-
Conflict of interest: The authors declare no conflicts of interest regarding this article.
-
Research funding: This research was supported by collaborative horizontal project between Dongchang College and Huainan Muqi Pharmaceutical Technology Co., Ltd under Grant 2025DCHX012, and Shandong Provincial Natural Science Foundation under Grant ZR2024QB181.
-
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
References
1. BRUKER. Saint, Apex2 and Sadabs; Bruker AXS Inc.: Madison, Wisconsin, USA, 2009.Search in Google Scholar
2. Sheldrick, G. M. A Short History of Shelx. Acta Cryst. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Search in Google Scholar PubMed
3. Sheldrick, G. M. Crystal Structure Refinement with Shelxl. Acta Cryst. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. Olex2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
5. Lubitz, W.; Ogata, H.; Rüdiger, O.; Reijerse, E. Hydrogenases. Chem. Rev. 2014, 114, 4081–4148; https://doi.org/10.1021/cr4005814.Search in Google Scholar PubMed
6. Li, Y. L.; Rauchfuss, T. B. Synthesis of Diiron(I) Dithiolato Carbonyl Complexes. Chem. Rev. 2016, 116, 7043–7077; https://doi.org/10.1021/acs.chemrev.5b00669.Search in Google Scholar PubMed PubMed Central
7. Seyferth, D.; Womack, G. B.; Gallagher, M. K.; Cowie, M.; Hames, B. W.; Fackler, J. P.; Mazany, A. M. Novel Anionic Rearrangements in Hexacarbonyldiiron Complexes of Chelating Organosulfur Ligands. Organometallics 1987, 6, 283–294; https://doi.org/10.1021/om00145a009.Search in Google Scholar
8. Lü, S.; Huang, H.-L.; Zhang, R.-F.; Ma, C.-L.; Li, Q.-L.; He, J.; Yang, J.; Li, T.; Li, Y.-L. Phosphine-Substituted Fe–Te Clusters Related to the Active Site of [FeFe]–H2ases. Inorg. Chem. Front. 2020, 7, 2352–2361; https://doi.org/10.1039/d0qi00276c.Search in Google Scholar
9. Bai, S.-F.; Du, X.-M.; Tian, W.-J.; Xu, H.; Zhang, R.-F.; Ma, C.-L.; Wang, Y.-L.; Lü, S.; Li, Q.-L.; Li, Y.-L. Di-Tri-and Tetraphosphine-Substituted Fe/Se Carbonyls: Synthesis, Characterization and Electrochemical Properties. Dalton Trans. 2022, 51, 11125–11134; https://doi.org/10.1039/d2dt01376b.Search in Google Scholar PubMed
10. Gao, X.-P.; Bai, S.-F.; Wang, Y.-L.; Lü, S.; Li, Q.-L. Facile Access to Tetra-Substituted FeIIFeII Biomimetics for the Oxidized State Active Site of [FeFe]-Hydrogenases. Inorg. Chem. Front. 2024, 11, 2372–2680; https://doi.org/10.1039/d4qi00773e.Search in Google Scholar
11. Bai, S.-F.; Ma, J.-W.; Guo, Y.-N.; Du, X.-M.; Wang, Y.-L.; Li, Q.-L.; Lü, S. Aminophosphine-Substituted Fe/E (E = S, Se) Carbonyls Related to [FeFe]-Hydrogenases: Synthesis, Protonation, and Electrocatalytic Proton Reduction. J. Mol. Struct. 2023, 1283, 135827; https://doi.org/10.1016/j.molstruc.2023.135287.Search in Google Scholar
12. Lü, S.; Bai, S.-F.; Gao, X.-P.; Wang, Y.-L.; Li, Q.-L. Aminodiphosphine Substituted 2Fe2Se Complex as New Precursor to Single and Double Butterfly Fe/Se Models Related to FeFe Hydrogenase Models. J. Mol. Struct. 2023, 1290, 135939; https://doi.org/10.1016/j.molstruc.2023.135939.Search in Google Scholar
13. Nicolet, Y.; Piras, C.; Legrand, P.; Hatchikian, C. E.; Fontecilla-Camps, J. C. Desulfovibrio Desulfuricans Iron Hydrogenase: The Structure Shows Unusual Coordination to an Active Site Fe Binuclear Center. Structure 1999, 1290, 13–23.10.1016/S0969-2126(99)80005-7Search in Google Scholar PubMed
14. Liu, X.-F.; Li, Y.-L.; Liu, X.-H. Synthesis, Characterization, Electrocatalytic Properties, and Antifungal Activity of Isoxazole-Containing Di-Iron Complexes. Chin. J. Inorg. Chem. 2023, 12, 2367–2376.Search in Google Scholar
15. Zhu, L.-J.; Liu, X.-F. Electrocatalytic Hydrogen Evolution Performance of Tetra-Iron Complexes with Bridging Diphosphine Ligands. Chin. J. Inorg. Chem. 2025, 2, 321–328.Search in Google Scholar
16. Zhu, L.-J.; Liu, X.-F. Synthesis, Characterization and Electrocatalytic Hydrogen Evolution of Two Di-Iron Complexes Containing a Phosphine Ligand with a Pendant Amine. Chin. J. Inorg. Chem. 2025, 5, 939–947.Search in Google Scholar
17. Lü, S.; Gong, S.; Qin, C.-R.; Li, Q.-L. PNP Bridged Diiron Carbonyls Containing Fe/E (E = S and Se) ‘Cluster Core Related to the Active Site of [FeFe]–H2ases. J. Organomet. Chem. 2022, 929, 121581.10.1016/j.jorganchem.2020.121581Search in Google Scholar
18. Wu, M. G.; Liu, X. F. The Crystal Structure of tris(carbonyl)-bis(carbonyl)-[μ-propane-1,2- dithiolato]-(benzyldiphenylphosphine)diiron (Fe–Fe). Z. Kristallogr. - N. Cryst. Struct 2023, 238, 133–135 https://doi.org/10.1515/ncrs-2022-0520.Search in Google Scholar
19. Daraosheh, A. Q.; Apfel, U.-P.; Gorls, H.; Friebe, C.; Schubert, U. S.; El-khateeb, M.; Mloston, G.; Weigand, W. New Approach to [FeFe]–Hydrogenase Models Using Aromatic Thioketones. Eur. J. Inorg. Chem. 2012, 318; https://doi.org/10.1002/ejic.201101032.Search in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of (S)-N-(10-((2,2-dimethoxyethyl)amino)-1,2,3-trimethoxy-9-oxo-5,6,7,9-tetrahydrobenzo[a]heptalen-7-yl)acetamide, C25H32N2O7
- The crystal structure of 6,6′-difluoro-3,3′-dimethyl-5,5′-di(10H-phenoxazin-10-yl)- [1,1′-biphenyl]-2,2′-dicarbonitrile, C40H24F2N4O2
- Crystal structure of poly[(di-ethylenediamine-κ2N,N′)cadmium(II) tetradedocyloxidohexavanadate] (V4+/V5+ = 2/1), C4H16CdN4O14V6
- The crystal structure of poly[bis(dimethylformamide-κ1N)-(μ4-2′,3,3″,5′-tetrakis(trifluoromethyl)-[1,1′:4′,1″-terphenyl]-4,4″-dicarboxylato-κ4 O,O′: O″,O‴)dicadmium(II)], C27H15CdF12NO5
- Crystal structure of bis(μ2-ferrocenylcarboxylato-O,O′)-(μ3-oxido-κ3O:O:O)-bis(μ2-salicyladoximato-κ2N,O,O′)-(μ2-isopropoxo)-tris(isopropoxy-κ1O trititanium(IV)), C48H55N2O13Fe2Ti3
- Crystal structure of 3-(diethylamino)-7,9,11-trimethyl-8-phenyl-6H,13H-12λ4,13λ4-chromeno[3′,4′:4,5]pyrrolo[1,2-c]pyrrolo[2,1-f][1,3,2]diazaborinin-6-one, C28H26BF2N3O2
- The crystal structure of catena-poly[aqua-μ2-2-nitro-benzene-1,3-dicarboxylato-κ2O,O′)-(1,10-phenanthroline-κ2N,N′)-zinc(II)], C20H13N3O7Zn
- Crystal structure of poly[diaqua-{μ3-1-(3-carboxylatophenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylato-κ4O,O′:O′′:O′′′′}manganese(II)] hydrate
- Crystal structure of N′-((1-hydroxycyclohexyl)(phenyl)methyl)-2-methoxybenzohydrazide methanol solvate, C22H28N2O4
- The cocrystal of caffeic acid — progesterone — water (1/2/1), C51H70O9
- Crystal structure of (((oxido(quinolin-6-yl)methoxy)triphenyl-λ5-stibanyl)oxy)(quinolin-7-yl)methanolate
- Crystal structure of [(E)-6′-(diethylamino)-2-(2-(((E)-pyren-1-ylmethylene)amino)ethyl)-4′-(2-((E)-1,3,3-trimethylindolin-2-ylidene)ethylidene)-1′,2′,3′,4′-tetrahydrospiro[isoindoline-1,9′-xanthen]-3-one]-methanol, solvate C57H56N4O3
- The crystal structure of 1-(acridin-9-yl)pyrrolidine-2,5-dione, C17H22N2O2
- Crystal structure of N-(4-acetylphenyl)-2-(6-methoxynaphthalen-2-yl)propanamide, C22H21NO3
- The crystal structure of 5,10,15,20-tetrakis(4-(1H-1,2,4-triazol-1-yl)phenyl)porphyrin, C52H34N16
- Crystal structure of hexacarbonyl-μ2-[phenylmethanedithiolato-κ4S:S,S′:S′]diiron (Fe–Fe) C13H6Fe2O6S2
- Crystal structure of diiodo-bis(1-((2-propyl-1H-benzo[d]imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κ1N)cadmium(II), C34H34CdI2N10
- Crystal structure of (E)-(3-(3-bromophenyl)acryloyl)ferrocene, C19H15BrFeO
- Crystal structure of catena-poly(μ2-6-chloropyridine-2-carboxylato-κ3N,O:O′)(6-chloropyridine-2-carboxylato-κ2O,N)copper(II), C12H6Cl2N2O4Cu
- Crystal structure of poly[diaqua-μ 3-(5-(3,5-dicarboxy-2,4,6-trimethylbenzyl)-2,4,6-trimethylisophthalato)-κ 6O,O′:O″,O‴:O‴′,O‴″) terbium(III)-monohydrate], C23H28TbO12
- Crystal structure of (E)-2-(((5-chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)methylene)amino)-3′,6′-dihydroxyspiro[isoindoline-1,9′-xanthen]-3-one – ethanol (1/2), C35H33ClN4O6
- The crystal structure of 3-(5-amino-3-phenylisoxazol-4-yl)-4-chloro-3-hydroxyindolin-2-one, C17H12ClN3O3
- The crystal structure of dimethylammonium 4-[2-(4-fluorophenyl)-4, 5-diphenyl-1H-imidazol-1-yl]benzenesulfonate, C29H26FN3O3S
- Crystal structure of (R)-2-ammonio-3-((5-carboxypentyl)thio)propanoate
- Crystal structure of 4-cyclohexyl-5-(thiophen-2-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione, C12H15N3S2
- The crystal structure of 4,6-bis(dimethylamino)-2-fluoroisophthalonitrile, C12H13FN4
- Hydrogen bonding in the crystal structure of nicotin-1,1′-dium tetrabromidomanganate(II)
- The crystal structure of bis(2-bromobenzyl)(2-((2-oxybenzylidene)amino)-4-methylpentanoato-κ3N, O,O′)tin(IV), C27H27Br2NO3Sn
- Crystal structure of (E)-(3-(p-tolyl)acryloyl)ferrocene, C20H18FeO
- Crystal structure of (E)-7-fluoro-2-((5-(4-methylpiperazin-1-yl)pyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C21H22FN3O
- Crystal structure of (E)-7-methoxy-2-((5-(4-methylpiperazin-1-yl)pyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C22H25N3O2
- The crystal structure of poly(bis(μ2-1,3,5-tri(1H-imidazol-1-yl)benzene-κ2N:N′)-(μ2-2,3,5,6-tetrafluoroterephthalato-κ2O:O′)-manganese(II), C38H24F4N12O4Mn
- Crystal structure of (3,4-dimethoxybenzyl)triphenylphosphonium bromide ethanol solvate, C29H32BrO3P
- Crystal structure of tetraethylammonium hydrogencarbonate – (diaminomethylene)thiourea – water (2/1/3)
- Crystal structure of N, N-Dimethyl-N′-tosylformimidamide, C10H14N2O2S
- The crystal structure of ethyl 2-methyl-5-oxo-4-(2-methoxyphenyl)-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C20H23N2O4
- Crystal structure of bis(μ2-1,5-bis[(E)-1-(2-hydroxyphenyl)ethylidene] thiocarbonohydrazide)-bis(dimethylformamide)-dizinc(II) dimethylformamide solvate, C40H46N10O6S2Zn2⋅C3H7NO
- Crystal structure of azido-κ1N{hydridotris(3-tert-butyl-5-methylpyrazol-1-yl)borato-κ3N,N′,N″}copper(II), C24H40BCuN9
- The crystal structure of fac-tricarbonyl(1,10-phenanthroline-κ2N,N′)-(azido-κ1N)rhenium(I), C15H8N5O3Re
- Crystal structure of 4-((triphenylphosphonio)methyl)pyridin-1-ium tetrachloridozincate(II), C24H22Cl4NPZn
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of (S)-N-(10-((2,2-dimethoxyethyl)amino)-1,2,3-trimethoxy-9-oxo-5,6,7,9-tetrahydrobenzo[a]heptalen-7-yl)acetamide, C25H32N2O7
- The crystal structure of 6,6′-difluoro-3,3′-dimethyl-5,5′-di(10H-phenoxazin-10-yl)- [1,1′-biphenyl]-2,2′-dicarbonitrile, C40H24F2N4O2
- Crystal structure of poly[(di-ethylenediamine-κ2N,N′)cadmium(II) tetradedocyloxidohexavanadate] (V4+/V5+ = 2/1), C4H16CdN4O14V6
- The crystal structure of poly[bis(dimethylformamide-κ1N)-(μ4-2′,3,3″,5′-tetrakis(trifluoromethyl)-[1,1′:4′,1″-terphenyl]-4,4″-dicarboxylato-κ4 O,O′: O″,O‴)dicadmium(II)], C27H15CdF12NO5
- Crystal structure of bis(μ2-ferrocenylcarboxylato-O,O′)-(μ3-oxido-κ3O:O:O)-bis(μ2-salicyladoximato-κ2N,O,O′)-(μ2-isopropoxo)-tris(isopropoxy-κ1O trititanium(IV)), C48H55N2O13Fe2Ti3
- Crystal structure of 3-(diethylamino)-7,9,11-trimethyl-8-phenyl-6H,13H-12λ4,13λ4-chromeno[3′,4′:4,5]pyrrolo[1,2-c]pyrrolo[2,1-f][1,3,2]diazaborinin-6-one, C28H26BF2N3O2
- The crystal structure of catena-poly[aqua-μ2-2-nitro-benzene-1,3-dicarboxylato-κ2O,O′)-(1,10-phenanthroline-κ2N,N′)-zinc(II)], C20H13N3O7Zn
- Crystal structure of poly[diaqua-{μ3-1-(3-carboxylatophenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylato-κ4O,O′:O′′:O′′′′}manganese(II)] hydrate
- Crystal structure of N′-((1-hydroxycyclohexyl)(phenyl)methyl)-2-methoxybenzohydrazide methanol solvate, C22H28N2O4
- The cocrystal of caffeic acid — progesterone — water (1/2/1), C51H70O9
- Crystal structure of (((oxido(quinolin-6-yl)methoxy)triphenyl-λ5-stibanyl)oxy)(quinolin-7-yl)methanolate
- Crystal structure of [(E)-6′-(diethylamino)-2-(2-(((E)-pyren-1-ylmethylene)amino)ethyl)-4′-(2-((E)-1,3,3-trimethylindolin-2-ylidene)ethylidene)-1′,2′,3′,4′-tetrahydrospiro[isoindoline-1,9′-xanthen]-3-one]-methanol, solvate C57H56N4O3
- The crystal structure of 1-(acridin-9-yl)pyrrolidine-2,5-dione, C17H22N2O2
- Crystal structure of N-(4-acetylphenyl)-2-(6-methoxynaphthalen-2-yl)propanamide, C22H21NO3
- The crystal structure of 5,10,15,20-tetrakis(4-(1H-1,2,4-triazol-1-yl)phenyl)porphyrin, C52H34N16
- Crystal structure of hexacarbonyl-μ2-[phenylmethanedithiolato-κ4S:S,S′:S′]diiron (Fe–Fe) C13H6Fe2O6S2
- Crystal structure of diiodo-bis(1-((2-propyl-1H-benzo[d]imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κ1N)cadmium(II), C34H34CdI2N10
- Crystal structure of (E)-(3-(3-bromophenyl)acryloyl)ferrocene, C19H15BrFeO
- Crystal structure of catena-poly(μ2-6-chloropyridine-2-carboxylato-κ3N,O:O′)(6-chloropyridine-2-carboxylato-κ2O,N)copper(II), C12H6Cl2N2O4Cu
- Crystal structure of poly[diaqua-μ 3-(5-(3,5-dicarboxy-2,4,6-trimethylbenzyl)-2,4,6-trimethylisophthalato)-κ 6O,O′:O″,O‴:O‴′,O‴″) terbium(III)-monohydrate], C23H28TbO12
- Crystal structure of (E)-2-(((5-chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)methylene)amino)-3′,6′-dihydroxyspiro[isoindoline-1,9′-xanthen]-3-one – ethanol (1/2), C35H33ClN4O6
- The crystal structure of 3-(5-amino-3-phenylisoxazol-4-yl)-4-chloro-3-hydroxyindolin-2-one, C17H12ClN3O3
- The crystal structure of dimethylammonium 4-[2-(4-fluorophenyl)-4, 5-diphenyl-1H-imidazol-1-yl]benzenesulfonate, C29H26FN3O3S
- Crystal structure of (R)-2-ammonio-3-((5-carboxypentyl)thio)propanoate
- Crystal structure of 4-cyclohexyl-5-(thiophen-2-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione, C12H15N3S2
- The crystal structure of 4,6-bis(dimethylamino)-2-fluoroisophthalonitrile, C12H13FN4
- Hydrogen bonding in the crystal structure of nicotin-1,1′-dium tetrabromidomanganate(II)
- The crystal structure of bis(2-bromobenzyl)(2-((2-oxybenzylidene)amino)-4-methylpentanoato-κ3N, O,O′)tin(IV), C27H27Br2NO3Sn
- Crystal structure of (E)-(3-(p-tolyl)acryloyl)ferrocene, C20H18FeO
- Crystal structure of (E)-7-fluoro-2-((5-(4-methylpiperazin-1-yl)pyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C21H22FN3O
- Crystal structure of (E)-7-methoxy-2-((5-(4-methylpiperazin-1-yl)pyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C22H25N3O2
- The crystal structure of poly(bis(μ2-1,3,5-tri(1H-imidazol-1-yl)benzene-κ2N:N′)-(μ2-2,3,5,6-tetrafluoroterephthalato-κ2O:O′)-manganese(II), C38H24F4N12O4Mn
- Crystal structure of (3,4-dimethoxybenzyl)triphenylphosphonium bromide ethanol solvate, C29H32BrO3P
- Crystal structure of tetraethylammonium hydrogencarbonate – (diaminomethylene)thiourea – water (2/1/3)
- Crystal structure of N, N-Dimethyl-N′-tosylformimidamide, C10H14N2O2S
- The crystal structure of ethyl 2-methyl-5-oxo-4-(2-methoxyphenyl)-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C20H23N2O4
- Crystal structure of bis(μ2-1,5-bis[(E)-1-(2-hydroxyphenyl)ethylidene] thiocarbonohydrazide)-bis(dimethylformamide)-dizinc(II) dimethylformamide solvate, C40H46N10O6S2Zn2⋅C3H7NO
- Crystal structure of azido-κ1N{hydridotris(3-tert-butyl-5-methylpyrazol-1-yl)borato-κ3N,N′,N″}copper(II), C24H40BCuN9
- The crystal structure of fac-tricarbonyl(1,10-phenanthroline-κ2N,N′)-(azido-κ1N)rhenium(I), C15H8N5O3Re
- Crystal structure of 4-((triphenylphosphonio)methyl)pyridin-1-ium tetrachloridozincate(II), C24H22Cl4NPZn

