The crystal structure of 1,1′-(((2 (dimethylamino)ethyl)azanediyl)bis(methylene)) bis(naphthalen-2-olato-κ4 N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)- titanium(IV) ─ dichloromethane (2/1), C33H29N3O6Ti
Abstract
C33H29N3O6Ti, monoclinic, C2/c (no. 15), a = 27.4022(15) Å, b = 14.0345(7) Å, c = 17.0047(10) Å, β = 116.009(3)°, V = 5877.3(6) Å3, Z = 8, R gt (F) = 0.0369, wRref (F 2) = 0.1125, T = 100(2) K.
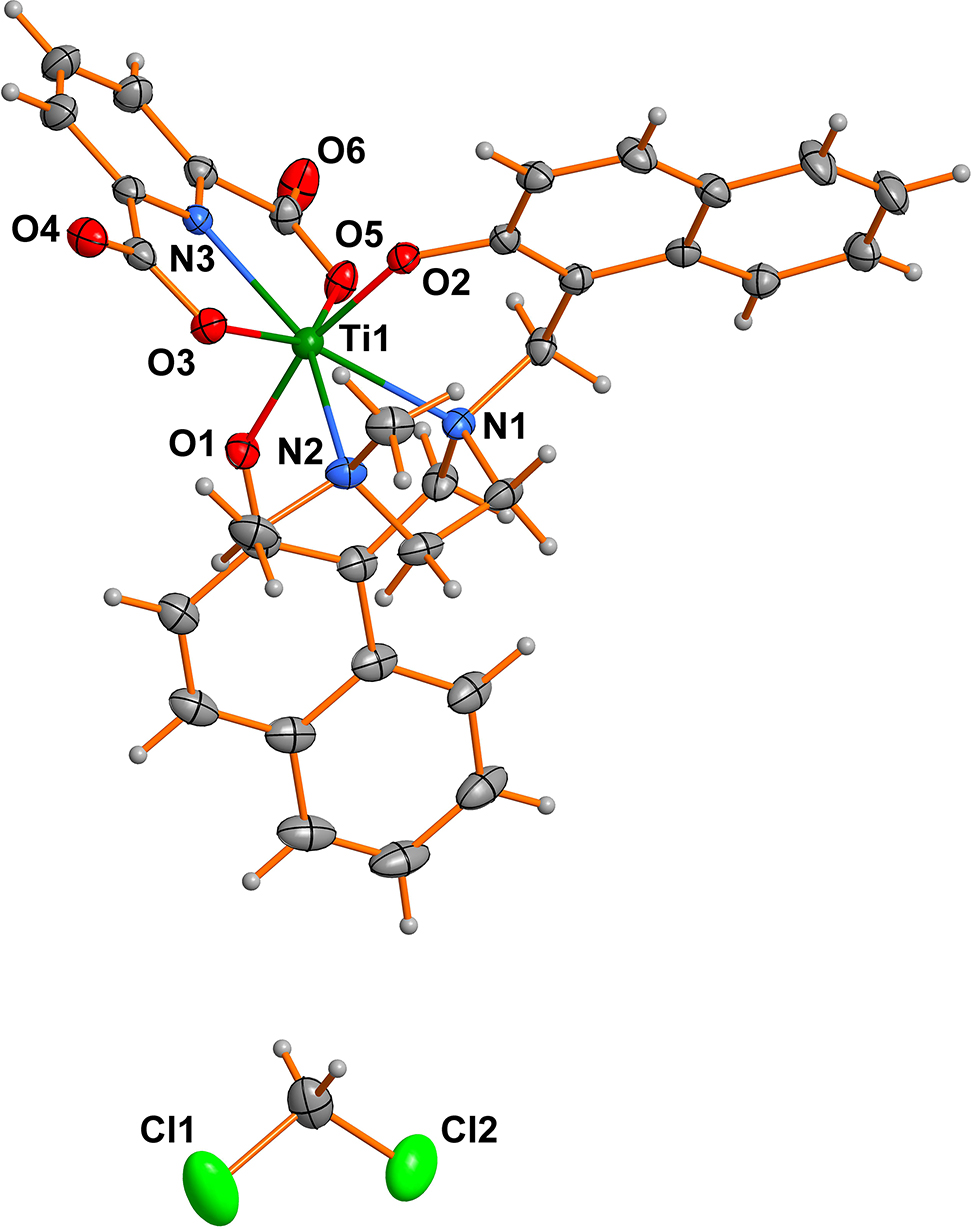
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Orange block |
| Size: | 0.12 × 0.10 × 0.10 mm |
| Wavelength: | Ga Kα radiation (1.34138 Å) |
| μ: | 2.51 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| θ max, completeness: | 57.1°, >99% |
| N(hkl)measured , N(hkl)unique, R int: | 38312, 6027, 0.052 |
| Criterion for I obs, N(hkl)gt: | I obs > 2 σ(I obs), 5177 |
| N(param)refined: | 418 |
| Programs: | Bruker [1], SHELX [2] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| C1 | 0.60189 (7) | 0.50113 (13) | 0.58013 (12) | 0.0233 (4) |
| C2 | 0.58945 (7) | 0.51514 (13) | 0.64972 (12) | 0.0216 (4) |
| C3 | 0.54729 (7) | 0.46373 (14) | 0.65755 (13) | 0.0257 (4) |
| H3 | 0.539570 | 0.474178 | 0.706156 | 0.031* |
| C4 | 0.51786 (7) | 0.39903 (14) | 0.59457 (13) | 0.0285 (4) |
| H4 | 0.489233 | 0.365464 | 0.599549 | 0.034* |
| C5 | 0.52879 (7) | 0.38076 (14) | 0.52254 (13) | 0.0274 (4) |
| C6 | 0.57149 (7) | 0.43256 (14) | 0.51411 (13) | 0.0255 (4) |
| C7 | 0.58010 (7) | 0.41355 (15) | 0.43924 (14) | 0.0315 (5) |
| H7 | 0.607797 | 0.447275 | 0.431655 | 0.038* |
| C8 | 0.54954 (8) | 0.34773 (16) | 0.37731 (15) | 0.0379 (5) |
| H8 | 0.555907 | 0.337312 | 0.327385 | 0.046* |
| C9 | 0.50878 (8) | 0.29568 (16) | 0.38777 (16) | 0.0397 (6) |
| H9 | 0.488380 | 0.248968 | 0.345741 | 0.048* |
| C10 | 0.49865 (8) | 0.31234 (14) | 0.45804 (15) | 0.0352 (5) |
| H10 | 0.470790 | 0.277395 | 0.464063 | 0.042* |
| C11 | 0.64373 (7) | 0.56345 (14) | 0.57011 (12) | 0.0231 (4) |
| H11A | 0.651376 | 0.536388 | 0.522901 | 0.028* |
| H11B | 0.627497 | 0.627221 | 0.550497 | 0.028* |
| C12 | 0.72841 (7) | 0.64323 (13) | 0.62014 (11) | 0.0203 (4) |
| H12A | 0.707714 | 0.703407 | 0.600574 | 0.024* |
| H12B | 0.731116 | 0.614728 | 0.568945 | 0.024* |
| C13 | 0.78488 (7) | 0.66628 (12) | 0.68869 (12) | 0.0185 (4) |
| C14 | 0.79433 (7) | 0.68331 (12) | 0.77415 (12) | 0.0185 (4) |
| C15 | 0.84694 (7) | 0.70365 (13) | 0.84046 (12) | 0.0215 (4) |
| H15 | 0.852252 | 0.714900 | 0.898766 | 0.026* |
| C16 | 0.88981 (7) | 0.70705 (14) | 0.82051 (13) | 0.0254 (4) |
| H16 | 0.925271 | 0.718108 | 0.865577 | 0.030* |
| C17 | 0.88219 (7) | 0.69428 (13) | 0.73334 (12) | 0.0233 (4) |
| C18 | 0.82922 (7) | 0.67450 (12) | 0.66575 (12) | 0.0186 (4) |
| C19 | 0.82341 (7) | 0.66746 (13) | 0.57918 (12) | 0.0225 (4) |
| H19 | 0.788488 | 0.655293 | 0.533004 | 0.027* |
| C20 | 0.86674 (8) | 0.67768 (14) | 0.55999 (13) | 0.0266 (4) |
| H20 | 0.861538 | 0.672721 | 0.501104 | 0.032* |
| C21 | 0.91865 (8) | 0.69541 (16) | 0.62681 (14) | 0.0347 (5) |
| H21 | 0.948694 | 0.701718 | 0.613397 | 0.042* |
| C22 | 0.92602 (8) | 0.70360 (16) | 0.71120 (14) | 0.0337 (5) |
| H22 | 0.961378 | 0.715836 | 0.756097 | 0.040* |
| C23 | 0.72777 (7) | 0.48444 (13) | 0.67352 (13) | 0.0241 (4) |
| H23A | 0.767214 | 0.497935 | 0.702948 | 0.029* |
| H23B | 0.720080 | 0.448072 | 0.619458 | 0.029* |
| C24 | 0.71249 (7) | 0.42561 (13) | 0.73265 (13) | 0.0262 (4) |
| H24A | 0.674471 | 0.403672 | 0.700276 | 0.031* |
| H24B | 0.736164 | 0.368694 | 0.752106 | 0.031* |
| C25 | 0.77619 (7) | 0.48459 (14) | 0.87507 (13) | 0.0281 (4) |
| H25A | 0.789033 | 0.419579 | 0.893957 | 0.042* |
| H25B | 0.779958 | 0.522532 | 0.925811 | 0.042* |
| H25C | 0.797847 | 0.513437 | 0.848288 | 0.042* |
| C26 | 0.68845 (8) | 0.42931 (14) | 0.85141 (14) | 0.0306 (4) |
| H26A | 0.649620 | 0.428229 | 0.811402 | 0.046* |
| H26B | 0.693933 | 0.461093 | 0.905982 | 0.046* |
| H26C | 0.702170 | 0.363861 | 0.864108 | 0.046* |
| C27 | 0.66641 (7) | 0.77403 (13) | 0.89390 (11) | 0.0197 (4) |
| C28 | 0.65133 (8) | 0.85380 (14) | 0.92549 (13) | 0.0274 (4) |
| H28 | 0.658098 | 0.857718 | 0.985179 | 0.033* |
| C29 | 0.62611 (9) | 0.92803 (14) | 0.86822 (14) | 0.0316 (5) |
| H29 | 0.615201 | 0.983574 | 0.888247 | 0.038* |
| C30 | 0.61690 (8) | 0.92068 (14) | 0.78133 (13) | 0.0266 (4) |
| H30 | 0.599543 | 0.970676 | 0.741017 | 0.032* |
| C31 | 0.63362 (7) | 0.83877 (13) | 0.75503 (12) | 0.0195 (4) |
| C32 | 0.69347 (7) | 0.68601 (13) | 0.94366 (12) | 0.0205 (4) |
| C33 | 0.62923 (7) | 0.81744 (13) | 0.66596 (12) | 0.0216 (4) |
| N1 | 0.69711 (5) | 0.57630 (10) | 0.65008 (10) | 0.0192 (3) |
| N2 | 0.71818 (6) | 0.48192 (11) | 0.81012 (10) | 0.0225 (3) |
| N3 | 0.65750 (5) | 0.76734 (10) | 0.81067 (9) | 0.0169 (3) |
| O1 | 0.61798 (5) | 0.57717 (9) | 0.71411 (8) | 0.0217 (3) |
| O2 | 0.75358 (5) | 0.68096 (9) | 0.79766 (8) | 0.0197 (3) |
| O3 | 0.69884 (5) | 0.62172 (9) | 0.89296 (8) | 0.0218 (3) |
| O4 | 0.70781 (5) | 0.67830 (10) | 1.02224 (8) | 0.0276 (3) |
| O5 | 0.65263 (5) | 0.73842 (9) | 0.66427 (8) | 0.0236 (3) |
| O6 | 0.60584 (6) | 0.87121 (10) | 0.60429 (9) | 0.0353 (4) |
| Ti1 | 0.68385 (2) | 0.63935 (2) | 0.76620 (2) | 0.01642 (11) |
| C1Sa | 0.51005 (19) | 0.0260 (3) | 0.5073 (4) | 0.0388 (11) |
| H1SAa | 0.494042 | 0.090634 | 0.497869 | 0.047* |
| H1SBa | 0.548342 | 0.030660 | 0.551686 | 0.047* |
| Cl1a | 0.47435 (7) | −0.04917 (14) | 0.54657 (14) | 0.0779 (5) |
| Cl2a | 0.50780 (5) | −0.01697 (13) | 0.40881 (9) | 0.0598 (4) |
-
aOccupancy: 0.5.
Source of material
The ONON ligand L1: (1,1′-(((2-(dimethyl-amino)ethyl)azanediyl)bis (methylene))bis(naphthalen-2-ol)) was prepared following the literature reported method by prolonging the reaction time from 2 to 6 h [3]. The corresponding Ti(IV) complex was synthesized according to lit. Under a nitrogen atmosphere, L1 (1 mmol, 400 mg) was dissolved in anhydrous THF (15 mL), Ti(OiPr)4 (0.95 mmol, 0.28 mL) was then added to the solution. The reaction was heated to 50 °C and kept stirring for 2 h, Dipic (pyridine-2,6-dicarboxylic acid) (0.95 mmol, 158.8 mg) was added and the reaction was left stirring for another 10 h and monitored by TLC. When the reaction was complete, [L1Ti(IV)(Dipic)] was separated by flash column chromatography (CH2Cl2/CH3OH = 30/1) to obtain a red solid (0.80 mmol, 492.1 mg, 85%). Suitable crystals for X-ray diffraction measurements were obtained by slow diffusion of n–hexane to a dichloromethane solution of [L1Ti(IV)(Dipic)] at room temperature.
Experimental details
H atoms bonded to C atoms were positioned geometrically with C–H = 0.95 Å (aromatic), 0.99 Å (methylene) and 0.98 Å (methyl) and refined in a riding mode [U iso(H) = 1.2U eqC (aromatic and methylene)], 1.5U eqC(methyl).
Comment
Various ONNO ligands stabilizing Ti(IV) complexes containing different substituents have been synthesized and tested for antitumor activities in recent years [4]. The use of pyridine-2,6-dicarboxylate as a second chelator gives a novel heptacoordinated Ti(IV) complex with enhanced aqueous stability and cytotoxicity [5, 6], which is considered as a new generation of anti-tumor Ti complexes [7]. The isotopic titanium complex [45Ti][(Salan)Ti(IV)(Dipic)] could be synthesized based on this ligand system and studied as PET probes [8, 9]. ONNO Ti(IV) complexes are structurally close to ONON Ti(IV) complexes, which exhibit much higher space flexibility because of the amino side arm [10]. However, only one report is related to the ONON Ti(IV) complex due to the difficult synthesis of ligands [10], no reports are found on the ONON Ti(IV) complexes containing a naphthalene moiety.
As shown in the Figure, the central Ti cation is seven-coordinated by three nitrogen atoms and four oxygen atoms, forming a distorted pentagonal bipyramidal geometry. The bond lengths of four Ti–O and three Ti–N bonds are ranging from 1.8390(12) to 2.0902(13) Å and from 2.1906(14) to 2.3891(15) Å, respectively, which is in accordance with the literature [7]. Surprisingly, the Mannich reaction of naphthalen-2-ol gave a rearranged ONON ligand L1, thus giving the titled ONON–Ti(IV) compound. The CH2Cl2 is disordered by an inversion center.
In the crystal packing, the molecules are linked together by some weak intermolecular interactions such as π⃛π and C–H⃛π. For instance, the centroid-to-centroid distance between two inversion-related C1–C6 aromatic rings is only 3.596(1) Å, indicating a π⃛π interaction.
Funding source: Natural Science Foundation of Gansu Province of China
Award Identifier / Grant number: 20JR5RA470
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: Natural Science Foundation of Gansu Province of China (Nos. 20JR5RA470).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. SMART and SAINT for Windows NT Software Reference Manuals. version 5.0; Bruker Analytical X–Ray Systems: Madison, WI, 1997.Search in Google Scholar
2. Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Search in Google Scholar PubMed
3. Slitikov, P. V., Rasadkina, E. N. Aminomethylation of naphthalen-2-ol and naphthalene-2,7-diol. Russ. J. Org. Chem. 2016, 52, 1432–1435; https://doi.org/10.1134/s1070428016100109.Search in Google Scholar
4. Immel, T. A., Groth, U., Huhn, T. Cytotoxic titanium salan complexes: surprising Interaction of salan and alkoxy ligands. Chem. Eur J. 2010, 16, 2775–2789; https://doi.org/10.1002/chem.200902312.Search in Google Scholar PubMed
5. Grützke, M., Zhao, T., Immel, T. A., Huhn, T. Heptacoordinate heteroleptic salan (ONNO) and thiosalan (OSSO) titanium(IV) complexes: investigation of stability and cytotoxicity. Inorg. Chem. 2015, 54, 6697–6706; https://doi.org/10.1021/acs.inorgchem.5b00690.Search in Google Scholar PubMed
6. Zhao, T., Grützke, M., Götz, K. H., Druzhenko, T., Huhn, T. Synthesis and X-ray structure analysis of cytotoxic heptacoordinate sulfonamide salan titanium(IV)-bis-Chelates. Dalton Trans. 2015, 44, 16475–16485; https://doi.org/10.1039/C5DT01618E.Search in Google Scholar PubMed
7. Immel, T. A., Grützke, M., Späte, A.–K., Groth, U., Öhlschläger, P., Huhn, T. Synthesis and X-ray structure analysis of a heptacoordinate titanium(IV)-bis-chelate with enhanced in vivo antitumor efficacy. Chem. Commun. 2012, 48, 5790–5792; https://doi.org/10.1039/c2cc31624b.Search in Google Scholar PubMed
8. Søborg Pedersen, K., Baun, C., Michaelsen Nielsen, K., Thisgaard, H., Ingemann Jensen, A., Zhuravlev, F. Design, synthesis, computational, and preclinical evaluation of natTi/45Ti-labeled urea-based glutamate PSMA ligand. Molecules 2020, 52, 1104; https://doi.org/10.3390/molecules25051104.Search in Google Scholar PubMed PubMed Central
9. Severin, G. W., Nielsen, C. H., Jensen, A. I., Fonslet, J., Kjar, A., Zhuravlev, F. Bringing radiotracing to titanium-based antineoplastics: solid phase radiosynthesis, PET and ex vivo evaluation of antitumor agent [45Ti](salan)Ti(dipic). J. Med. Chem. 2015, 58, 7591–7595; https://doi.org/10.1021/acs.jmedchem.5b01167.Search in Google Scholar PubMed
10. Peri, D., Manna, C. M., Shavit, M., Tshuva, E. Y. TiIV complexes of branched diamine bis(phenolato) ligands: hydrolysis and cytotoxicity. Eur. J. Inorg. Chem. 2011, 2011, 4896–4900; https://doi.org/10.1002/ejic.201100725.Search in Google Scholar
© 2021 Tiankun Zhao et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of [aqua-(4-iodopyridine-2,6-dicarboxylato-κ3 O,N,O′)-(1,10-phenanothroline-κ2 N,N′)copper(II)] dihydrate, C19H16O7N3CuI
- The crystal structure of tetrakis(1-isopropyl-1H-imidazolium) octamolybdate, C24H44Mo8N8O26
- Crystal structure of catena-poly[bis(µ2-3,5-bis(1-imidazolyl)pyridine-κ2 N:N′)-(µ2-3-nitrophthalato-k3 O,O′:O″)cadmium(II)] dihydrate, C30H25N11O8Cd
- The crystal structure of diaqua-bis(2-(3-(1H-pyrazol-4-yl)-1H-1,2,4-triazol-5-yl)pyridine-κ2 N:N′)-bis(3,5-dicarboxybenzoato-κ1 O)cobalt(II), C38H30CoN12O14
- Crystal structure of the nickel(II) complex aqua-(2,6-di(pyrazin-2-yl)-4,4′-bipyridine-κ3 N,N′,N′′)-(phthalato-κ2 O,O′)nickel(II) tetrahydrate, C26H26N6O9Ni
- The crystal structure of 1-[5-(2-fluorophenyl)-1-(pyridine-3-sulfonyl)-1H-pyrrol-3-yl]-N-methylmethanaminium 3-carboxyprop-2-enoate, C21H20FN3O6S
- The crystal structure of 1,2-bis(4-pyridyl)ethane - 4,4-dihydroxydiphenylmethane (1/1), C25H21N2O2
- Crystal structure of bis(2-((E)-5-chloro-2-hydroxybenzylidene)hydrazineyl)methaniminium trifluoroacetate dihydrate, C34H36Cl4N10O12
- Crystal structure of 1-cyclopropyl-7-ethoxy-6,8-difluoro-4-oxo-1,4-dihydro-quinoline-3-carboxylic acid, C15H13F2NO4
- Crystal structure of methyl 3-(1H-naphtho[1,8-de][1,3,2]diazaborinin-2(3H)-yl)benzoate, C18H15BN2O2
- Crystal structure of (E)-N′-(2-chloro-6-hydroxybenzylidene)-2-hydroxybenzohydrazide, C14H11ClN2O3
- Crystal structure of Al-rich fluorophlogopite, K1.0(Mg2.8Al0.2)(Si2.8Al1.2)O10F2
- The crystal structure of 4,5-diiodo-1,3-dimesityl-1H-1,2,3-triazol-3-ium hexafluoridoantimonate(V), C20H22F6I2N3Sb
- Crystal structure of tris(3-iodopyridin-1-ium) catena-poly[(hexachlorido-κ1 Cl)-(μ2-trichlorido-κ2 Cl:Cl)diantimony(III)], C15H15Cl9I3N3Sb2
- Crystal structure of methyl 2-(1H-naphtho[1,8-de][1.3.2]diazaborinin-2(3H-yl)benzoate C18H15BN2O2
- The crystal structure of 1,8-bis(4-methoxybenzoyl)naphthalene-2,7-diyl dibenzoate, C40H28O8
- Crystal structure of 2-bromo-1,3,6,8-tetramethylBOPHY (BOPHY = bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl)methylene)hydrazine), C14H15B2BrF4N4
- The crystal structure of (E)-3-chloro-2-(2-(2-fluorobenzylidene)hydrazinyl)pyridine, C12H9ClFN3
- Crystal structure of bis(µ2- 4-iodopyridine-2,6-dicarboxylato-κ3O:N:O′)-bis(4-iodopyridine-2,6-dicarboxylato-κ3O:N:O′)-bis(µ2-1-(4-pyridyl)piperazine-κ2N:N′)-hexa-aqua-tetra-copper(II), C46H46Cu4I4N10O22
- Crystal structure of poly[diaqua-(μ2-2,5-dihydroxyterephthalato-κ2O:O′)(μ2-bis(4-pyridylformyl)piperazine-κ2N:N′)cadmium(II)] dihydrate, C24H28CdN4O12
- Crystal structure of poly[aqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)-(μ3-2,3,5,6-tetrafluoroterephthalato-κ3O:O′:O′′)cadmium(II)], C17H14N4O5F4Cd
- Crystal structure of 6-(quinolin-8-yl)benzo[a]phenanthridin-5(6H)-one, C26H16N2O
- The crystal structure of aqua-bis(6-chloropicolinato-κ2N,O)copper(II), C12H8Cl2N2O5Cu
- Crystal structure of catena-poly[diaqua-bis(μ2-4,4′-bipyridyl-κ2N:N′) disilver(I)] 4-oxidopyridine-3-sulfonate trihydrate, C25H29Ag2N5O9S
- The crystal structure of 4-(3-bromophenyl)pyrimidin-2-amine, C10H8BrN3
- Crystal structure of 6-oxo-4-phenyl-1-propyl-1,6-dihydropyridine-3-carbonitrile, C15H14N2O
- Crystal structure of 4-(2,2-difluoroethyl)-2,4-dimethyl-6-(trifluoromethyl)isoquinoline-1,3(2H,4H)-dione, C14H12F5NO2
- Crystal structure of dibromido-(1-methyl-1H-imidazole-κ1N)-(3-(3-methyl-1H-imidazol-3-ium-1-yl)propanoato-κ1O)zinc(II), C11H16Br2N4O2Zn
- The crystal structure of 1,1′-(((2 (dimethylamino)ethyl)azanediyl)bis(methylene)) bis(naphthalen-2-olato-κ4 N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)- titanium(IV) ─ dichloromethane (2/1), C33H29N3O6Ti
- The layered crystal structure of bis(theophyllinium) hexachloridostannate (IV), C14H18N8O8SnCl6
- The crystal structre of 3-(1-ethenyl-1H-imidazol-3-ium-3-yl)propane-1-sulfonate, C8H12N2O3S
- Synthesis and crystal structure of di-tert-butyl 1″-acetyl-2,2″,9′-trioxo-4a′,9a′-dihydro-1′H,3′H,9′H-dispiro[indoline-3,2′-xanthene-4′,3″-indoline]-1,3′-dicarboxylate, C39H38N2O9
- The crystal structure of 4-chloro-2-(quinolin-8-yl)isoindoline-1,3-dione, C17H9ClN2O2
- The crystal structure of 1-fluoro-4-(p-tolylethynyl)benzene, C15H11F
- The crystal structure of bis[4-bromo-2-(1H-pyrazol-3-yl) phenolato-κ2N,O] copper(II), C18H12Br2CuN4O2
- The crystal structure of poly[(μ 3-imidazolato-κ 3 N:N:N′)(tetrahydrofuran- κ 1 O)lithium(I)], C7H11LiN2O
- Crystal structure of N′,N′′′-((1E,1′E)-(propane-2,2-diylbis(1H-pyrrole-5,2diyl))bis(methaneylylidene))di(nicotinohydrazide) pentahydrate, C25H24N8O2·5H2O
- Crystal structure of 3-(2-ethoxy-2-oxoethyl)-1-ethyl-1H-imidazol-3-ium hexafluoridophos-phate(V), C9H15F6N2O2P
- Crystal structure of (1,10-phenanthroline-κ2N,N′)-bis(3-thiophenecarboxylato-κ2O,O′)copper(II), C22H14N2O4S2Cu
- The crystal structure of 2-amino-3-carboxypyridin-1-ium iodide hemihydrate, C6H8IN2O2.5
- Crystal structure of (E)-7-methoxy-2-((6-methoxypyridin-2-yl)methylene)-tetralone, C18H17NO3
- The crystal structure of [μ-hydroxido-bis[(5,5′-dimethyl-2,2′-bipyridine-κ2N,N′)-tricarbonylrhenium(I)] bromide hemihydrate, C30H26N4O9Re2Br
- The crystal structure of 2,5-bis(3,5-dimethylphenyl)thiazolo[5,4-d]thiazole, C20H18N2S2
- The crystal structure of 5-benzoyl-1-[(E)-(4-fluorobenzylidene)amino]-4-phenylpyrimidin-2(1H)-one, C24H16FN3O2
- Crystal structure of monocarbonyl(N-nitroso-N-oxido-phenylamine-κ 2 O,O′)(tricyclohexylphosphine-κP)rhodium(I), C25H39N2O3PRh
- Crystal structure of poly[bis[μ3-1,3,5-tris[(1H-imidazol-1-yl)methyl]benzene-κ3N:N′:N″]nickel(II)] hexafluorosilicate, C36H36N12NiSiF6
- The crystal structure of 13-(pyrazole-1-yl-4-carbonitrile)-matrine, C19H25N5O
- Crystal structure of 3,5-bis((E)-4-methoxy-2-(trifluoromethyl)benzylidene)-1-methylpiperidin-4-one, C24H21F6NO3
- The crystal structure of N,N′-(Disulfanediyldi-2,1-phenylene)di(6′-methylpyridine)-2-carboxamide, C26H22N4O2S2
- Crystal structure of (E)-7-fluoro-2-(4-methoxy-2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C19H14F4O2
- Crystal structure of ethyl 1-(4-fluorophenyl)-4-phenyl-1H-pyrrole-3-carboxylate, C19H16FNO2
- The crystal structure of cis-diaqua-bis (N-butyl-N-(pyridin-2-yl)pyridin-2-amine-κ2N,N′)cobalt(II)] dichloride trihydrate, C28H44Cl2N6O5Co
- Crystal structure of (E)-7-methoxy-2-((6-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C18H17NO3
- Crystal structure of (E)-2-((3-fluoropyridin-4-yl)methylene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- The crystal structure of 6-bromohexanoic acid, C6H11BrO2
- The crystal structure of 4-chloro-thiophenol, C6H5ClS
- The crystal structure of 4-bromobenzyl chloride, C7H6BrCl
- The crystal structure of di-tert-butyl dicarbonate, C10H18O5
- The crystal structure of (2-(4-chlorophenyl)-5-methyl-1,3-dioxan-5-yl)methanol, C12H15ClO3
- The crystal structure of the co-crystal: 2-hydroxybenzoic acid – N′-(butan-2-ylidene)pyridine-4-carbohydrazide, C10H13N3O·C7H6O3
- Crystal structure and anti-inflammatory activity of (E)-7-fluoro-2-((5-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- Crystal structure of (E)-7-fluoro-2-((6-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- Crystal structure of 1,1′-(butane-1,4-diyl)bis(3-propyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C32H56F24N8P4
- The crystal structure of dichlorido-bis(3-methyl-3-imidazolium-1-ylpropionato-κ2)-cadmium(II), C14H20CdCl2N4O4
- Crystal structure of 1-(2-cyanobenzyl)-3-cyano-4-phenyl-4-(2-cyanobenzyl)-1,4-dihydropyridine monohydrate, C56H42N8O
- The crystal structure of 3-(carboxymethyl)-1-ethenyl-1H-imidazol-3-ium chloride, C7H9N2O2Cl
- The crystal structure of adamantylmethoxydiphenylsilane, C23H28OSi
- Redetermination of the crystal structure of (2E,4Z,13E,15Z)-3,5,14,16-tetramethyl-2,6,13,17-tetraazatricyclo[16.4.0.07,12]docosa-1(22),2,4,7,9,11,13,15,18,20-decaene, C22H24N4
- Crystal structure of (E)-7-hydroxy-2-((6-methoxypyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H15NO3
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2 N:N′)cobalt(II)] dinitrate, C18H28N10O8Co
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of [aqua-(4-iodopyridine-2,6-dicarboxylato-κ3 O,N,O′)-(1,10-phenanothroline-κ2 N,N′)copper(II)] dihydrate, C19H16O7N3CuI
- The crystal structure of tetrakis(1-isopropyl-1H-imidazolium) octamolybdate, C24H44Mo8N8O26
- Crystal structure of catena-poly[bis(µ2-3,5-bis(1-imidazolyl)pyridine-κ2 N:N′)-(µ2-3-nitrophthalato-k3 O,O′:O″)cadmium(II)] dihydrate, C30H25N11O8Cd
- The crystal structure of diaqua-bis(2-(3-(1H-pyrazol-4-yl)-1H-1,2,4-triazol-5-yl)pyridine-κ2 N:N′)-bis(3,5-dicarboxybenzoato-κ1 O)cobalt(II), C38H30CoN12O14
- Crystal structure of the nickel(II) complex aqua-(2,6-di(pyrazin-2-yl)-4,4′-bipyridine-κ3 N,N′,N′′)-(phthalato-κ2 O,O′)nickel(II) tetrahydrate, C26H26N6O9Ni
- The crystal structure of 1-[5-(2-fluorophenyl)-1-(pyridine-3-sulfonyl)-1H-pyrrol-3-yl]-N-methylmethanaminium 3-carboxyprop-2-enoate, C21H20FN3O6S
- The crystal structure of 1,2-bis(4-pyridyl)ethane - 4,4-dihydroxydiphenylmethane (1/1), C25H21N2O2
- Crystal structure of bis(2-((E)-5-chloro-2-hydroxybenzylidene)hydrazineyl)methaniminium trifluoroacetate dihydrate, C34H36Cl4N10O12
- Crystal structure of 1-cyclopropyl-7-ethoxy-6,8-difluoro-4-oxo-1,4-dihydro-quinoline-3-carboxylic acid, C15H13F2NO4
- Crystal structure of methyl 3-(1H-naphtho[1,8-de][1,3,2]diazaborinin-2(3H)-yl)benzoate, C18H15BN2O2
- Crystal structure of (E)-N′-(2-chloro-6-hydroxybenzylidene)-2-hydroxybenzohydrazide, C14H11ClN2O3
- Crystal structure of Al-rich fluorophlogopite, K1.0(Mg2.8Al0.2)(Si2.8Al1.2)O10F2
- The crystal structure of 4,5-diiodo-1,3-dimesityl-1H-1,2,3-triazol-3-ium hexafluoridoantimonate(V), C20H22F6I2N3Sb
- Crystal structure of tris(3-iodopyridin-1-ium) catena-poly[(hexachlorido-κ1 Cl)-(μ2-trichlorido-κ2 Cl:Cl)diantimony(III)], C15H15Cl9I3N3Sb2
- Crystal structure of methyl 2-(1H-naphtho[1,8-de][1.3.2]diazaborinin-2(3H-yl)benzoate C18H15BN2O2
- The crystal structure of 1,8-bis(4-methoxybenzoyl)naphthalene-2,7-diyl dibenzoate, C40H28O8
- Crystal structure of 2-bromo-1,3,6,8-tetramethylBOPHY (BOPHY = bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl)methylene)hydrazine), C14H15B2BrF4N4
- The crystal structure of (E)-3-chloro-2-(2-(2-fluorobenzylidene)hydrazinyl)pyridine, C12H9ClFN3
- Crystal structure of bis(µ2- 4-iodopyridine-2,6-dicarboxylato-κ3O:N:O′)-bis(4-iodopyridine-2,6-dicarboxylato-κ3O:N:O′)-bis(µ2-1-(4-pyridyl)piperazine-κ2N:N′)-hexa-aqua-tetra-copper(II), C46H46Cu4I4N10O22
- Crystal structure of poly[diaqua-(μ2-2,5-dihydroxyterephthalato-κ2O:O′)(μ2-bis(4-pyridylformyl)piperazine-κ2N:N′)cadmium(II)] dihydrate, C24H28CdN4O12
- Crystal structure of poly[aqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)-(μ3-2,3,5,6-tetrafluoroterephthalato-κ3O:O′:O′′)cadmium(II)], C17H14N4O5F4Cd
- Crystal structure of 6-(quinolin-8-yl)benzo[a]phenanthridin-5(6H)-one, C26H16N2O
- The crystal structure of aqua-bis(6-chloropicolinato-κ2N,O)copper(II), C12H8Cl2N2O5Cu
- Crystal structure of catena-poly[diaqua-bis(μ2-4,4′-bipyridyl-κ2N:N′) disilver(I)] 4-oxidopyridine-3-sulfonate trihydrate, C25H29Ag2N5O9S
- The crystal structure of 4-(3-bromophenyl)pyrimidin-2-amine, C10H8BrN3
- Crystal structure of 6-oxo-4-phenyl-1-propyl-1,6-dihydropyridine-3-carbonitrile, C15H14N2O
- Crystal structure of 4-(2,2-difluoroethyl)-2,4-dimethyl-6-(trifluoromethyl)isoquinoline-1,3(2H,4H)-dione, C14H12F5NO2
- Crystal structure of dibromido-(1-methyl-1H-imidazole-κ1N)-(3-(3-methyl-1H-imidazol-3-ium-1-yl)propanoato-κ1O)zinc(II), C11H16Br2N4O2Zn
- The crystal structure of 1,1′-(((2 (dimethylamino)ethyl)azanediyl)bis(methylene)) bis(naphthalen-2-olato-κ4 N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)- titanium(IV) ─ dichloromethane (2/1), C33H29N3O6Ti
- The layered crystal structure of bis(theophyllinium) hexachloridostannate (IV), C14H18N8O8SnCl6
- The crystal structre of 3-(1-ethenyl-1H-imidazol-3-ium-3-yl)propane-1-sulfonate, C8H12N2O3S
- Synthesis and crystal structure of di-tert-butyl 1″-acetyl-2,2″,9′-trioxo-4a′,9a′-dihydro-1′H,3′H,9′H-dispiro[indoline-3,2′-xanthene-4′,3″-indoline]-1,3′-dicarboxylate, C39H38N2O9
- The crystal structure of 4-chloro-2-(quinolin-8-yl)isoindoline-1,3-dione, C17H9ClN2O2
- The crystal structure of 1-fluoro-4-(p-tolylethynyl)benzene, C15H11F
- The crystal structure of bis[4-bromo-2-(1H-pyrazol-3-yl) phenolato-κ2N,O] copper(II), C18H12Br2CuN4O2
- The crystal structure of poly[(μ 3-imidazolato-κ 3 N:N:N′)(tetrahydrofuran- κ 1 O)lithium(I)], C7H11LiN2O
- Crystal structure of N′,N′′′-((1E,1′E)-(propane-2,2-diylbis(1H-pyrrole-5,2diyl))bis(methaneylylidene))di(nicotinohydrazide) pentahydrate, C25H24N8O2·5H2O
- Crystal structure of 3-(2-ethoxy-2-oxoethyl)-1-ethyl-1H-imidazol-3-ium hexafluoridophos-phate(V), C9H15F6N2O2P
- Crystal structure of (1,10-phenanthroline-κ2N,N′)-bis(3-thiophenecarboxylato-κ2O,O′)copper(II), C22H14N2O4S2Cu
- The crystal structure of 2-amino-3-carboxypyridin-1-ium iodide hemihydrate, C6H8IN2O2.5
- Crystal structure of (E)-7-methoxy-2-((6-methoxypyridin-2-yl)methylene)-tetralone, C18H17NO3
- The crystal structure of [μ-hydroxido-bis[(5,5′-dimethyl-2,2′-bipyridine-κ2N,N′)-tricarbonylrhenium(I)] bromide hemihydrate, C30H26N4O9Re2Br
- The crystal structure of 2,5-bis(3,5-dimethylphenyl)thiazolo[5,4-d]thiazole, C20H18N2S2
- The crystal structure of 5-benzoyl-1-[(E)-(4-fluorobenzylidene)amino]-4-phenylpyrimidin-2(1H)-one, C24H16FN3O2
- Crystal structure of monocarbonyl(N-nitroso-N-oxido-phenylamine-κ 2 O,O′)(tricyclohexylphosphine-κP)rhodium(I), C25H39N2O3PRh
- Crystal structure of poly[bis[μ3-1,3,5-tris[(1H-imidazol-1-yl)methyl]benzene-κ3N:N′:N″]nickel(II)] hexafluorosilicate, C36H36N12NiSiF6
- The crystal structure of 13-(pyrazole-1-yl-4-carbonitrile)-matrine, C19H25N5O
- Crystal structure of 3,5-bis((E)-4-methoxy-2-(trifluoromethyl)benzylidene)-1-methylpiperidin-4-one, C24H21F6NO3
- The crystal structure of N,N′-(Disulfanediyldi-2,1-phenylene)di(6′-methylpyridine)-2-carboxamide, C26H22N4O2S2
- Crystal structure of (E)-7-fluoro-2-(4-methoxy-2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C19H14F4O2
- Crystal structure of ethyl 1-(4-fluorophenyl)-4-phenyl-1H-pyrrole-3-carboxylate, C19H16FNO2
- The crystal structure of cis-diaqua-bis (N-butyl-N-(pyridin-2-yl)pyridin-2-amine-κ2N,N′)cobalt(II)] dichloride trihydrate, C28H44Cl2N6O5Co
- Crystal structure of (E)-7-methoxy-2-((6-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C18H17NO3
- Crystal structure of (E)-2-((3-fluoropyridin-4-yl)methylene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- The crystal structure of 6-bromohexanoic acid, C6H11BrO2
- The crystal structure of 4-chloro-thiophenol, C6H5ClS
- The crystal structure of 4-bromobenzyl chloride, C7H6BrCl
- The crystal structure of di-tert-butyl dicarbonate, C10H18O5
- The crystal structure of (2-(4-chlorophenyl)-5-methyl-1,3-dioxan-5-yl)methanol, C12H15ClO3
- The crystal structure of the co-crystal: 2-hydroxybenzoic acid – N′-(butan-2-ylidene)pyridine-4-carbohydrazide, C10H13N3O·C7H6O3
- Crystal structure and anti-inflammatory activity of (E)-7-fluoro-2-((5-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- Crystal structure of (E)-7-fluoro-2-((6-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- Crystal structure of 1,1′-(butane-1,4-diyl)bis(3-propyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C32H56F24N8P4
- The crystal structure of dichlorido-bis(3-methyl-3-imidazolium-1-ylpropionato-κ2)-cadmium(II), C14H20CdCl2N4O4
- Crystal structure of 1-(2-cyanobenzyl)-3-cyano-4-phenyl-4-(2-cyanobenzyl)-1,4-dihydropyridine monohydrate, C56H42N8O
- The crystal structure of 3-(carboxymethyl)-1-ethenyl-1H-imidazol-3-ium chloride, C7H9N2O2Cl
- The crystal structure of adamantylmethoxydiphenylsilane, C23H28OSi
- Redetermination of the crystal structure of (2E,4Z,13E,15Z)-3,5,14,16-tetramethyl-2,6,13,17-tetraazatricyclo[16.4.0.07,12]docosa-1(22),2,4,7,9,11,13,15,18,20-decaene, C22H24N4
- Crystal structure of (E)-7-hydroxy-2-((6-methoxypyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H15NO3
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2 N:N′)cobalt(II)] dinitrate, C18H28N10O8Co

