Abstract
C18H17NO3, monoclinic, P21/n (no. 14), a = 14.702(3) Å, b = 7.0421(11) Å, c = 15.303(3) Å, β = 113.32(2)°, V = 1454.9(5) Å3, Z = 4, R gt (F) = 0.0522, wR ref (F2) = 0.1274, T = 100.03(18) K.
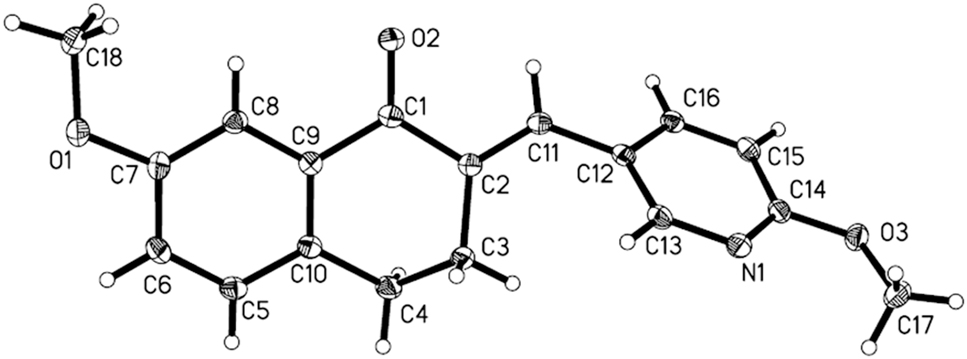
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.15 × 0.13 × 0.12 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.09 mm−1 |
| Diffractometer, scan mode: | SuperNova, |
| θmax, completeness: | 25.5°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 5780, 2706, 0.037 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 2210 |
| N(param)refined: | 201 |
| Programs: | CrysAlisPRO [1], SHELX [2], [3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1 | 0.60522 (13) | 0.1959 (3) | 0.74043 (13) | 0.0209 (4) |
| C2 | 0.58280 (13) | 0.0543 (3) | 0.80206 (13) | 0.0203 (4) |
| C3 | 0.48628 (13) | 0.0807 (3) | 0.81337 (14) | 0.0246 (5) |
| H3A | 0.493063 | 0.182582 | 0.858191 | 0.029* |
| H3B | 0.470026 | −0.034743 | 0.838653 | 0.029* |
| C4 | 0.40264 (14) | 0.1283 (3) | 0.71743 (15) | 0.0271 (5) |
| H4A | 0.391924 | 0.021682 | 0.674354 | 0.033* |
| H4B | 0.341807 | 0.150548 | 0.726427 | 0.033* |
| C5 | 0.35718 (14) | 0.4364 (3) | 0.62377 (13) | 0.0243 (4) |
| H5 | 0.291225 | 0.416245 | 0.613545 | 0.029* |
| C6 | 0.38296 (14) | 0.5988 (3) | 0.58844 (14) | 0.0264 (5) |
| H6 | 0.334722 | 0.687687 | 0.555707 | 0.032* |
| C7 | 0.48127 (14) | 0.6295 (3) | 0.60179 (13) | 0.0238 (4) |
| C8 | 0.55255 (13) | 0.4971 (3) | 0.64931 (13) | 0.0223 (4) |
| H8 | 0.617785 | 0.515581 | 0.656541 | 0.027* |
| C9 | 0.52652 (13) | 0.3344 (3) | 0.68674 (13) | 0.0203 (4) |
| C10 | 0.42824 (13) | 0.3020 (3) | 0.67457 (13) | 0.0216 (4) |
| C11 | 0.64810 (13) | −0.0856 (3) | 0.83997 (13) | 0.0213 (4) |
| H11 | 0.701958 | −0.088119 | 0.822580 | 0.026* |
| C12 | 0.64561 (13) | −0.2361 (3) | 0.90567 (13) | 0.0207 (4) |
| C13 | 0.61552 (14) | −0.2073 (3) | 0.97986 (14) | 0.0232 (4) |
| H13 | 0.593660 | −0.086562 | 0.986845 | 0.028* |
| C14 | 0.64567 (13) | −0.5127 (3) | 1.03041 (13) | 0.0227 (4) |
| C15 | 0.67955 (13) | −0.5589 (3) | 0.95984 (14) | 0.0238 (4) |
| H15 | 0.700922 | −0.681162 | 0.954833 | 0.029* |
| C16 | 0.68035 (13) | −0.4186 (3) | 0.89816 (13) | 0.0220 (4) |
| H16 | 0.703933 | −0.444214 | 0.851255 | 0.026* |
| C17 | 0.62767 (15) | −0.5983 (3) | 1.17267 (15) | 0.0311 (5) |
| H17A | 0.669149 | −0.492383 | 1.203339 | 0.047* |
| H17B | 0.641718 | −0.702547 | 1.216473 | 0.047* |
| H17C | 0.559400 | −0.562400 | 1.152595 | 0.047* |
| C18 | 0.59711 (14) | 0.8274 (3) | 0.57214 (15) | 0.0292 (5) |
| H18A | 0.642524 | 0.824837 | 0.637674 | 0.044* |
| H18B | 0.600831 | 0.948658 | 0.545038 | 0.044* |
| H18C | 0.614129 | 0.729117 | 0.537841 | 0.044* |
| N1 | 0.61569 (11) | −0.3415 (2) | 1.04243 (11) | 0.0240 (4) |
| O1 | 0.49892 (10) | 0.79735 (18) | 0.56603 (10) | 0.0313 (4) |
| O2 | 0.68674 (9) | 0.20122 (18) | 0.73600 (10) | 0.0281 (4) |
| O3 | 0.64672 (10) | −0.65428 (18) | 1.09140 (9) | 0.0271 (3) |
Source of material
The title compound was synthesized via a one-pot Claisen–Schmidt condensation reaction [4]. To a stirred solution of 7–methoxy-3,4-dihydronaphthalen-1(2H)-one (0.52 g, 3.0 mmol) and 6-methoxy-3-pyridinecarbaldehyde (0.41 g, 3.0 mmol) in methanol (10 mL), 20% NaOH solution (3 mL) was added. The mixture was stirred at room temperature for 5 h. The process of the reaction was monitored by thin layer chromatography (TLC). The yellow precipitated solids were collected by filtration, washed with 5 mL of cold methanol and dried in vacuum. The crude product was purified by column chromatography using petroleum ether and ethyl acetate (2:1, v/v). Crystals were prepared by slow evaporation of methanol solution under ambient temperature.
Experimental details
The C–H atoms were then constrained to ideal geometries with C–H distances of 0.93–0.97 Å. The Uiso values of the hydrogen atoms of methyl groups were set to 1.5Ueq(C) and the Uiso values of all other hydrogen atoms were set to 1.2Ueq(C).
Comment
Chalcone and its various derivatives have attracted intense interest not only due to the widespread distribution in plants, but also because of their potential biological properties including antimicrobial, antifungal, anti-inflammatory, antioxidant and anti-mutagenic activities [5], [6], [7], [8]. Generally, each chalcone is composed of two aromatic rings and a three-carbon α,β-unsaturated carbonyl system. The pharmacophore of α,β-unsaturated keto possesses a characteristic functionality, which can bind bio-thiols from susceptible neoplasms with lower toxicity toward normal cell [9]. In order to synthesize novel pharmaceutical agents, the aryl rings of chalcones may be modified by functional groups to improve their biological activities [10], [11], [12]. As an important class of compounds, 3,4-dihydronaphthalen-1(2H)-ones were used to synthesize chalcone derivatives and the corresponding compounds possess important biological activities [13], [14]. As one chalcone analogue, (2E)-2-(3,4-dichlorobenzylidene)-7-hydroxy-3,4-dihydronaphthalen-1(2H)-one was shown to act as a potent inhibitor of the monoamine oxidase enzymes [15]. It was reported that some chalcone-like derivatives containing 6-methoxy-3,4-dihydronaphthalenone possessed potential antioxidant properties [16]. Additionally, chalcones with methoxy groups and pyridine groups demonstrated antibiotic-resistant bacteria [17]. However, it is rarely reported that 3,4-dihydronaphthalenone-based chalcones can be used to treat neuroinflammatory diseases [18]. As a part of our continuing study on anti-neuroinflammatory agents for the treatment of inflammatory diseases in the central nervous system, a new chalcone with 3,4-dihydronapthalen-1(2H)-one and functional groups of methoxy was designed and synthesized.
X-ray crystallographic analysis shows that the title compound contains one independent molecule in the asymmetric unit (cf. the figure). All bond lengths and bond angles are all in the normal ranges []. Due to the arrangement of the pyridine ring and carbonyl group around the C2=C11 olefinic bonds, the title compound adopts the E stereochemistry. In the molecule, the cyclohexenonyl ring of the 3,4-dihydronaphthalen-1(2H)-one shows a half-chair conformation, which may be attributed to the conjugated relationship of the carbonyl group with the adjacent benzo moiety. The pyridyl and the benzo moety enclose a dihedral angle of 59.56(7)°. There are no classical hydrogen bonds found in the crystal, but molecules are connected to form layer structures through weak C18–H18B···O1 and C18–H18A···O2 hydrogen bonds. It is remarkable that the dihydronaphthalenone and methoxy group of the title compound can increase molecular lipophilicy and may effectively establish hydrophobic interactions with certain proteins. The unique structure will enable the title compound to possess a potential property in bioactivities.
Funding source: Shandong Province Higher Educational Science and Technology Program http://dx.doi.org/10.13039/501100015642
Award Identifier / Grant number: J18KA092
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work was supported by Project of the Shandong Province Higher Educational Science and Technology Program (No. J18KA092).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Rigaku, O. D. CrysAlisPRO; Rigaku Oxford Diffraction Ltd: Yarnton, Oxfordshire, England, 2017.Search in Google Scholar
2. Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Search in Google Scholar PubMed
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Wang, F., Zhang, R., Cui, Y., Sheng, L., Sun, Y., Tian, W., Liu, X., Liang, S. Design, synthesis and biological evaluation of 3,4-dihydronaphthalen-1(2H)-one derivatives as Bcl-2 inhibitors. Res. Chem. Intermed. 2017, 43, 5933–5942; https://doi.org/10.1007/s11164-017-2972-x.Search in Google Scholar
5. Gupta, D., Jain, D. K. Chalcone derivatives as potential antifungal agents: synthesis, and antifungal activity. J. Adv. Pharm. Technol. Res. 2015, 6, 114–117; https://doi.org/10.4103/2231-4040.161507.Search in Google Scholar PubMed PubMed Central
6. Katila, P., Shrestha, A., Shrestha, A., Shrestha, R., Park, P. H., Lee, E. S. Introduction of amino moiety enhances the inhibitory potency of 1-tetralone chalcone derivatives against LPS-stimulated reactive oxygen species production in RAW 264.7 macrophages. Bioorg. Chem. 2019, 87, 495–505; https://doi.org/10.1016/j.bioorg.2019.03.055.Search in Google Scholar PubMed
7. Zhuang, C., Zhang, W., Sheng, C., Zhang, W., Xing, C., Miao, Z. Chalcone: a privileged structure in medicinal chemistry. Chem. Rev. 2017, 117, 7762–7810; https://doi.org/10.1021/acs.chemrev.7b00020.Search in Google Scholar PubMed PubMed Central
8. Rashid, H., Xu, Y., Ahmad, N., Muhammad, Y., Wang, L. Promising anti-inflammatory effects of chalcones via inhibition of cyclooxygenase, prostaglandin E2, inducible NO synthase and nuclear factor κb activities. Bioorg. Chem. 2019, 87, 335–365; https://doi.org/10.1016/j.bioorg.2019.03.033.Search in Google Scholar PubMed
9. Sun, Y., Gao, Z., Wang, C., Hou, G. Synthesis, crystal structures and anti-inflammatory activity of fluorine-substituted 1,4,5,6-tetrahydrobenzo[h]quinazolin-2-amine derivatives. Acta Crystallogr. 2019, C75, 1157–1165; https://doi.org/10.1107/s2053229619010118.Search in Google Scholar
10. Elamathi, P., Chandrasekar, G., Balamurali, M. M. Nanoporous AlSBA-15 catalysed Claisen–Schmidt condensation for the synthesis of novel and biologically active chalcones. J. Porous Mater. 2020, 27, 817–829; https://doi.org/10.1007/s10934-019-00854-3.Search in Google Scholar
11. Park, S., Kim, E. H., Kim, J., Kim, S. H., Kim, I. Biological evaluation of indolizine-chalcone hybrids as new anticancer agents. Eur. J. Med. Chem. 2018, 144, 435–443; https://doi.org/10.1016/j.ejmech.2017.12.056.Search in Google Scholar PubMed
12. Gil, H. N., Koh, D., Lim, Y., Lee, Y. H., Shin, S. Y. The synthetic chalcone derivative 2-hydroxy-,5,-trimethoxychalcone induces unfolded protein response-mediated apoptosis in A549 lung cancer cells. Bioorg. Med. Chem. Lett 2018, 28, 2969–2975; https://doi.org/10.1016/j.bmcl.2018.07.003.Search in Google Scholar PubMed
13. Leng, J., Qin, H. L., Zhu, K., Jantan, I., Hussain, M. A., Sher, M., Amjad, M. W., Naeem-ul–Hassan, M., Ahmad, W., Bukhari, S. N. A. Evaluation of multifunctional synthetic tetralone derivatives for treatment of Alzheimer’s disease. Chem. Biol. Drug Des. 2016, 88, 889–898; https://doi.org/10.1111/cbdd.12822.Search in Google Scholar PubMed
14. Amakali, K. T., Legoabe, L. J., Petzer, A., Petzer, J. P. Synthesis and in vitro evaluation of 2-heteroarylidene-1-tetralone derivatives as monoamine oxidase inhibitors. Drug Res. 2018, 68, 687–695; https://doi.org/10.1055/a-0620-8309.Search in Google Scholar PubMed
15. Amakali, K. T., Legoabe, L. J., Petzer, A., Petzer, J. P. Synthesis and evaluation of 2-benzylidene-1-tetralone derivatives for monoamine oxidase inhibitory activity. Cent. Nerv. Syst. Agents Med. Chem. 2018, 18, 136–149; https://doi.org/10.2174/1871524918666180501121638.Search in Google Scholar PubMed
16. Ranjbar, S., Akbari, A., Edraki, N., Khoshneviszadeh, M., Hemmatian, H., Firuzi, O., Khoshneviszadeh, M. 6–Methoxy-3,4-dihydronaphthalenone chalcone-like derivatives as potent tyrosinase inhibitors and radical scavengers. Lett. Drug Des. Discov. 2018, 15, 1170–1179; https://doi.org/10.2174/1570180815666180219155027.Search in Google Scholar
17. Gibson, M. Z., Nguyen, M. A., Zingales, S. K. Design, synthesis, and evaluation of (2-(pyridinyl)methylene)-1-tetralone chalcones for anticancer and antimicrobial activity. Med. Chem. 2018, 14, 333–343; https://doi.org/10.2174/1573406413666171020121244.Search in Google Scholar PubMed
18. Sun, Y., Zhou, Y. Q., Liu, Y. K., Zhang, H. Q., Hou, G. G., Meng, Q. G., Hou, Y. Potential anti-neuroinflammatory NF-κB inhibitors based on 3,4-dihydronaphthalen-1(2H)-one derivatives. J. Enzym. Inhib. Med. Chem. 2020, 35, 1631–1640; https://doi.org/10.1080/14756366.2020.1804899.Search in Google Scholar PubMed PubMed Central
19. El–Sayed, N. N. E., Almaneai, N. M., Ghabbour, H. A., Alafeefy, A. M. Crystal structure of (E)-2-(4-hydroxy-3-methoxybenzylidene)-6- methoxy-3,4-dihydronaphthalen-1(2H)-one, C19H18O4. Z. Kristallogr. N. Cryst. Struct. 2017, 232, 203–205; https://doi.org/10.1515/ncrs-2016-0195.Search in Google Scholar
20. Luan, M.-Z., Meng, Q.-G. Crystal structure of (E)-7-methoxy-2-((5- methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C18H17NO3. Z. Kristallogr. N. Cryst. Struct. 2021, 236, 387–389; https://doi.org/10.1515/ncrs-2020-0602.Search in Google Scholar
21. Zhang, X.-F., Meng, Q.-G. Crystal structure of (E)-2-((2-methoxy-3- pyridyl)methylene)-7-fluoro-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2. Z. Kristallogr. N. Cryst. Struct. 2021, 236, 507–509; https://doi.org/10.1515/ncrs-2020-0603.Search in Google Scholar
© 2021 Lei Wang et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of [aqua-(4-iodopyridine-2,6-dicarboxylato-κ3 O,N,O′)-(1,10-phenanothroline-κ2 N,N′)copper(II)] dihydrate, C19H16O7N3CuI
- The crystal structure of tetrakis(1-isopropyl-1H-imidazolium) octamolybdate, C24H44Mo8N8O26
- Crystal structure of catena-poly[bis(µ2-3,5-bis(1-imidazolyl)pyridine-κ2 N:N′)-(µ2-3-nitrophthalato-k3 O,O′:O″)cadmium(II)] dihydrate, C30H25N11O8Cd
- The crystal structure of diaqua-bis(2-(3-(1H-pyrazol-4-yl)-1H-1,2,4-triazol-5-yl)pyridine-κ2 N:N′)-bis(3,5-dicarboxybenzoato-κ1 O)cobalt(II), C38H30CoN12O14
- Crystal structure of the nickel(II) complex aqua-(2,6-di(pyrazin-2-yl)-4,4′-bipyridine-κ3 N,N′,N′′)-(phthalato-κ2 O,O′)nickel(II) tetrahydrate, C26H26N6O9Ni
- The crystal structure of 1-[5-(2-fluorophenyl)-1-(pyridine-3-sulfonyl)-1H-pyrrol-3-yl]-N-methylmethanaminium 3-carboxyprop-2-enoate, C21H20FN3O6S
- The crystal structure of 1,2-bis(4-pyridyl)ethane - 4,4-dihydroxydiphenylmethane (1/1), C25H21N2O2
- Crystal structure of bis(2-((E)-5-chloro-2-hydroxybenzylidene)hydrazineyl)methaniminium trifluoroacetate dihydrate, C34H36Cl4N10O12
- Crystal structure of 1-cyclopropyl-7-ethoxy-6,8-difluoro-4-oxo-1,4-dihydro-quinoline-3-carboxylic acid, C15H13F2NO4
- Crystal structure of methyl 3-(1H-naphtho[1,8-de][1,3,2]diazaborinin-2(3H)-yl)benzoate, C18H15BN2O2
- Crystal structure of (E)-N′-(2-chloro-6-hydroxybenzylidene)-2-hydroxybenzohydrazide, C14H11ClN2O3
- Crystal structure of Al-rich fluorophlogopite, K1.0(Mg2.8Al0.2)(Si2.8Al1.2)O10F2
- The crystal structure of 4,5-diiodo-1,3-dimesityl-1H-1,2,3-triazol-3-ium hexafluoridoantimonate(V), C20H22F6I2N3Sb
- Crystal structure of tris(3-iodopyridin-1-ium) catena-poly[(hexachlorido-κ1 Cl)-(μ2-trichlorido-κ2 Cl:Cl)diantimony(III)], C15H15Cl9I3N3Sb2
- Crystal structure of methyl 2-(1H-naphtho[1,8-de][1.3.2]diazaborinin-2(3H-yl)benzoate C18H15BN2O2
- The crystal structure of 1,8-bis(4-methoxybenzoyl)naphthalene-2,7-diyl dibenzoate, C40H28O8
- Crystal structure of 2-bromo-1,3,6,8-tetramethylBOPHY (BOPHY = bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl)methylene)hydrazine), C14H15B2BrF4N4
- The crystal structure of (E)-3-chloro-2-(2-(2-fluorobenzylidene)hydrazinyl)pyridine, C12H9ClFN3
- Crystal structure of bis(µ2- 4-iodopyridine-2,6-dicarboxylato-κ3O:N:O′)-bis(4-iodopyridine-2,6-dicarboxylato-κ3O:N:O′)-bis(µ2-1-(4-pyridyl)piperazine-κ2N:N′)-hexa-aqua-tetra-copper(II), C46H46Cu4I4N10O22
- Crystal structure of poly[diaqua-(μ2-2,5-dihydroxyterephthalato-κ2O:O′)(μ2-bis(4-pyridylformyl)piperazine-κ2N:N′)cadmium(II)] dihydrate, C24H28CdN4O12
- Crystal structure of poly[aqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)-(μ3-2,3,5,6-tetrafluoroterephthalato-κ3O:O′:O′′)cadmium(II)], C17H14N4O5F4Cd
- Crystal structure of 6-(quinolin-8-yl)benzo[a]phenanthridin-5(6H)-one, C26H16N2O
- The crystal structure of aqua-bis(6-chloropicolinato-κ2N,O)copper(II), C12H8Cl2N2O5Cu
- Crystal structure of catena-poly[diaqua-bis(μ2-4,4′-bipyridyl-κ2N:N′) disilver(I)] 4-oxidopyridine-3-sulfonate trihydrate, C25H29Ag2N5O9S
- The crystal structure of 4-(3-bromophenyl)pyrimidin-2-amine, C10H8BrN3
- Crystal structure of 6-oxo-4-phenyl-1-propyl-1,6-dihydropyridine-3-carbonitrile, C15H14N2O
- Crystal structure of 4-(2,2-difluoroethyl)-2,4-dimethyl-6-(trifluoromethyl)isoquinoline-1,3(2H,4H)-dione, C14H12F5NO2
- Crystal structure of dibromido-(1-methyl-1H-imidazole-κ1N)-(3-(3-methyl-1H-imidazol-3-ium-1-yl)propanoato-κ1O)zinc(II), C11H16Br2N4O2Zn
- The crystal structure of 1,1′-(((2 (dimethylamino)ethyl)azanediyl)bis(methylene)) bis(naphthalen-2-olato-κ4 N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)- titanium(IV) ─ dichloromethane (2/1), C33H29N3O6Ti
- The layered crystal structure of bis(theophyllinium) hexachloridostannate (IV), C14H18N8O8SnCl6
- The crystal structre of 3-(1-ethenyl-1H-imidazol-3-ium-3-yl)propane-1-sulfonate, C8H12N2O3S
- Synthesis and crystal structure of di-tert-butyl 1″-acetyl-2,2″,9′-trioxo-4a′,9a′-dihydro-1′H,3′H,9′H-dispiro[indoline-3,2′-xanthene-4′,3″-indoline]-1,3′-dicarboxylate, C39H38N2O9
- The crystal structure of 4-chloro-2-(quinolin-8-yl)isoindoline-1,3-dione, C17H9ClN2O2
- The crystal structure of 1-fluoro-4-(p-tolylethynyl)benzene, C15H11F
- The crystal structure of bis[4-bromo-2-(1H-pyrazol-3-yl) phenolato-κ2N,O] copper(II), C18H12Br2CuN4O2
- The crystal structure of poly[(μ 3-imidazolato-κ 3 N:N:N′)(tetrahydrofuran- κ 1 O)lithium(I)], C7H11LiN2O
- Crystal structure of N′,N′′′-((1E,1′E)-(propane-2,2-diylbis(1H-pyrrole-5,2diyl))bis(methaneylylidene))di(nicotinohydrazide) pentahydrate, C25H24N8O2·5H2O
- Crystal structure of 3-(2-ethoxy-2-oxoethyl)-1-ethyl-1H-imidazol-3-ium hexafluoridophos-phate(V), C9H15F6N2O2P
- Crystal structure of (1,10-phenanthroline-κ2N,N′)-bis(3-thiophenecarboxylato-κ2O,O′)copper(II), C22H14N2O4S2Cu
- The crystal structure of 2-amino-3-carboxypyridin-1-ium iodide hemihydrate, C6H8IN2O2.5
- Crystal structure of (E)-7-methoxy-2-((6-methoxypyridin-2-yl)methylene)-tetralone, C18H17NO3
- The crystal structure of [μ-hydroxido-bis[(5,5′-dimethyl-2,2′-bipyridine-κ2N,N′)-tricarbonylrhenium(I)] bromide hemihydrate, C30H26N4O9Re2Br
- The crystal structure of 2,5-bis(3,5-dimethylphenyl)thiazolo[5,4-d]thiazole, C20H18N2S2
- The crystal structure of 5-benzoyl-1-[(E)-(4-fluorobenzylidene)amino]-4-phenylpyrimidin-2(1H)-one, C24H16FN3O2
- Crystal structure of monocarbonyl(N-nitroso-N-oxido-phenylamine-κ 2 O,O′)(tricyclohexylphosphine-κP)rhodium(I), C25H39N2O3PRh
- Crystal structure of poly[bis[μ3-1,3,5-tris[(1H-imidazol-1-yl)methyl]benzene-κ3N:N′:N″]nickel(II)] hexafluorosilicate, C36H36N12NiSiF6
- The crystal structure of 13-(pyrazole-1-yl-4-carbonitrile)-matrine, C19H25N5O
- Crystal structure of 3,5-bis((E)-4-methoxy-2-(trifluoromethyl)benzylidene)-1-methylpiperidin-4-one, C24H21F6NO3
- The crystal structure of N,N′-(Disulfanediyldi-2,1-phenylene)di(6′-methylpyridine)-2-carboxamide, C26H22N4O2S2
- Crystal structure of (E)-7-fluoro-2-(4-methoxy-2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C19H14F4O2
- Crystal structure of ethyl 1-(4-fluorophenyl)-4-phenyl-1H-pyrrole-3-carboxylate, C19H16FNO2
- The crystal structure of cis-diaqua-bis (N-butyl-N-(pyridin-2-yl)pyridin-2-amine-κ2N,N′)cobalt(II)] dichloride trihydrate, C28H44Cl2N6O5Co
- Crystal structure of (E)-7-methoxy-2-((6-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C18H17NO3
- Crystal structure of (E)-2-((3-fluoropyridin-4-yl)methylene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- The crystal structure of 6-bromohexanoic acid, C6H11BrO2
- The crystal structure of 4-chloro-thiophenol, C6H5ClS
- The crystal structure of 4-bromobenzyl chloride, C7H6BrCl
- The crystal structure of di-tert-butyl dicarbonate, C10H18O5
- The crystal structure of (2-(4-chlorophenyl)-5-methyl-1,3-dioxan-5-yl)methanol, C12H15ClO3
- The crystal structure of the co-crystal: 2-hydroxybenzoic acid – N′-(butan-2-ylidene)pyridine-4-carbohydrazide, C10H13N3O·C7H6O3
- Crystal structure and anti-inflammatory activity of (E)-7-fluoro-2-((5-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- Crystal structure of (E)-7-fluoro-2-((6-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- Crystal structure of 1,1′-(butane-1,4-diyl)bis(3-propyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C32H56F24N8P4
- The crystal structure of dichlorido-bis(3-methyl-3-imidazolium-1-ylpropionato-κ2)-cadmium(II), C14H20CdCl2N4O4
- Crystal structure of 1-(2-cyanobenzyl)-3-cyano-4-phenyl-4-(2-cyanobenzyl)-1,4-dihydropyridine monohydrate, C56H42N8O
- The crystal structure of 3-(carboxymethyl)-1-ethenyl-1H-imidazol-3-ium chloride, C7H9N2O2Cl
- The crystal structure of adamantylmethoxydiphenylsilane, C23H28OSi
- Redetermination of the crystal structure of (2E,4Z,13E,15Z)-3,5,14,16-tetramethyl-2,6,13,17-tetraazatricyclo[16.4.0.07,12]docosa-1(22),2,4,7,9,11,13,15,18,20-decaene, C22H24N4
- Crystal structure of (E)-7-hydroxy-2-((6-methoxypyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H15NO3
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2 N:N′)cobalt(II)] dinitrate, C18H28N10O8Co
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of [aqua-(4-iodopyridine-2,6-dicarboxylato-κ3 O,N,O′)-(1,10-phenanothroline-κ2 N,N′)copper(II)] dihydrate, C19H16O7N3CuI
- The crystal structure of tetrakis(1-isopropyl-1H-imidazolium) octamolybdate, C24H44Mo8N8O26
- Crystal structure of catena-poly[bis(µ2-3,5-bis(1-imidazolyl)pyridine-κ2 N:N′)-(µ2-3-nitrophthalato-k3 O,O′:O″)cadmium(II)] dihydrate, C30H25N11O8Cd
- The crystal structure of diaqua-bis(2-(3-(1H-pyrazol-4-yl)-1H-1,2,4-triazol-5-yl)pyridine-κ2 N:N′)-bis(3,5-dicarboxybenzoato-κ1 O)cobalt(II), C38H30CoN12O14
- Crystal structure of the nickel(II) complex aqua-(2,6-di(pyrazin-2-yl)-4,4′-bipyridine-κ3 N,N′,N′′)-(phthalato-κ2 O,O′)nickel(II) tetrahydrate, C26H26N6O9Ni
- The crystal structure of 1-[5-(2-fluorophenyl)-1-(pyridine-3-sulfonyl)-1H-pyrrol-3-yl]-N-methylmethanaminium 3-carboxyprop-2-enoate, C21H20FN3O6S
- The crystal structure of 1,2-bis(4-pyridyl)ethane - 4,4-dihydroxydiphenylmethane (1/1), C25H21N2O2
- Crystal structure of bis(2-((E)-5-chloro-2-hydroxybenzylidene)hydrazineyl)methaniminium trifluoroacetate dihydrate, C34H36Cl4N10O12
- Crystal structure of 1-cyclopropyl-7-ethoxy-6,8-difluoro-4-oxo-1,4-dihydro-quinoline-3-carboxylic acid, C15H13F2NO4
- Crystal structure of methyl 3-(1H-naphtho[1,8-de][1,3,2]diazaborinin-2(3H)-yl)benzoate, C18H15BN2O2
- Crystal structure of (E)-N′-(2-chloro-6-hydroxybenzylidene)-2-hydroxybenzohydrazide, C14H11ClN2O3
- Crystal structure of Al-rich fluorophlogopite, K1.0(Mg2.8Al0.2)(Si2.8Al1.2)O10F2
- The crystal structure of 4,5-diiodo-1,3-dimesityl-1H-1,2,3-triazol-3-ium hexafluoridoantimonate(V), C20H22F6I2N3Sb
- Crystal structure of tris(3-iodopyridin-1-ium) catena-poly[(hexachlorido-κ1 Cl)-(μ2-trichlorido-κ2 Cl:Cl)diantimony(III)], C15H15Cl9I3N3Sb2
- Crystal structure of methyl 2-(1H-naphtho[1,8-de][1.3.2]diazaborinin-2(3H-yl)benzoate C18H15BN2O2
- The crystal structure of 1,8-bis(4-methoxybenzoyl)naphthalene-2,7-diyl dibenzoate, C40H28O8
- Crystal structure of 2-bromo-1,3,6,8-tetramethylBOPHY (BOPHY = bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl)methylene)hydrazine), C14H15B2BrF4N4
- The crystal structure of (E)-3-chloro-2-(2-(2-fluorobenzylidene)hydrazinyl)pyridine, C12H9ClFN3
- Crystal structure of bis(µ2- 4-iodopyridine-2,6-dicarboxylato-κ3O:N:O′)-bis(4-iodopyridine-2,6-dicarboxylato-κ3O:N:O′)-bis(µ2-1-(4-pyridyl)piperazine-κ2N:N′)-hexa-aqua-tetra-copper(II), C46H46Cu4I4N10O22
- Crystal structure of poly[diaqua-(μ2-2,5-dihydroxyterephthalato-κ2O:O′)(μ2-bis(4-pyridylformyl)piperazine-κ2N:N′)cadmium(II)] dihydrate, C24H28CdN4O12
- Crystal structure of poly[aqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)-(μ3-2,3,5,6-tetrafluoroterephthalato-κ3O:O′:O′′)cadmium(II)], C17H14N4O5F4Cd
- Crystal structure of 6-(quinolin-8-yl)benzo[a]phenanthridin-5(6H)-one, C26H16N2O
- The crystal structure of aqua-bis(6-chloropicolinato-κ2N,O)copper(II), C12H8Cl2N2O5Cu
- Crystal structure of catena-poly[diaqua-bis(μ2-4,4′-bipyridyl-κ2N:N′) disilver(I)] 4-oxidopyridine-3-sulfonate trihydrate, C25H29Ag2N5O9S
- The crystal structure of 4-(3-bromophenyl)pyrimidin-2-amine, C10H8BrN3
- Crystal structure of 6-oxo-4-phenyl-1-propyl-1,6-dihydropyridine-3-carbonitrile, C15H14N2O
- Crystal structure of 4-(2,2-difluoroethyl)-2,4-dimethyl-6-(trifluoromethyl)isoquinoline-1,3(2H,4H)-dione, C14H12F5NO2
- Crystal structure of dibromido-(1-methyl-1H-imidazole-κ1N)-(3-(3-methyl-1H-imidazol-3-ium-1-yl)propanoato-κ1O)zinc(II), C11H16Br2N4O2Zn
- The crystal structure of 1,1′-(((2 (dimethylamino)ethyl)azanediyl)bis(methylene)) bis(naphthalen-2-olato-κ4 N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)- titanium(IV) ─ dichloromethane (2/1), C33H29N3O6Ti
- The layered crystal structure of bis(theophyllinium) hexachloridostannate (IV), C14H18N8O8SnCl6
- The crystal structre of 3-(1-ethenyl-1H-imidazol-3-ium-3-yl)propane-1-sulfonate, C8H12N2O3S
- Synthesis and crystal structure of di-tert-butyl 1″-acetyl-2,2″,9′-trioxo-4a′,9a′-dihydro-1′H,3′H,9′H-dispiro[indoline-3,2′-xanthene-4′,3″-indoline]-1,3′-dicarboxylate, C39H38N2O9
- The crystal structure of 4-chloro-2-(quinolin-8-yl)isoindoline-1,3-dione, C17H9ClN2O2
- The crystal structure of 1-fluoro-4-(p-tolylethynyl)benzene, C15H11F
- The crystal structure of bis[4-bromo-2-(1H-pyrazol-3-yl) phenolato-κ2N,O] copper(II), C18H12Br2CuN4O2
- The crystal structure of poly[(μ 3-imidazolato-κ 3 N:N:N′)(tetrahydrofuran- κ 1 O)lithium(I)], C7H11LiN2O
- Crystal structure of N′,N′′′-((1E,1′E)-(propane-2,2-diylbis(1H-pyrrole-5,2diyl))bis(methaneylylidene))di(nicotinohydrazide) pentahydrate, C25H24N8O2·5H2O
- Crystal structure of 3-(2-ethoxy-2-oxoethyl)-1-ethyl-1H-imidazol-3-ium hexafluoridophos-phate(V), C9H15F6N2O2P
- Crystal structure of (1,10-phenanthroline-κ2N,N′)-bis(3-thiophenecarboxylato-κ2O,O′)copper(II), C22H14N2O4S2Cu
- The crystal structure of 2-amino-3-carboxypyridin-1-ium iodide hemihydrate, C6H8IN2O2.5
- Crystal structure of (E)-7-methoxy-2-((6-methoxypyridin-2-yl)methylene)-tetralone, C18H17NO3
- The crystal structure of [μ-hydroxido-bis[(5,5′-dimethyl-2,2′-bipyridine-κ2N,N′)-tricarbonylrhenium(I)] bromide hemihydrate, C30H26N4O9Re2Br
- The crystal structure of 2,5-bis(3,5-dimethylphenyl)thiazolo[5,4-d]thiazole, C20H18N2S2
- The crystal structure of 5-benzoyl-1-[(E)-(4-fluorobenzylidene)amino]-4-phenylpyrimidin-2(1H)-one, C24H16FN3O2
- Crystal structure of monocarbonyl(N-nitroso-N-oxido-phenylamine-κ 2 O,O′)(tricyclohexylphosphine-κP)rhodium(I), C25H39N2O3PRh
- Crystal structure of poly[bis[μ3-1,3,5-tris[(1H-imidazol-1-yl)methyl]benzene-κ3N:N′:N″]nickel(II)] hexafluorosilicate, C36H36N12NiSiF6
- The crystal structure of 13-(pyrazole-1-yl-4-carbonitrile)-matrine, C19H25N5O
- Crystal structure of 3,5-bis((E)-4-methoxy-2-(trifluoromethyl)benzylidene)-1-methylpiperidin-4-one, C24H21F6NO3
- The crystal structure of N,N′-(Disulfanediyldi-2,1-phenylene)di(6′-methylpyridine)-2-carboxamide, C26H22N4O2S2
- Crystal structure of (E)-7-fluoro-2-(4-methoxy-2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C19H14F4O2
- Crystal structure of ethyl 1-(4-fluorophenyl)-4-phenyl-1H-pyrrole-3-carboxylate, C19H16FNO2
- The crystal structure of cis-diaqua-bis (N-butyl-N-(pyridin-2-yl)pyridin-2-amine-κ2N,N′)cobalt(II)] dichloride trihydrate, C28H44Cl2N6O5Co
- Crystal structure of (E)-7-methoxy-2-((6-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C18H17NO3
- Crystal structure of (E)-2-((3-fluoropyridin-4-yl)methylene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- The crystal structure of 6-bromohexanoic acid, C6H11BrO2
- The crystal structure of 4-chloro-thiophenol, C6H5ClS
- The crystal structure of 4-bromobenzyl chloride, C7H6BrCl
- The crystal structure of di-tert-butyl dicarbonate, C10H18O5
- The crystal structure of (2-(4-chlorophenyl)-5-methyl-1,3-dioxan-5-yl)methanol, C12H15ClO3
- The crystal structure of the co-crystal: 2-hydroxybenzoic acid – N′-(butan-2-ylidene)pyridine-4-carbohydrazide, C10H13N3O·C7H6O3
- Crystal structure and anti-inflammatory activity of (E)-7-fluoro-2-((5-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- Crystal structure of (E)-7-fluoro-2-((6-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- Crystal structure of 1,1′-(butane-1,4-diyl)bis(3-propyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C32H56F24N8P4
- The crystal structure of dichlorido-bis(3-methyl-3-imidazolium-1-ylpropionato-κ2)-cadmium(II), C14H20CdCl2N4O4
- Crystal structure of 1-(2-cyanobenzyl)-3-cyano-4-phenyl-4-(2-cyanobenzyl)-1,4-dihydropyridine monohydrate, C56H42N8O
- The crystal structure of 3-(carboxymethyl)-1-ethenyl-1H-imidazol-3-ium chloride, C7H9N2O2Cl
- The crystal structure of adamantylmethoxydiphenylsilane, C23H28OSi
- Redetermination of the crystal structure of (2E,4Z,13E,15Z)-3,5,14,16-tetramethyl-2,6,13,17-tetraazatricyclo[16.4.0.07,12]docosa-1(22),2,4,7,9,11,13,15,18,20-decaene, C22H24N4
- Crystal structure of (E)-7-hydroxy-2-((6-methoxypyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H15NO3
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2 N:N′)cobalt(II)] dinitrate, C18H28N10O8Co

