Abstract
C9H15F6N2O2P, monoclinic, P21/n (no. 14), a = 8.3781(15) Å, b = 13.970(3) Å, c = 12.904(2) Å, β = 104.500(2)°, V = 1462.3(5) Å3, Z = 4, R gt (F) = 0.0677, wR ref(F 2) = 0.1977, T = 296(2) K.
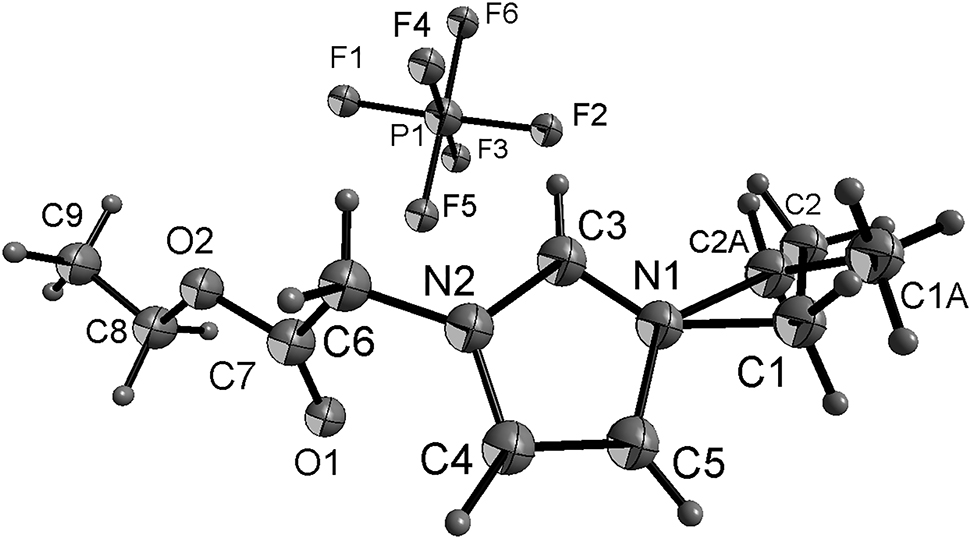
The molecular structure is shown in the figure (Atoms are shown with arbitrary radii). Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colorless block |
| Size: | 0.21 × 0.17 × 0.14 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.26 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| θ max, completeness: | 25.5°, >99% |
| N(hkl) measured, N(hkl)unique, R int: | 11050, 2724, 0.020 |
| Criterion for I obs, N(hkl)gt: | I obs > 2 σ(I obs), 2028 |
| N(param)refined: | 205 |
| Programs: | Bruker [1], SHELX [2], [3], Diamond [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| C1 | −0.215 (2) | 0.6235 (18) | 0.341 (2) | 0.107 (8) |
| H1A | −0.2679 | 0.6186 | 0.4000 | 0.128* |
| H1B | −0.2162 | 0.5615 | 0.3074 | 0.128* |
| C2 | −0.294 (3) | 0.7010 (18) | 0.2611 (17) | 0.112 (8) |
| H2A | −0.2258 | 0.7131 | 0.2130 | 0.168* |
| H2B | −0.3060 | 0.7588 | 0.2988 | 0.168* |
| H2C | −0.4009 | 0.6797 | 0.2212 | 0.168* |
| C3 | 0.0885 (5) | 0.6878 (3) | 0.3473 (3) | 0.0744 (10) |
| H3 | 0.0904 | 0.7073 | 0.2787 | 0.089* |
| C4 | 0.1674 (6) | 0.6492 (3) | 0.5167 (3) | 0.0874 (12) |
| H4 | 0.2332 | 0.6380 | 0.5851 | 0.105* |
| C5 | 0.0042 (7) | 0.6381 (4) | 0.4832 (5) | 0.1069 (17) |
| H5 | −0.0651 | 0.6171 | 0.5246 | 0.128* |
| C6 | 0.3859 (4) | 0.7006 (2) | 0.4253 (3) | 0.0654 (9) |
| H6A | 0.3879 | 0.7139 | 0.3518 | 0.078* |
| H6B | 0.4534 | 0.6444 | 0.4486 | 0.078* |
| C7 | 0.4584 (5) | 0.7843 (3) | 0.4944 (3) | 0.0683 (9) |
| C8 | 0.6852 (7) | 0.8905 (4) | 0.5296 (5) | 0.1161 (18) |
| H8A | 0.6118 | 0.9450 | 0.5239 | 0.139* |
| H8B | 0.7240 | 0.8738 | 0.6047 | 0.139* |
| C9 | 0.8191 (10) | 0.9145 (6) | 0.4887 (7) | 0.186 (4) |
| H9A | 0.8884 | 0.8595 | 0.4910 | 0.280* |
| H9B | 0.8810 | 0.9650 | 0.5310 | 0.280* |
| H9C | 0.7798 | 0.9357 | 0.4160 | 0.280* |
| N1 | −0.0432 (4) | 0.6629 (3) | 0.3780 (3) | 0.0974 (12) |
| N2 | 0.2178 (4) | 0.6806 (2) | 0.4296 (2) | 0.0625 (7) |
| O1 | 0.4026 (4) | 0.8196 (2) | 0.5618 (2) | 0.0909 (9) |
| O2 | 0.5963 (3) | 0.80970 (19) | 0.4704 (2) | 0.0791 (8) |
| P1 | 0.21382 (14) | 0.92432 (7) | 0.20331 (8) | 0.0741 (4) |
| F1 | 0.3934 (4) | 0.9605 (3) | 0.2159 (4) | 0.1680 (16) |
| F2 | 0.0353 (4) | 0.8861 (3) | 0.1873 (3) | 0.1488 (14) |
| F3 | 0.1626 (4) | 1.02882 (19) | 0.2258 (2) | 0.1221 (11) |
| F4 | 0.2675 (5) | 0.8199 (2) | 0.1817 (3) | 0.1370 (13) |
| F5 | 0.2497 (5) | 0.9010 (2) | 0.3253 (2) | 0.1282 (12) |
| F6 | 0.1809 (5) | 0.9463 (3) | 0.0801 (2) | 0.1323 (12) |
| C2A | −0.2120 (9) | 0.6706 (8) | 0.3010 (8) | 0.111 (3) |
| H2AA | −0.2871 | 0.7049 | 0.3340 | 0.133* |
| H2AB | −0.2049 | 0.7045 | 0.2367 | 0.133* |
| C1A | −0.2678 (12) | 0.5757 (7) | 0.2759 (10) | 0.155 (4) |
| H1AA | −0.3757 | 0.5770 | 0.2279 | 0.233* |
| H1AB | −0.2723 | 0.5428 | 0.3404 | 0.233* |
| H1AC | −0.1931 | 0.5430 | 0.2425 | 0.233* |
-
aOccupancy: 0.265(15), bOccupancy: 0.735(15).
Source of material
Ethyl bromoacetate (7.5 mL, 0.067 mol) was added dropwise to a stirred solution of 1-ethylimidazole (5.77 g, 0.06 mol) in THF (60 mL). The mixture was stirred vigorously at −5 °C for 1.5 h, then at room temperature for 5–8 h. After the reaction completed (monitored by TLC), the THF top phase was decanted and the product washed with ethyl acetate and diethyl ether three times, respectively. Then residual solvent was removed, and the product was dried in vacuo at 60 °C for 1 h to give a white powder solid in 85.5% yield. Then the intermediate (3-(2-ethoxy-2-oxoethyl)-1-ethyl-1H-imidazol-3-ium bromide) (1.32 g, 0.005 mol) and KPF6 (1.08 g, 0.0058 mol) were dissolved in water (25 mL). The mixture stirred at 80 °C for 14 h, and then cooled slowly. The crystals were obtained in 25% yield.
Experimental details
All H atoms were included in calculated positions and refined as riding atoms, with C–H = 0.90–0.97 Å with U iso(H) = 1.5 U eq(C) for methyl H atoms and 1.2 U eq(C) for all other H atoms. The ethyl group is disordered (see Table 2 and the Figure).
Comment
As a room temperature molten salt, some ionic liquids have been recognized as a new type of substance [5]. Most of them exist in liquid form at relatively low temperatures (below 100 °C) or room temperature [6]. Due to their wide electrochemical window [7], they have a wide application prospect in extraction [8], biocatalysis [9], and material synthesis [10] compared to traditional solvents. In recent years, some hexafluorophosphate ionic liquids have been extensively studied due to their superior physical and chemical properties [11], [12]. Therefore, in order to keep up with the pace of green chemistry, we are committed to find hexafluorophosphate ionic liquids with better catalytic and recycled utilization efficiency [5, 13–17].
In the title structure bond lengths and angles within 3-(2-ethoxy-2-oxoethyl)-1-ethyl-1H-imidazol-3-ium and in the counter anion hexafluoridophosphate(V) are very similar to those given in the literature for 3-(2-ethoxy-2-oxoethyl)-1-vinyl-1H-imidazol-3-ium hexafluoridophosphate(V) [5], [18]. The atoms of the imidazole moiety are coplanar, and the dihedral angle of the imidazole ring and the carboxylate group is 58.8(2)°. The torsion angles of C3–N2–C6–C7, N2–C6–C7–O2, C6–C7–O2–C8, and C7–O2–C8–C9 are 116.0(3)°, −167.8(2)°, −179.9(3)° and 174.3(4)°, respectively.
Funding source: National Natural Science Foundation of China 10.13039/501100001809
Award Identifier / Grant number: 31760193
Funding source: Natural Science Foundation of Jiangxi Province of China
Award Identifier / Grant number: 20202BABL205003
Funding source: Key Research Foundation of Education Department of Jiangxi Province of China
Award Identifier / Grant number: GJJ190181, GJJ200404
Acknowledgements
X-ray data were collected at Instrumental Analysis Center Nanchang Hangkong University, Nanchang, 330063, People’s Republic of China.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work was supported by the National Natural Science Foundation of China (No. 31760193), the Natural Science Foundation of Jiangxi Province of China (No. 20202BABL205003) and the Key Research Foundation of Education Department of Jiangxi Province of China [GJJ190181, GJJ200404].
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. APEX2, SAINT and SADABS; Bruker AXS Inc.: Madison, Wisconsin, USA, 2009.Search in Google Scholar
2. Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Search in Google Scholar PubMed
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar PubMed PubMed Central
4. Brandenburg, K. DIAMOND. Visual Crystal Structure Information System. Ver. 4.0; Crystal Impact: Bonn, Germany, 2015.Search in Google Scholar
5. Huang, T., Nie, X. L., Chen, J., Zhao, W., Xiong, W. M. Crystal structure of 3-(2-ethoxy-2-oxoethyl)-1-methyl-1H-imidazol-3- iumhexafluoridophosphate(V), C8H13F6N2O2P. Z. Kristallogr. N. Cryst. Struct. 2019, 234, 1077–1079; https://doi.org/10.1515/ncrs-2019-0273.Search in Google Scholar
6. Zhang, Q. H., Shreeve, J. M. Energetic ionic liquids as explosives and propellant fuels: a new journey of ionic liquid chemistry. Chem. Rev. 2014, 114, 10527–10574; https://doi.org/10.1021/cr500364t.Search in Google Scholar PubMed
7. Huang, T., Zhao, W., Zhang, X. H., Nie, X. L., Chen, J., Xiong, W. M. Synthesis and characterization of diimidazole-based hexafluorophosphate ionic liquids. J. Mol. Liq. 2020, 320, 114465; https://doi.org/10.1016/j.molliq.2020.114465.Search in Google Scholar
8. Matsumoto, M., Mochiduki, K., Fukunishi, K., Kondo, K. Extraction of organic acids using imidazolium-based ionic liquids and their toxicity to Lactobacillus rhamnosus. Separ. Purif. Technol. 2014, 40, 97–101.10.1016/j.seppur.2004.01.009Search in Google Scholar
9. Lutz–Wahl, S., Trost, E. M., Wagner, B., Manns, A., Fischer, L. Performance of D-amino acid oxidase in presence of ionic liquids. J. Biotechnol. 2006, 124, 163–171; https://doi.org/10.1016/j.jbiotec.2006.01.023.Search in Google Scholar PubMed
10. Cao, J. M., Fang, B. Q., Wang, J., Zheng, M. B., Deng, S. G., Ma, X. J. Ionic liquids for the convenient synthesis of functional inorganic nanomaterials. Prog. Chem. 2005, 06, 82–87.Search in Google Scholar
11. Bakkar, A., Neubert, V. Electrodeposition and corrosion characterisation of micro- and nano-crystalline aluminium from AlCl3/1-ethyl-3-methylimidazolium chloride ionic liquid. Electrochim. Acta 2013, 103, 211–218; https://doi.org/10.1016/j.electacta.2013.03.198.Search in Google Scholar
12. DePasquale, J., White, N. J., Ennis, E. J., Zeller, M., Foley, J. P., Papish, E. T. Synthesis of chiral N-heterocyclic carbene (NHC) ligand precursors and formation of ruthenium(II) complexes for transfer hydrogenation catalysts. Polyhedron 2013, 58, 162–170; https://doi.org/10.1016/j.poly.2012.10.010.Search in Google Scholar
13. Kong, J. H., Lan, Y. D., Chen, J., Huang, C. G., Xiong, W. M. Preparation and component analysis of biodiesel catalyzed by functionalized dication ionic liquid. Acta Agric. Univ. Jiangxiensis 2016, 38, 386–390.Search in Google Scholar
14. Zhou, Y. H., Huang, T., Nie, X. L., Chen, J., Xiong, W. M. Crystal structure of 3,3′-(1,2-phenylenebis(methylene))bis(1-methyl-1H- imidazol-3-ium) bis(hexafluoridophosphate), C16H20F12N4P2. Z. Kristallogr. N. Cryst. Struct. 2020, 235, 1217–1219.10.1515/ncrs-2020-0268Search in Google Scholar
15. Xiong, W. M., Chen, J., Zhao, W., Zhou, Y. H., Jing, C., Nie, X. L. Crystal structure of 1,1′-methylenebis(3-ethyl-1H-imidazol-3-ium) bis(hexafluorophosphate(V)), C11H18F12N4P2. Z. Kristallogr. N. Cryst. Struct. 2020, 235, 7–9; https://doi.org/10.1515/ncrs-2020-0147.Search in Google Scholar
16. Zhao, W., Liu, X. T., Wu, S. Q., Xiong, W. M., Nie, X. L. Crystal structure of 3,3′-(1,2-phenylene-bis(methylene))bis(1-ethyl- 1H-imidazol-3-ium) bis(hexafluorophosphate), C18H24F12N4P2. Z. Kristallogr. N. Cryst. Struct. 2021, 236, 545–547; https://doi.org/10.1515/ncrs-2020-0639.Search in Google Scholar
17. Huang, T., Chen, J. Z., Nie, X. L., Chen, J., Xiong, W. M. Crystal structure of 3,3′-(1,2-phenylene-bis(methylene))bis(1-vinyl- 1H-imidazol-3-ium) bis(hexafluorophosphate)(V), C18H20F12N4P2. Z. Kristallogr. N. Cryst. Struct. 2021, 236, 369–371.10.1515/ncrs-2020-0555Search in Google Scholar
18. Xiong, W. M., Huang, T., Liao, S., Chen, J., Nie, X. L. Crystal structure of 3-(2-ethoxy-2-oxoethyl)-1-vinyl-1H-imidazol-3-ium hexafluoridophosphate(V), C9H13F6N2O2P. Z. Kristallogr. N. Cryst. Struct. 2020, 235, 1029–1031; https://doi.org/10.1515/ncrs-2020-0147.Search in Google Scholar
© 2021 Kun Yuan et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of [aqua-(4-iodopyridine-2,6-dicarboxylato-κ3 O,N,O′)-(1,10-phenanothroline-κ2 N,N′)copper(II)] dihydrate, C19H16O7N3CuI
- The crystal structure of tetrakis(1-isopropyl-1H-imidazolium) octamolybdate, C24H44Mo8N8O26
- Crystal structure of catena-poly[bis(µ2-3,5-bis(1-imidazolyl)pyridine-κ2 N:N′)-(µ2-3-nitrophthalato-k3 O,O′:O″)cadmium(II)] dihydrate, C30H25N11O8Cd
- The crystal structure of diaqua-bis(2-(3-(1H-pyrazol-4-yl)-1H-1,2,4-triazol-5-yl)pyridine-κ2 N:N′)-bis(3,5-dicarboxybenzoato-κ1 O)cobalt(II), C38H30CoN12O14
- Crystal structure of the nickel(II) complex aqua-(2,6-di(pyrazin-2-yl)-4,4′-bipyridine-κ3 N,N′,N′′)-(phthalato-κ2 O,O′)nickel(II) tetrahydrate, C26H26N6O9Ni
- The crystal structure of 1-[5-(2-fluorophenyl)-1-(pyridine-3-sulfonyl)-1H-pyrrol-3-yl]-N-methylmethanaminium 3-carboxyprop-2-enoate, C21H20FN3O6S
- The crystal structure of 1,2-bis(4-pyridyl)ethane - 4,4-dihydroxydiphenylmethane (1/1), C25H21N2O2
- Crystal structure of bis(2-((E)-5-chloro-2-hydroxybenzylidene)hydrazineyl)methaniminium trifluoroacetate dihydrate, C34H36Cl4N10O12
- Crystal structure of 1-cyclopropyl-7-ethoxy-6,8-difluoro-4-oxo-1,4-dihydro-quinoline-3-carboxylic acid, C15H13F2NO4
- Crystal structure of methyl 3-(1H-naphtho[1,8-de][1,3,2]diazaborinin-2(3H)-yl)benzoate, C18H15BN2O2
- Crystal structure of (E)-N′-(2-chloro-6-hydroxybenzylidene)-2-hydroxybenzohydrazide, C14H11ClN2O3
- Crystal structure of Al-rich fluorophlogopite, K1.0(Mg2.8Al0.2)(Si2.8Al1.2)O10F2
- The crystal structure of 4,5-diiodo-1,3-dimesityl-1H-1,2,3-triazol-3-ium hexafluoridoantimonate(V), C20H22F6I2N3Sb
- Crystal structure of tris(3-iodopyridin-1-ium) catena-poly[(hexachlorido-κ1 Cl)-(μ2-trichlorido-κ2 Cl:Cl)diantimony(III)], C15H15Cl9I3N3Sb2
- Crystal structure of methyl 2-(1H-naphtho[1,8-de][1.3.2]diazaborinin-2(3H-yl)benzoate C18H15BN2O2
- The crystal structure of 1,8-bis(4-methoxybenzoyl)naphthalene-2,7-diyl dibenzoate, C40H28O8
- Crystal structure of 2-bromo-1,3,6,8-tetramethylBOPHY (BOPHY = bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl)methylene)hydrazine), C14H15B2BrF4N4
- The crystal structure of (E)-3-chloro-2-(2-(2-fluorobenzylidene)hydrazinyl)pyridine, C12H9ClFN3
- Crystal structure of bis(µ2- 4-iodopyridine-2,6-dicarboxylato-κ3O:N:O′)-bis(4-iodopyridine-2,6-dicarboxylato-κ3O:N:O′)-bis(µ2-1-(4-pyridyl)piperazine-κ2N:N′)-hexa-aqua-tetra-copper(II), C46H46Cu4I4N10O22
- Crystal structure of poly[diaqua-(μ2-2,5-dihydroxyterephthalato-κ2O:O′)(μ2-bis(4-pyridylformyl)piperazine-κ2N:N′)cadmium(II)] dihydrate, C24H28CdN4O12
- Crystal structure of poly[aqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)-(μ3-2,3,5,6-tetrafluoroterephthalato-κ3O:O′:O′′)cadmium(II)], C17H14N4O5F4Cd
- Crystal structure of 6-(quinolin-8-yl)benzo[a]phenanthridin-5(6H)-one, C26H16N2O
- The crystal structure of aqua-bis(6-chloropicolinato-κ2N,O)copper(II), C12H8Cl2N2O5Cu
- Crystal structure of catena-poly[diaqua-bis(μ2-4,4′-bipyridyl-κ2N:N′) disilver(I)] 4-oxidopyridine-3-sulfonate trihydrate, C25H29Ag2N5O9S
- The crystal structure of 4-(3-bromophenyl)pyrimidin-2-amine, C10H8BrN3
- Crystal structure of 6-oxo-4-phenyl-1-propyl-1,6-dihydropyridine-3-carbonitrile, C15H14N2O
- Crystal structure of 4-(2,2-difluoroethyl)-2,4-dimethyl-6-(trifluoromethyl)isoquinoline-1,3(2H,4H)-dione, C14H12F5NO2
- Crystal structure of dibromido-(1-methyl-1H-imidazole-κ1N)-(3-(3-methyl-1H-imidazol-3-ium-1-yl)propanoato-κ1O)zinc(II), C11H16Br2N4O2Zn
- The crystal structure of 1,1′-(((2 (dimethylamino)ethyl)azanediyl)bis(methylene)) bis(naphthalen-2-olato-κ4 N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)- titanium(IV) ─ dichloromethane (2/1), C33H29N3O6Ti
- The layered crystal structure of bis(theophyllinium) hexachloridostannate (IV), C14H18N8O8SnCl6
- The crystal structre of 3-(1-ethenyl-1H-imidazol-3-ium-3-yl)propane-1-sulfonate, C8H12N2O3S
- Synthesis and crystal structure of di-tert-butyl 1″-acetyl-2,2″,9′-trioxo-4a′,9a′-dihydro-1′H,3′H,9′H-dispiro[indoline-3,2′-xanthene-4′,3″-indoline]-1,3′-dicarboxylate, C39H38N2O9
- The crystal structure of 4-chloro-2-(quinolin-8-yl)isoindoline-1,3-dione, C17H9ClN2O2
- The crystal structure of 1-fluoro-4-(p-tolylethynyl)benzene, C15H11F
- The crystal structure of bis[4-bromo-2-(1H-pyrazol-3-yl) phenolato-κ2N,O] copper(II), C18H12Br2CuN4O2
- The crystal structure of poly[(μ 3-imidazolato-κ 3 N:N:N′)(tetrahydrofuran- κ 1 O)lithium(I)], C7H11LiN2O
- Crystal structure of N′,N′′′-((1E,1′E)-(propane-2,2-diylbis(1H-pyrrole-5,2diyl))bis(methaneylylidene))di(nicotinohydrazide) pentahydrate, C25H24N8O2·5H2O
- Crystal structure of 3-(2-ethoxy-2-oxoethyl)-1-ethyl-1H-imidazol-3-ium hexafluoridophos-phate(V), C9H15F6N2O2P
- Crystal structure of (1,10-phenanthroline-κ2N,N′)-bis(3-thiophenecarboxylato-κ2O,O′)copper(II), C22H14N2O4S2Cu
- The crystal structure of 2-amino-3-carboxypyridin-1-ium iodide hemihydrate, C6H8IN2O2.5
- Crystal structure of (E)-7-methoxy-2-((6-methoxypyridin-2-yl)methylene)-tetralone, C18H17NO3
- The crystal structure of [μ-hydroxido-bis[(5,5′-dimethyl-2,2′-bipyridine-κ2N,N′)-tricarbonylrhenium(I)] bromide hemihydrate, C30H26N4O9Re2Br
- The crystal structure of 2,5-bis(3,5-dimethylphenyl)thiazolo[5,4-d]thiazole, C20H18N2S2
- The crystal structure of 5-benzoyl-1-[(E)-(4-fluorobenzylidene)amino]-4-phenylpyrimidin-2(1H)-one, C24H16FN3O2
- Crystal structure of monocarbonyl(N-nitroso-N-oxido-phenylamine-κ 2 O,O′)(tricyclohexylphosphine-κP)rhodium(I), C25H39N2O3PRh
- Crystal structure of poly[bis[μ3-1,3,5-tris[(1H-imidazol-1-yl)methyl]benzene-κ3N:N′:N″]nickel(II)] hexafluorosilicate, C36H36N12NiSiF6
- The crystal structure of 13-(pyrazole-1-yl-4-carbonitrile)-matrine, C19H25N5O
- Crystal structure of 3,5-bis((E)-4-methoxy-2-(trifluoromethyl)benzylidene)-1-methylpiperidin-4-one, C24H21F6NO3
- The crystal structure of N,N′-(Disulfanediyldi-2,1-phenylene)di(6′-methylpyridine)-2-carboxamide, C26H22N4O2S2
- Crystal structure of (E)-7-fluoro-2-(4-methoxy-2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C19H14F4O2
- Crystal structure of ethyl 1-(4-fluorophenyl)-4-phenyl-1H-pyrrole-3-carboxylate, C19H16FNO2
- The crystal structure of cis-diaqua-bis (N-butyl-N-(pyridin-2-yl)pyridin-2-amine-κ2N,N′)cobalt(II)] dichloride trihydrate, C28H44Cl2N6O5Co
- Crystal structure of (E)-7-methoxy-2-((6-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C18H17NO3
- Crystal structure of (E)-2-((3-fluoropyridin-4-yl)methylene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- The crystal structure of 6-bromohexanoic acid, C6H11BrO2
- The crystal structure of 4-chloro-thiophenol, C6H5ClS
- The crystal structure of 4-bromobenzyl chloride, C7H6BrCl
- The crystal structure of di-tert-butyl dicarbonate, C10H18O5
- The crystal structure of (2-(4-chlorophenyl)-5-methyl-1,3-dioxan-5-yl)methanol, C12H15ClO3
- The crystal structure of the co-crystal: 2-hydroxybenzoic acid – N′-(butan-2-ylidene)pyridine-4-carbohydrazide, C10H13N3O·C7H6O3
- Crystal structure and anti-inflammatory activity of (E)-7-fluoro-2-((5-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- Crystal structure of (E)-7-fluoro-2-((6-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- Crystal structure of 1,1′-(butane-1,4-diyl)bis(3-propyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C32H56F24N8P4
- The crystal structure of dichlorido-bis(3-methyl-3-imidazolium-1-ylpropionato-κ2)-cadmium(II), C14H20CdCl2N4O4
- Crystal structure of 1-(2-cyanobenzyl)-3-cyano-4-phenyl-4-(2-cyanobenzyl)-1,4-dihydropyridine monohydrate, C56H42N8O
- The crystal structure of 3-(carboxymethyl)-1-ethenyl-1H-imidazol-3-ium chloride, C7H9N2O2Cl
- The crystal structure of adamantylmethoxydiphenylsilane, C23H28OSi
- Redetermination of the crystal structure of (2E,4Z,13E,15Z)-3,5,14,16-tetramethyl-2,6,13,17-tetraazatricyclo[16.4.0.07,12]docosa-1(22),2,4,7,9,11,13,15,18,20-decaene, C22H24N4
- Crystal structure of (E)-7-hydroxy-2-((6-methoxypyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H15NO3
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2 N:N′)cobalt(II)] dinitrate, C18H28N10O8Co
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of [aqua-(4-iodopyridine-2,6-dicarboxylato-κ3 O,N,O′)-(1,10-phenanothroline-κ2 N,N′)copper(II)] dihydrate, C19H16O7N3CuI
- The crystal structure of tetrakis(1-isopropyl-1H-imidazolium) octamolybdate, C24H44Mo8N8O26
- Crystal structure of catena-poly[bis(µ2-3,5-bis(1-imidazolyl)pyridine-κ2 N:N′)-(µ2-3-nitrophthalato-k3 O,O′:O″)cadmium(II)] dihydrate, C30H25N11O8Cd
- The crystal structure of diaqua-bis(2-(3-(1H-pyrazol-4-yl)-1H-1,2,4-triazol-5-yl)pyridine-κ2 N:N′)-bis(3,5-dicarboxybenzoato-κ1 O)cobalt(II), C38H30CoN12O14
- Crystal structure of the nickel(II) complex aqua-(2,6-di(pyrazin-2-yl)-4,4′-bipyridine-κ3 N,N′,N′′)-(phthalato-κ2 O,O′)nickel(II) tetrahydrate, C26H26N6O9Ni
- The crystal structure of 1-[5-(2-fluorophenyl)-1-(pyridine-3-sulfonyl)-1H-pyrrol-3-yl]-N-methylmethanaminium 3-carboxyprop-2-enoate, C21H20FN3O6S
- The crystal structure of 1,2-bis(4-pyridyl)ethane - 4,4-dihydroxydiphenylmethane (1/1), C25H21N2O2
- Crystal structure of bis(2-((E)-5-chloro-2-hydroxybenzylidene)hydrazineyl)methaniminium trifluoroacetate dihydrate, C34H36Cl4N10O12
- Crystal structure of 1-cyclopropyl-7-ethoxy-6,8-difluoro-4-oxo-1,4-dihydro-quinoline-3-carboxylic acid, C15H13F2NO4
- Crystal structure of methyl 3-(1H-naphtho[1,8-de][1,3,2]diazaborinin-2(3H)-yl)benzoate, C18H15BN2O2
- Crystal structure of (E)-N′-(2-chloro-6-hydroxybenzylidene)-2-hydroxybenzohydrazide, C14H11ClN2O3
- Crystal structure of Al-rich fluorophlogopite, K1.0(Mg2.8Al0.2)(Si2.8Al1.2)O10F2
- The crystal structure of 4,5-diiodo-1,3-dimesityl-1H-1,2,3-triazol-3-ium hexafluoridoantimonate(V), C20H22F6I2N3Sb
- Crystal structure of tris(3-iodopyridin-1-ium) catena-poly[(hexachlorido-κ1 Cl)-(μ2-trichlorido-κ2 Cl:Cl)diantimony(III)], C15H15Cl9I3N3Sb2
- Crystal structure of methyl 2-(1H-naphtho[1,8-de][1.3.2]diazaborinin-2(3H-yl)benzoate C18H15BN2O2
- The crystal structure of 1,8-bis(4-methoxybenzoyl)naphthalene-2,7-diyl dibenzoate, C40H28O8
- Crystal structure of 2-bromo-1,3,6,8-tetramethylBOPHY (BOPHY = bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl)methylene)hydrazine), C14H15B2BrF4N4
- The crystal structure of (E)-3-chloro-2-(2-(2-fluorobenzylidene)hydrazinyl)pyridine, C12H9ClFN3
- Crystal structure of bis(µ2- 4-iodopyridine-2,6-dicarboxylato-κ3O:N:O′)-bis(4-iodopyridine-2,6-dicarboxylato-κ3O:N:O′)-bis(µ2-1-(4-pyridyl)piperazine-κ2N:N′)-hexa-aqua-tetra-copper(II), C46H46Cu4I4N10O22
- Crystal structure of poly[diaqua-(μ2-2,5-dihydroxyterephthalato-κ2O:O′)(μ2-bis(4-pyridylformyl)piperazine-κ2N:N′)cadmium(II)] dihydrate, C24H28CdN4O12
- Crystal structure of poly[aqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)-(μ3-2,3,5,6-tetrafluoroterephthalato-κ3O:O′:O′′)cadmium(II)], C17H14N4O5F4Cd
- Crystal structure of 6-(quinolin-8-yl)benzo[a]phenanthridin-5(6H)-one, C26H16N2O
- The crystal structure of aqua-bis(6-chloropicolinato-κ2N,O)copper(II), C12H8Cl2N2O5Cu
- Crystal structure of catena-poly[diaqua-bis(μ2-4,4′-bipyridyl-κ2N:N′) disilver(I)] 4-oxidopyridine-3-sulfonate trihydrate, C25H29Ag2N5O9S
- The crystal structure of 4-(3-bromophenyl)pyrimidin-2-amine, C10H8BrN3
- Crystal structure of 6-oxo-4-phenyl-1-propyl-1,6-dihydropyridine-3-carbonitrile, C15H14N2O
- Crystal structure of 4-(2,2-difluoroethyl)-2,4-dimethyl-6-(trifluoromethyl)isoquinoline-1,3(2H,4H)-dione, C14H12F5NO2
- Crystal structure of dibromido-(1-methyl-1H-imidazole-κ1N)-(3-(3-methyl-1H-imidazol-3-ium-1-yl)propanoato-κ1O)zinc(II), C11H16Br2N4O2Zn
- The crystal structure of 1,1′-(((2 (dimethylamino)ethyl)azanediyl)bis(methylene)) bis(naphthalen-2-olato-κ4 N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)- titanium(IV) ─ dichloromethane (2/1), C33H29N3O6Ti
- The layered crystal structure of bis(theophyllinium) hexachloridostannate (IV), C14H18N8O8SnCl6
- The crystal structre of 3-(1-ethenyl-1H-imidazol-3-ium-3-yl)propane-1-sulfonate, C8H12N2O3S
- Synthesis and crystal structure of di-tert-butyl 1″-acetyl-2,2″,9′-trioxo-4a′,9a′-dihydro-1′H,3′H,9′H-dispiro[indoline-3,2′-xanthene-4′,3″-indoline]-1,3′-dicarboxylate, C39H38N2O9
- The crystal structure of 4-chloro-2-(quinolin-8-yl)isoindoline-1,3-dione, C17H9ClN2O2
- The crystal structure of 1-fluoro-4-(p-tolylethynyl)benzene, C15H11F
- The crystal structure of bis[4-bromo-2-(1H-pyrazol-3-yl) phenolato-κ2N,O] copper(II), C18H12Br2CuN4O2
- The crystal structure of poly[(μ 3-imidazolato-κ 3 N:N:N′)(tetrahydrofuran- κ 1 O)lithium(I)], C7H11LiN2O
- Crystal structure of N′,N′′′-((1E,1′E)-(propane-2,2-diylbis(1H-pyrrole-5,2diyl))bis(methaneylylidene))di(nicotinohydrazide) pentahydrate, C25H24N8O2·5H2O
- Crystal structure of 3-(2-ethoxy-2-oxoethyl)-1-ethyl-1H-imidazol-3-ium hexafluoridophos-phate(V), C9H15F6N2O2P
- Crystal structure of (1,10-phenanthroline-κ2N,N′)-bis(3-thiophenecarboxylato-κ2O,O′)copper(II), C22H14N2O4S2Cu
- The crystal structure of 2-amino-3-carboxypyridin-1-ium iodide hemihydrate, C6H8IN2O2.5
- Crystal structure of (E)-7-methoxy-2-((6-methoxypyridin-2-yl)methylene)-tetralone, C18H17NO3
- The crystal structure of [μ-hydroxido-bis[(5,5′-dimethyl-2,2′-bipyridine-κ2N,N′)-tricarbonylrhenium(I)] bromide hemihydrate, C30H26N4O9Re2Br
- The crystal structure of 2,5-bis(3,5-dimethylphenyl)thiazolo[5,4-d]thiazole, C20H18N2S2
- The crystal structure of 5-benzoyl-1-[(E)-(4-fluorobenzylidene)amino]-4-phenylpyrimidin-2(1H)-one, C24H16FN3O2
- Crystal structure of monocarbonyl(N-nitroso-N-oxido-phenylamine-κ 2 O,O′)(tricyclohexylphosphine-κP)rhodium(I), C25H39N2O3PRh
- Crystal structure of poly[bis[μ3-1,3,5-tris[(1H-imidazol-1-yl)methyl]benzene-κ3N:N′:N″]nickel(II)] hexafluorosilicate, C36H36N12NiSiF6
- The crystal structure of 13-(pyrazole-1-yl-4-carbonitrile)-matrine, C19H25N5O
- Crystal structure of 3,5-bis((E)-4-methoxy-2-(trifluoromethyl)benzylidene)-1-methylpiperidin-4-one, C24H21F6NO3
- The crystal structure of N,N′-(Disulfanediyldi-2,1-phenylene)di(6′-methylpyridine)-2-carboxamide, C26H22N4O2S2
- Crystal structure of (E)-7-fluoro-2-(4-methoxy-2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C19H14F4O2
- Crystal structure of ethyl 1-(4-fluorophenyl)-4-phenyl-1H-pyrrole-3-carboxylate, C19H16FNO2
- The crystal structure of cis-diaqua-bis (N-butyl-N-(pyridin-2-yl)pyridin-2-amine-κ2N,N′)cobalt(II)] dichloride trihydrate, C28H44Cl2N6O5Co
- Crystal structure of (E)-7-methoxy-2-((6-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C18H17NO3
- Crystal structure of (E)-2-((3-fluoropyridin-4-yl)methylene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- The crystal structure of 6-bromohexanoic acid, C6H11BrO2
- The crystal structure of 4-chloro-thiophenol, C6H5ClS
- The crystal structure of 4-bromobenzyl chloride, C7H6BrCl
- The crystal structure of di-tert-butyl dicarbonate, C10H18O5
- The crystal structure of (2-(4-chlorophenyl)-5-methyl-1,3-dioxan-5-yl)methanol, C12H15ClO3
- The crystal structure of the co-crystal: 2-hydroxybenzoic acid – N′-(butan-2-ylidene)pyridine-4-carbohydrazide, C10H13N3O·C7H6O3
- Crystal structure and anti-inflammatory activity of (E)-7-fluoro-2-((5-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- Crystal structure of (E)-7-fluoro-2-((6-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- Crystal structure of 1,1′-(butane-1,4-diyl)bis(3-propyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C32H56F24N8P4
- The crystal structure of dichlorido-bis(3-methyl-3-imidazolium-1-ylpropionato-κ2)-cadmium(II), C14H20CdCl2N4O4
- Crystal structure of 1-(2-cyanobenzyl)-3-cyano-4-phenyl-4-(2-cyanobenzyl)-1,4-dihydropyridine monohydrate, C56H42N8O
- The crystal structure of 3-(carboxymethyl)-1-ethenyl-1H-imidazol-3-ium chloride, C7H9N2O2Cl
- The crystal structure of adamantylmethoxydiphenylsilane, C23H28OSi
- Redetermination of the crystal structure of (2E,4Z,13E,15Z)-3,5,14,16-tetramethyl-2,6,13,17-tetraazatricyclo[16.4.0.07,12]docosa-1(22),2,4,7,9,11,13,15,18,20-decaene, C22H24N4
- Crystal structure of (E)-7-hydroxy-2-((6-methoxypyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H15NO3
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2 N:N′)cobalt(II)] dinitrate, C18H28N10O8Co

