Abstract
Chrysotile asbestos has excellent physical and chemical properties, but its high biotoxicity is very damaging to human health, due to its durability, high aspect ratio, and the exist of iron. By simple chemical treatment of chrysotile asbestos, silicon oxide nanofibers rich in hydroxyl groups on the surface can be prepared.
In this study, we synthesized a series of polyurethane/silicon oxide nano-composite coatings with special performance following a blending method that introduced silicon oxide nanofibers into polyurethane. Measurement results revealed that, silicon oxide nanofibers can be used as reinforcing filler to increase the hardness, shear strength, wear resistance, thermal stability and chemical resistance of polyurethane coatings. And the optical performance test results indicate that the transmittance of the polyurethane coating decreased with increasing of silicon oxide nanofibers contents. However, the ultraviolet shielding effect of the polyurethane coating was improved to a certain extent with the addition of silicon oxide nanofibers.
1 Introduction
Silicon oxide nanofibers have useful properties, and they have a great potential for application and development in new materials [1, 2]. In the structure of silicon oxide nanofibers, atoms silicon and oxygen are in amorphous rules arranged in a three-dimensional reticular structure, showing a one-dimensional mesoscopic morphology. And they typically have diameters less than 100nm, and lengths ranging from micrometers to millimeters. Silicon oxide nanofibers can be used as a reinforcing material of polymers, offering new special performance to such materials. For example, the density of silica nanofibers is smaller than that of common fillers, which is helpful for reducing the specific gravity of composites. In addition, the thermal conductivity is higher than that of common fillers, which is beneficial to the heat dissipation of composites and increasing the thermal stability of composites. Moreover, the refractive index is closer to that of common resins, which is beneficial for preparation of transparent composites. Finally, they have high chemical stability and can be introduced into matrix material via chemical bonding due to the hydroxyl groups at the surface [3, 4, 5, 6].
Chrysotile asbestos is a kind of silicate mineral, with an ideal molecular structure of Mg6(Si4O10)(OH)8. It has excellent properties, such as heat resistance, tensile resistance, and transparent under visible light, and is used widely in modern industries such as construction, chemical industry, metallurgy, and machinery. Chrysotile asbestos has a one-dimensional tubular structure, and its low cost of natural output is unmatched by other artificial materials. However, the biological toxicity of chrysotile asbestos causes great damage to human health with chronic contact, the adverse environmental and health effects of asbestos are very well documented in literature [7, 8, 9, 10, 11]. Therefore, it is important to inactivate the harmful properties of chrysotile asbestos while, taking advantage of its low cost and unique performance. Maravelaki-Kalaitzaki et al investigated potential detoxification of pure chrysotile asbestos via a combined treatment of oxalic acid dihydratewith silicates, such as tetraethoxysilane (TEOS) and pure water glass, the results indicated that all
of the applied treatments destructed the chrysotile structure, yielded silica with amorphous phase and each of the proposed formulations can be applied for the detoxification of asbestos [12]. Willenbring et al examined whether exudates from bacteria and fungi can alter chrysotile and lower its toxicity, results indicated that siderophore can remove iron from chrysotile fibers at natural concentration levels, and organic acid exudates did not remove iron from chrysotile fibers [13].
In this study, we examined silicon oxide nanofibers rich in hydroxyl groups on the surface prepared from chrysotile asbestos via chemical dispersion in anionic surfactant, and then followed a hydrochloric acid leaching method to remove the Mg- (OH) O octahedral geometry and other metal ions [14]. Finally, we added them to a polyurethane matrix following a simple blending method and, synthesized a series of polyurethane/silicon oxide nano-composite coatings containing different weights of silicon oxide nanofibers, and investigated, the effects of the silicon oxide nanofibers on the properties of the polyurethane coating.
2 Materials and methods
2.1 Raw Materials
Adipic acid (AA), trimethylol propane (TMP), and 1,6-hexanediol (HDO) were purchased from Alfa Aesar (USA). 1,4-cyclohexanedicarboxylic acid (1,4-CHDA) and 1,4-cyclohexanedimethanol (1,4-CHDM) were obtained from SK Chemicals (Korea). Isophorone diisocyanate (IPDI) trimer (Desmodur Z4470) was supplied by Bayer Corporation (Germany). BYK141 (BYK Chemie, Germany) was used as a leveling agent. Silicon oxide nanofibers were supplied by the team of Professor Qiming Feng in Central South University (Changsha, China). All otherreagents were of analytical grade.
2.2 Polyester polyol synthesis
Polyester polyol was synthesized via a polycondensation reaction using AA, 1,4-CHDA, HDO, 1,4-CHDM, and TMP in a monomer molar ratio of 1:9:2.15:8.62:2.15. The reaction was performed in a 500-mL reactor equipped with a mechanical stirrer, nitrogen purging device, and Dean-Stark trap used for separating water and xylene. The reaction mixture was stirred continuously, and the reaction temperature was gradually increased to 210∘C, until the polyester resin reached an acid number below 1 (mg KOH/g resin). Then, the solvent wasremoved and the resin was cooled to room temperature. The acid number and hydroxyl number of the polyester was measured according to ASTM methods D1639-90 and D1957-09, respectively [15, 16].
2.3 Preparation of polyurethane/silicon oxide nano-composite coatings
Polyester polyol was dissolved in butyl acetate, and silicon oxide nanofibers was added and, dispersed in the mixture with a high speed disperser at 2000 min−1 for 10 min. Then, trimer of IPDI was mixed in the presence of a leveling agent (0.1wt% of reactants (polyester polyol, silicon oxide nanofibers and trimer of IPDI) and solvent (butyl acetate)) and catalyst (dibutyltin dilaurate, 0.01wt% of reactants and solvent). The molar ratio of functional groups (NCO/OH) in the system was 1.2:1. The solid content of the system was kept at 39∼40 wt%. The composite coating was prepared by casting the solution onto iron plates that had been degreased with acetone. The cast film was maintained at a thickness of 0.2∼0.3 mm with a drawbar, and was cured at 80∘C for 24 h. The cured films were kept under ambient atmospheric conditions for 7 days before testing and characterization were performed.
2.4 Characterization
Gel permeation chromatography (GPC; Series-200; Perkin Elmer, USA) was used to determine the molecular weight and molecular weight distribution of the polyester resin. Fourier transform infrared spectroscopy (FTIR) analysis was performed with a VECTOR-33 (Bruker, Germany) with, a spectral scanning range was 400 ~ 4000 cm−1.
Wide Angle X-ray diffraction (WAXD) patterns were obtained on Bruker-D8 ADVANCE at room temperature (Cu radiation, 40 kV/40 mA). Angel 2θ was scanned from 3∘ to 50∘ with the step size of 0.02∘ (2θ) and 0.1 s/step.
The aggregation structure of silicon oxide nanofibers was detected on Transmission Electron Microscope (TEM, Hitachi H-600, Hitachi Corporation). Samples were diluted with anhydrous ethanol and dripped on the copper wire mesh covered with acetate before tested.
The chemical composition of silicon oxide nanofibers was detected on Atomic Absorption Spectrophotometer (WFX-120; Beijing Beifen-Ruili, China).
Reverse impact resistance, flexibility, shear strength, abrasion resistance and shore hardness were performed according to GB/T 1732-93 [17], GB/T 1731-93 [18],GB/T10007-200 [19], GB/T1768-2006 [20] and GB/T531-1999 [21], respectively.
Dynamic mechanical analysis was performed on a dynamic mechanical thermal analysis system (DMA 242, NETZSCH, Germany) using standard dumbbell-shaped samples. The glass transition temperature (Tg) was measured in the stretching mode using a heating rate of 3∘C/min and a frequency of 1 Hz in the temperature range of 120∘C to 150∘C.
Optical transmittance was tested on an ultraviolet–visible spectrophotometer (LAMBDA950, PerkinElmer, USA) with an optical wavelength range of 200~900 nm.
3 Results and discussion
3.1 Characterization of polyester polyol
Fig. 1 shows the infrared spectrum of the polyester resin. A strong and wide peak at 3535 cm−1 represented the stretching vibration absorption peak of hydroxyls in the polyester resin. The absorption peaks at 2937 cm−1 and 2837 cm−1 were the asymmetric and symmetric stretching vibrations of –CH2–. The absorption peak at 1732 cm−1 was the stretching vibration of –C=O. The absorption peaks at 1452 cm−1 and 1383 cm−1 represented the deformation vibration of -CH2-. The vibrational absorption at 1169 cm−1 represented C–O in polyester. The vibrational absorption at 1038 cm−1 represented C–O–C. Finally, the vibrational absorption at 752 cm−1 represented (CH2), n ≥ 4 in polyester. The infrared analysis showed that hydroxyl terminated polyester resin was synthesized. The acid value was 0.76 mg KOH/g, and the hydroxyl value of polyester resin was 90.7 mg KOH/g, as tested according to ASTM methods D1639-90 and D1957-09, respectively. The molecularweight and molecular weightdistribution of polyester resin was 2854 g/mol and 1.26, respectively, which were measured via GPC.

FTIR spectra of polyester polyol.
3.2 Structural characterization of the silicon oxide nanofibers
Fig. 2 shows the infrared spectrum of the silicon oxide nanofibers. The strong and wide peak at 1092 cm−1 was the antisymmetric stretching vibration. The peaks at 800 cm−1 and 471 cm−1 represented the symmetric stretching vibration of Si–O. The peak at 3645 cm−1 represented the antisymmetric stretching vibration of –OH, which exists in the chemical structure water. The weak peaks near 1632 cm−1

FTIR spectra of silicon oxide nanofibers.
(unlabeled) represented the H–O–H bending vibration, which wasthe adsorbed wateron the silicon oxide surface. Finally, the bending vibration absorption peak at 959 cm−1 was Si–OH. The absorption peak at 2928 cm−1 disappeared after calcination, which might have been caused by the residual chemical dispersants in the silicon oxide nanofibers [14, 22, 24]. Fig. 3 shows the wide angle x-ray diffraction of silicon oxide nanofibers, which shows a broad diffraction peak with low intensity at 2θ = 15∼30∘, indicating that silicon oxide nanofibers synthesized in our experiment were basically amorphous structure. The aggregation structure of silicon oxide nanofibers was showed in Fig. 4. The natural output of chrysotile asbestos have fibrous shape with diameter of dozen nanometers to hundreds of nanometers. Silicon oxide nanofibers prepared from chrysotile asbestos via chemical dispersion in anionic surfactant and hydrochloric acid leaching basically preserved the structure of chrysotile asbestos, the surface of silicon oxide nanofibers was smooth and dispersed uniformly. The results showed that silicon oxide nanofibers were polycrystalline structure, dispersed uniformly, surface was smooth and contained a large amount of hydroxyl groups and, which could be used as reinforcing filler for polymer materials.
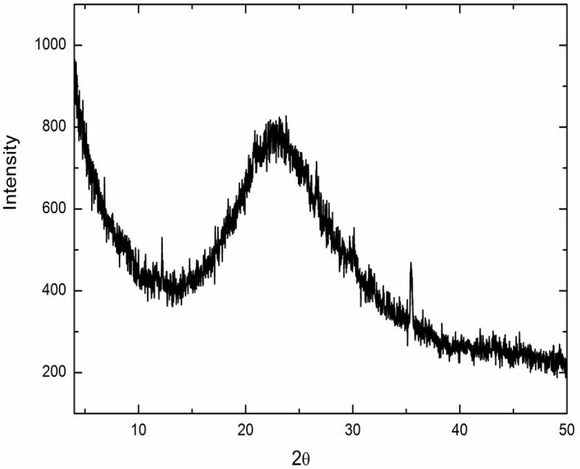
Wide Angle X-ray diffraction of silicon oxide nanofiber.

TEM of silicon oxide nanofibers.
3.3 Mechanical properties of the nano-composite coatings
Table 1 shows the mechanical properties of the nano-composite coatings (different contents of silicon oxide). A small amount of silicon oxide nanofibers had great influence on the mechanical properties of the polyurethane coating. Silicon oxide had a clear enhancement effect on
Mechanical properties of polyurethane/silicon oxide nano-composite coatings.
| Sample | Impact resistance (kg.cm) | Flexibility (mm) | Shore A hardness | Shear strength (MPa) |
|---|---|---|---|---|
| PU | 90 | 1 | 65.2 | 49.1 |
| 0.2% | 90 | 1 | 65.6 | 50.3 |
| 0.4% | 85 | 1 | 65.9 | 51.2 |
| 0.6% | 80 | 1 | 66.8 | 53.5 |
| 0.8% | 70 | 1 | 67.6 | 55.5 |
| 1% | 65 | 1 | 68.2 | 56.3 |
| 2% | 60 | 1 | 72.5 | 60.2 |
the hardness and shear strength of the composite coating, although the polyurethane matrix already had high hardness and shear strength. This was because the surface of the silicon oxide nanofibers were rich in hydroxyl groups, which had a great reinforcing effect on the polymer. However, the impact strength decreased with the addition of silicon oxide, possibly because the reinforcing effect of the silicon oxide gave the coating a higher hardness and lower toughness.
3.4 Abrasion resistance of the nano-composite coatings
The weight loss of the composite coating decreased as the content of silicon oxide increased (Fig. 5), and, the abrasion resistance of the polyurethane increased with the addition of silicon oxide (GB/T 1768-2006). The increase in the hardness gave the coating surface better abrasion resistance (Table 1). Therefore, the abrasion resistance of the coating could be improved by adding silicon oxide nanofibers.

Abrasion resistance of polyurethane/silicon oxide nano-composite coatings.
3.5 Chemical resistance of the nano-composite coatings
Table 2 shows the chemical resistance of the nano-composite coatings. Nano-composite polyurethane coatings had excellent chemical resistance properties. This was because silicon oxide nanofibers were stable in acids and alkalis, and they had good endurance to acids and alkalis in addition to hydrofluoric acid and strong alkali. Therefore, the chemical resistance of the nano-composite
Chemical resistance of polyurethane/silicon oxide nano-composite coatings (23∘C, 7 days).
| Compound | 0% | 0.2% | 0.4% | 0.6% | 0.8% | 1% | 2% |
|---|---|---|---|---|---|---|---|
| H2SO4, 5% | 4 | 5 | 5 | 5 | 5 | 5 | 5 |
| NaOH,10% | 4 | 5 | 5 | 5 | 5 | 5 | 5 |
| Toluene | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Xylene | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Acetone | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Methyl ethyl ketone | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
5=Film unaffected; 4=Change in color and loss in gloss; 3=Blistering of film; 2=Become soft;1=Part of the film fall off from the tinplate;0=Completely fall off from the tinplate
coatings was improved with the addition of silicon oxide nanofibers.
3.6 Water absorption of the nano-composite coatings
In this test, the polymer films were immersed in distilled water for 72 h, then took out and, subsequently wiped their surface water with filter paper. The weight increase percentage was calculated using the following expression: Wincrease=[(M-m)/M]×100% , where M is the sample weight after immersion in distilled water and, m is the sample weight after being dried in the oven (Table 3). The water absorption of the composite coating slightly increased with different qualities of added silicon oxides. This might caused by the rich hydroxyl groups on the surface of silicon oxide nanofibers which, did not form a dense crosslinking with polyurethane matrix
Water absorption of polyurethane/silicon oxide nano-composite coatings.
| Time | 0% | 0.4% | 0.6% | 0.8% | 1% | 2% |
|---|---|---|---|---|---|---|
| 7 days | 0.30 | 0.33 | 0.36 | 0.34 | 0.32 | 0.35 |
| 14 days | 0.32 | 0.33 | 0.38 | 0.35 | 0.31 | 0.35 |
| 21days | 0.33 | 0.34 | 0.39 | 0.35 | 0.32 | 0.36 |
3.7 Dynamic mechanical analysis of the nano-composite coatings
Fig. 6(a) and (b) display the storage modulus (E′) and tanδof the polyurethane/ silicon oxide nanofibers composite coatings at different temperatures, respectively. It can be seen from Fig. 6(a) that the storage modulus of polyurethane coatings embedded with silicon oxide nanofibers were higher than that of a pure polyurethane coating at temperatures over 10∘C, which was probably because of the strong interaction between organic and inorganic phases in composite coatings. And there was no significant change in storage modulus of polyurethane after different amounts of silicon oxide nanofibers were added, but a slight increase was observed for polyurethane with 0.4wt% silicon oxide nanofibers at temperatures over 45∘C. Fig. 6(b) shows that, the Tg of polyurethane coating increased with the addition of silicon oxide nanofibers, and the composite coating had the highest Tg value when the silicon oxide nanofiber concentration was 0.4wt%. Adding more, silicon oxide resulted in a decrease in Tg, where the degree of reduction was inversely proportional to the amount of silicon oxide added. This was probably because the amount of silicon oxide was small, and the surface hydroxyl groups were cross-linked with isocyanate, increasing the Tg of the composite. However, large amounts of silicon oxide were likely readily aggregated in the polyurethane matrix, resulting in the incomplete reaction of isocyanate with silicon oxide, and the interaction among the aggregated silicon oxide particles inhibited other silicon oxide particles reacting with isocyanate, thereby decreasing the influence on the Tg of the composite coating. Overall, as the silicon oxide content increased, the inhibition decreased and Tg increased to a certain degree [24, 25].

(a) DMA storage modulus vs. temperature at 1 Hz and (b) the corresponding tanδ of polyurethane/silicon oxide nano-composite coatings.
3.8 Optical properties of the nano-composite coatings
Fig. ?? shows the optical properties of the polyurethane/silicon oxide nano-composite coatings. The light transmittance of the coating decreased as the addition of silicon oxide nanofibers increased. The transmittance decreased sharply, and the surface became black as the quality of silicon oxide nanofibers reached or exceeded 1wt%, possibly due to the presence of some Fe, Mg, or other metal ions in the silicon oxide nanofibers causing a, difference in the refractive index between metal ions and polyurethane matrix due to light scattering on the polyurethane surface, resulting in a significant
decrease in transparency (Table 4). Therefore, to make a nano-composite coating with better transmittance, the amount of silicon oxide nanofibers should be controlled below 1wt%.
Metal element content of silicon oxide nanofiber.
| Metal element | Ca | Al | Mg | Fe | Pb |
|---|---|---|---|---|---|
| Content(wt%) | 0.26 | 0.11 | 0.24 | 2.00 | <0.005 |
4 Conclusions
Silicon oxide nanofibers rich in hydroxyl groups on the surface were prepared via the simple chemical treatment of serpentine asbestos. From this, we synthesized a series of polyurethane/silicon oxide nano-composite coatings with special performance following a simple blending method that introduced silicon oxide nanofibers into polyurethane. Silicon oxide nanofibers rich in hydroxyl groups on their surface were ideal reinforcing fillers that could improve the hardness, shear strength, wear resistance, thermal stability and chemical resistance of the coating; however, the toughness of the coating declined with increasing addition of silicon oxide nanofibers. The light transmittance of the coating decreased with increasing addition of silicon oxide nanofibers, possibly caused by the large difference in the refractive index of the polyurethane matrix and metal ion residues in the silicon oxide. The addition of silicon oxide nanofibers improved the ultraviolet light shielding effect. The simple chemical treatment of chrysotile make it as a reinforcing material for polymers, which expanding the scope of chrysotile applications, offering some new special characteristics to such materials.
Acknowledgements
This research was supported by Zhejiang Provincial Natural Science Foundation of China (LQ18E030014, LQ18E030013, LQ19F050004 and LQ14E030004 ), the National Natural Science Foundation of China (21504079).
References
[1] Jin CK, Won JH. Sensors 2010, 10, 4083-4099.10.3390/s100404083Search in Google Scholar PubMed PubMed Central
[2] Li T, Zeng W, Wang Z. Sensors & Actuators B Chemical 2015, 221, 1570-1585.10.1016/j.snb.2015.08.003Search in Google Scholar
[3] Jiang XC, Herricks T, Xia YN. Nano Letters 2002, 2, 1333-1338.10.1021/nl0257519Search in Google Scholar
[4] Chen GM, Wu LX, Xu JH, Xu GW, Huang YP. Science and Engineering of Composite Materials 2018, 25, 603-610.10.1515/secm-2015-0164Search in Google Scholar
[5] Kar S, Chaudhuri S. Solid State Communications 2005, 133, 151-155.10.1016/j.ssc.2004.10.026Search in Google Scholar
[6] Chen Z,Wang YX, He HP. Solid State Communications 2005, 135, 247-250.10.1016/j.ssc.2005.04.027Search in Google Scholar
[7] Yamamoto T, Kida A, Noma Y, Terazono A Sakai S. Waste Management 2014, 34, 536-541.10.1016/j.wasman.2013.11.008Search in Google Scholar PubMed
[8] Feder IS, Tischoff I, Theile A, Schmitz I Merget R, Tannapfel A. European Respiratory Journal 2017, 49, 02534-2016.10.1183/13993003.02534-2016Search in Google Scholar PubMed PubMed Central
[9] Kradin RL, Eng G, Christiani DC. American Journal of Industrial Medicine 2017, 60, 963-967.10.1002/ajim.22768Search in Google Scholar PubMed
[10] Turci F, M, Tomatis M, Mantegna S, Cravotto G, Fubini B. Journal of Toxicology and Environmental Health, Part A 2010, 73, 368-377.10.1080/15287390903442678Search in Google Scholar PubMed
[11] Loomis D, Dement J, Richardson D, Wolf S. Occupational Environmental Medicine 2010, 67, 580-584.10.1136/oem.2009.050120Search in Google Scholar PubMed PubMed Central
[12] Valouma A, Verganelaki A,Maravelaki-Kalaitzaki P Gidarakos E. Journal of Hazardous Materials 2016, 305, 164-170. ‘10.1016/j.jhazmat.2015.11.036Search in Google Scholar PubMed
[13] Mohanty SK, Gonneau C, Salamatipour A, Pietrofesa RA, Casper B, Christofidou-Solomidou M, Willenbring JK. Journal of Hazardous Materials 2018, 341, 290-296.10.1016/j.jhazmat.2017.07.033Search in Google Scholar PubMed PubMed Central
[14] Liu K, Feng QM, Yang YX, Zhang GF. Journal of the Chinese Ceramic Society 2007, 35, 164-169.Search in Google Scholar
[15] ASTM D1639-90, Standard Test Method for Acid Value of Organic Coating Materials.Search in Google Scholar
[16] ASTM-D1957 - 2009 - Hydroxyl Value of Fatty Oils and Acids.Search in Google Scholar
[17] GB/T 1732-93, Determination of impact resistance of film.Search in Google Scholar
[18] GB/T 1731-93, Determination of flexibility of films.Search in Google Scholar
[19] GB/T 10007-2008, Rigid cellular plastics-Determination of shear strength.Search in Google Scholar
[20] GB/T 1768-2006, Method of test for abrasion resistance of paint films.Search in Google Scholar
[21] GB/T 531-1999, Rubber-Determination of indentation hardness by means of pocket hardness meters.Search in Google Scholar
[22] Wypych F, Schreiner WH, Richard EJr. J Colloid Interface Sci 2004, 276, 167-173.10.1016/j.jcis.2004.03.022Search in Google Scholar PubMed
[23] Fonseca MG, Oliveira AS, Airoldi C. J Colloid Interface Sci 2001, 240, 533-538.10.1006/jcis.2001.7663Search in Google Scholar PubMed
[24] Yang CH, Liu FJ, Liu YP, Liao WT. J Colloid Interface Sci 2006, 302, 123-132.10.1016/j.jcis.2006.06.001Search in Google Scholar PubMed
[25] Zhang SW, Liu R, Jiang JQ,Yang C, Chen MQ, Liu XY. Progress in organic coatins 2011,70, 1-8.10.1016/j.porgcoat.2010.09.005Search in Google Scholar
© 2019 Junrui Zhang et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Analysis on the impact response of fiber-reinforced composite laminates: an emphasis on the FEM simulation
- Artificial neural network for predicting the flexural bond strength of FRP bars in concrete
- Cyclic behavior of GFRP strengthened infilled RC frames with low and normal strength concrete
- Durability of basalt fiber-reinforced polymer bars in wet-dry cycles alkali-salt corrosion
- Effect of B4C particle size on the mechanical properties of B4C reinforced aluminum matrix layered composite
- Enhanced dielectric properties of BaTiO3 ceramics with cerium doping, manganese doping and Ce-Mn co-doping
- Free and forced vibration analysis of rectangular/circular/annular plates made of carbon fiber-carbon nanotube-polymer hybrid composites
- Influence of nano-SiO2 on the bonding strength and wear resistance properties of polyurethane coating
- Investigation of wear behavior of nanoalumina and marble dust-reinforced dental composites
- Negative effect of clay fillers on the polyvinyl alcohol biodegradation: technical note
- Photocatalytic activity of Cu2O/ZnO nanocomposite for the decomposition of methyl orange under visible light irradiation
- Sub-surface mechanical properties and sub-surface creep behavior of wood-plastic composites reinforced by organoclay
- Surface integrity in wire-EDM tangential turning of in situ hybrid metal matrix composite A359/B4C/Al2O3
- The influence of the WC-Co composite microstructure model on stress field heterogeneity at the microstructure level: FEM based study
- Vibration-damping characterization of the basalt/epoxy composite laminates containing graphene nanopellets
- A review on nanocomposite hydrogels and their biomedical applications
- Optimization and simulation analysis of structure parameters of OPCM ultrasonic longitudinal wave actuating element
- Research Article
- Preparation of POSS-triol/wollastonite composite particles by Liquid phase mechanochemical method and its application in UV curable coatings
- Research on preload relaxation for composite pre-tightened tooth connections
- Dough moulding compound reinforced silicone rubber insulating composites using polymerized styrene butadiene rubber as a compatibilizer
- Hydration And Microstructure Of Astm Type I Cement Paste
- Effects of NiO content on the microstructure and mechanical properties of AgSnO2NiO composites
- Overall buckling behaviour of laminated CFRP tubes with off-axis ply orientation in axial compression
- UV sensing optode for composite materials environment monitoring
- On crushing characteristics of hybrid sandwich aluminum-cardboard panels reinforced with glass fiber composite rods
- Preparation and characterization of Ni-Cu composite nanoparticles for conductive paints
- A research on the preparation of oil-adsorbing hydrophobic porous resins by high internal phase emulsions (HIPEs) template
- Material characteristics of random glass-mat-reinforced thermoplastic under cryogenic thermal cycles
- Differentiation of non-black fillers in rubber composites using linear discriminant analysis of principal components
- Research Article
- Efficiency of TiO2 catalyst supported by modified waste fly ash during photodegradation of RR45 dye
- Synthesis and performance of polyurethane/silicon oxide nano-composite coatings
- Study on preparation of magnesium-rich composite coating and performance enhancement by graft modification of epoxy resin
- Research Article
- Mechanical and wear properties of polyetheretherketone composites filled with basalt fibres
- Mechanical Properties of Al 25 wt.% Cu Functionally Graded Material
- Research Article
- Weight reduction of a carbon fibre composite wheel
- Synthesis, electrical properties, and kinetic thermal analysis of polyaniline/ polyvinyl alcohol - magnetite nanocomposites film
- Seismic Behaviour of TRC-Strengthened RC Columns under Different Constraint Conditions
- Characterization of neat and modified asphalt binders and mixtures in relation to permanent deformation
- Microstructures, interface structure and room temperature tensile properties of magnesium materials reinforced by high content submicron SiCp
- Research Article
- Effect of Cutting Temperature on Bending Properties of Carbon Fibre Reinforced Plastics
- Mechanical and tribological properties of B-C-N coatings sliding against different wood balls
- Thermal conductivity of unidirectional composites consisting of randomly dispersed glass fibers and temperature-dependent polyethylene matrix
- Effects of Waste Eggshells addition on Microstructures, Mechanical and Tribological Properties of Green Metal Matrix Composite
- Investigation of porosity effect on flexural analysis of doubly curved FGM conoids
- Review Article
- Utilization of tailings in cement and concrete: A review
- Research Article
- Equivalent stiffness prediction and global buckling analysis using refined analytical model of composite laminated box beam
- Mechanochemical synthesis of zincite doped with cadmium in various amounts
- Size-dependent vibration analysis of graphene-PMMA lamina based on non-classical continuum theory
- Automated, Quality Assured and High Volume Oriented Production of Fiber Metal Laminates (FML) for the Next Generation of Passenger Aircraft Fuselage Shells
- Research Article
- An investigation of the stitching effect on single lap shear joints in laminated composites
- The low-velocity impact and compression after impact (CAI) behavior of foam core sandwich panels with shape memory alloy hybrid face-sheets
- Effect of granulometric distribution on electromagnetic shielding effectiveness for polymeric composite based on natural graphite
- The enhancement of filament winding in marine launching rubber gasbag
- Research on ELID Grinding Mechanism and Process Parameter Optimization of Aluminum-Based Diamond Composites for Electronic Packaging
Articles in the same Issue
- Analysis on the impact response of fiber-reinforced composite laminates: an emphasis on the FEM simulation
- Artificial neural network for predicting the flexural bond strength of FRP bars in concrete
- Cyclic behavior of GFRP strengthened infilled RC frames with low and normal strength concrete
- Durability of basalt fiber-reinforced polymer bars in wet-dry cycles alkali-salt corrosion
- Effect of B4C particle size on the mechanical properties of B4C reinforced aluminum matrix layered composite
- Enhanced dielectric properties of BaTiO3 ceramics with cerium doping, manganese doping and Ce-Mn co-doping
- Free and forced vibration analysis of rectangular/circular/annular plates made of carbon fiber-carbon nanotube-polymer hybrid composites
- Influence of nano-SiO2 on the bonding strength and wear resistance properties of polyurethane coating
- Investigation of wear behavior of nanoalumina and marble dust-reinforced dental composites
- Negative effect of clay fillers on the polyvinyl alcohol biodegradation: technical note
- Photocatalytic activity of Cu2O/ZnO nanocomposite for the decomposition of methyl orange under visible light irradiation
- Sub-surface mechanical properties and sub-surface creep behavior of wood-plastic composites reinforced by organoclay
- Surface integrity in wire-EDM tangential turning of in situ hybrid metal matrix composite A359/B4C/Al2O3
- The influence of the WC-Co composite microstructure model on stress field heterogeneity at the microstructure level: FEM based study
- Vibration-damping characterization of the basalt/epoxy composite laminates containing graphene nanopellets
- A review on nanocomposite hydrogels and their biomedical applications
- Optimization and simulation analysis of structure parameters of OPCM ultrasonic longitudinal wave actuating element
- Research Article
- Preparation of POSS-triol/wollastonite composite particles by Liquid phase mechanochemical method and its application in UV curable coatings
- Research on preload relaxation for composite pre-tightened tooth connections
- Dough moulding compound reinforced silicone rubber insulating composites using polymerized styrene butadiene rubber as a compatibilizer
- Hydration And Microstructure Of Astm Type I Cement Paste
- Effects of NiO content on the microstructure and mechanical properties of AgSnO2NiO composites
- Overall buckling behaviour of laminated CFRP tubes with off-axis ply orientation in axial compression
- UV sensing optode for composite materials environment monitoring
- On crushing characteristics of hybrid sandwich aluminum-cardboard panels reinforced with glass fiber composite rods
- Preparation and characterization of Ni-Cu composite nanoparticles for conductive paints
- A research on the preparation of oil-adsorbing hydrophobic porous resins by high internal phase emulsions (HIPEs) template
- Material characteristics of random glass-mat-reinforced thermoplastic under cryogenic thermal cycles
- Differentiation of non-black fillers in rubber composites using linear discriminant analysis of principal components
- Research Article
- Efficiency of TiO2 catalyst supported by modified waste fly ash during photodegradation of RR45 dye
- Synthesis and performance of polyurethane/silicon oxide nano-composite coatings
- Study on preparation of magnesium-rich composite coating and performance enhancement by graft modification of epoxy resin
- Research Article
- Mechanical and wear properties of polyetheretherketone composites filled with basalt fibres
- Mechanical Properties of Al 25 wt.% Cu Functionally Graded Material
- Research Article
- Weight reduction of a carbon fibre composite wheel
- Synthesis, electrical properties, and kinetic thermal analysis of polyaniline/ polyvinyl alcohol - magnetite nanocomposites film
- Seismic Behaviour of TRC-Strengthened RC Columns under Different Constraint Conditions
- Characterization of neat and modified asphalt binders and mixtures in relation to permanent deformation
- Microstructures, interface structure and room temperature tensile properties of magnesium materials reinforced by high content submicron SiCp
- Research Article
- Effect of Cutting Temperature on Bending Properties of Carbon Fibre Reinforced Plastics
- Mechanical and tribological properties of B-C-N coatings sliding against different wood balls
- Thermal conductivity of unidirectional composites consisting of randomly dispersed glass fibers and temperature-dependent polyethylene matrix
- Effects of Waste Eggshells addition on Microstructures, Mechanical and Tribological Properties of Green Metal Matrix Composite
- Investigation of porosity effect on flexural analysis of doubly curved FGM conoids
- Review Article
- Utilization of tailings in cement and concrete: A review
- Research Article
- Equivalent stiffness prediction and global buckling analysis using refined analytical model of composite laminated box beam
- Mechanochemical synthesis of zincite doped with cadmium in various amounts
- Size-dependent vibration analysis of graphene-PMMA lamina based on non-classical continuum theory
- Automated, Quality Assured and High Volume Oriented Production of Fiber Metal Laminates (FML) for the Next Generation of Passenger Aircraft Fuselage Shells
- Research Article
- An investigation of the stitching effect on single lap shear joints in laminated composites
- The low-velocity impact and compression after impact (CAI) behavior of foam core sandwich panels with shape memory alloy hybrid face-sheets
- Effect of granulometric distribution on electromagnetic shielding effectiveness for polymeric composite based on natural graphite
- The enhancement of filament winding in marine launching rubber gasbag
- Research on ELID Grinding Mechanism and Process Parameter Optimization of Aluminum-Based Diamond Composites for Electronic Packaging

