Abstract
C24H16N2O3S, triclinic, P
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
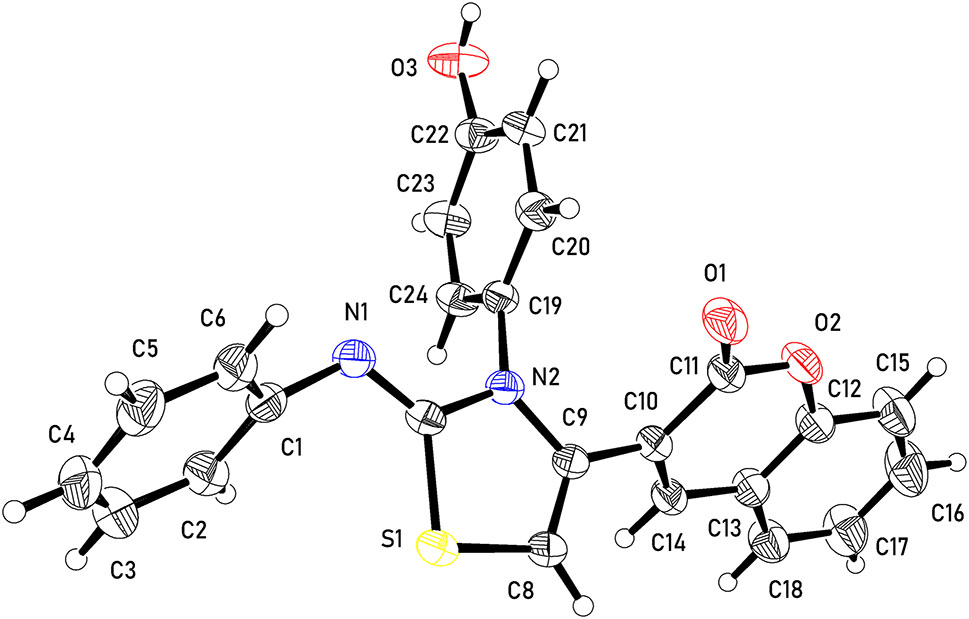
Oretep representation of C24H16N2O3S showing 50 % probability ellipsoids.
Data collection and handling.
| Crystal: | Needle |
| Size: | 0.51 × 0.18 × 0.09 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.20 mm−1 |
| Diffractometer, scan mode: | SuperNova, ω |
| θmax, completeness: | 29.8°, >99 % |
| N(hkl)measured, N(hkl)unique, Rint: | 9085, 4643, 0.027 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2σ(Iobs), 3456 |
| N(param)refined: | 272 |
| Programs: | CrysAlisPro [1], SHELX [2], WinGX/ORTEP [3], CHEMDRAW [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1 | 0.3230 (3) | 0.09965 (18) | 0.71824 (15) | 0.0383 (4) |
| C2 | 0.1327 (3) | 0.0800 (2) | 0.75093 (17) | 0.0472 (5) |
| H2 | 0.021607 | 0.118125 | 0.704308 | 0.057* |
| C3 | 0.1077 (4) | 0.0035 (2) | 0.85323 (19) | 0.0597 (6) |
| H3 | −0.020624 | −0.009146 | 0.874940 | 0.072* |
| C4 | 0.2710 (4) | −0.0540 (2) | 0.92320 (18) | 0.0599 (6) |
| H4 | 0.253591 | −0.105995 | 0.991549 | 0.072* |
| C5 | 0.4589 (4) | −0.0337 (2) | 0.89088 (17) | 0.0545 (6) |
| H5 | 0.569540 | −0.071982 | 0.937827 | 0.065* |
| C6 | 0.4864 (3) | 0.0429 (2) | 0.78950 (16) | 0.0453 (5) |
| H6 | 0.614734 | 0.056533 | 0.768821 | 0.054* |
| C7 | 0.3107 (3) | 0.15544 (17) | 0.53638 (15) | 0.0341 (4) |
| C8 | 0.2202 (3) | 0.07071 (19) | 0.40507 (16) | 0.0410 (5) |
| H8 | 0.181907 | 0.025773 | 0.367486 | 0.049* |
| C9 | 0.2674 (3) | 0.18276 (18) | 0.36010 (15) | 0.0352 (4) |
| C10 | 0.2666 (3) | 0.25009 (18) | 0.24588 (15) | 0.0372 (4) |
| C11 | 0.4554 (3) | 0.2818 (2) | 0.19874 (16) | 0.0417 (5) |
| C12 | 0.2898 (3) | 0.3519 (2) | 0.02815 (16) | 0.0452 (5) |
| C13 | 0.1133 (3) | 0.31867 (19) | 0.07210 (15) | 0.0419 (5) |
| C14 | 0.1051 (3) | 0.26890 (19) | 0.18428 (15) | 0.0399 (4) |
| H14 | −0.014458 | 0.249166 | 0.215191 | 0.048* |
| C15 | 0.3129 (4) | 0.3981 (3) | −0.07901 (18) | 0.0638 (7) |
| H15 | 0.432265 | 0.421168 | −0.107204 | 0.077* |
| C16 | 0.1541 (4) | 0.4092 (3) | −0.14293 (19) | 0.0754 (8) |
| H16 | 0.167762 | 0.438865 | −0.215479 | 0.090* |
| C17 | −0.0245 (4) | 0.3776 (3) | −0.10238 (19) | 0.0680 (7) |
| H17 | −0.130147 | 0.386257 | −0.147271 | 0.082* |
| C18 | −0.0465 (3) | 0.3334 (2) | 0.00444 (17) | 0.0538 (6) |
| H18 | −0.167949 | 0.313113 | 0.031971 | 0.065* |
| C19 | 0.3226 (3) | 0.36330 (17) | 0.40678 (14) | 0.0342 (4) |
| C20 | 0.5033 (3) | 0.40177 (19) | 0.39629 (16) | 0.0418 (5) |
| H20 | 0.625552 | 0.343916 | 0.401233 | 0.050* |
| C21 | 0.5014 (3) | 0.52668 (19) | 0.37840 (17) | 0.0447 (5) |
| H21 | 0.622755 | 0.553395 | 0.370296 | 0.054* |
| C22 | 0.3191 (3) | 0.61256 (18) | 0.37248 (15) | 0.0416 (5) |
| C23 | 0.1393 (3) | 0.57410 (19) | 0.37911 (17) | 0.0451 (5) |
| H23 | 0.016550 | 0.632289 | 0.371565 | 0.054* |
| C24 | 0.1414 (3) | 0.44943 (18) | 0.39693 (15) | 0.0402 (5) |
| H24 | 0.019877 | 0.423325 | 0.402319 | 0.048* |
| N1 | 0.3543 (2) | 0.18229 (15) | 0.61556 (12) | 0.0395 (4) |
| N2 | 0.3184 (2) | 0.23212 (14) | 0.43302 (12) | 0.0349 (4) |
| O1 | 0.6119 (2) | 0.26286 (16) | 0.24484 (12) | 0.0547 (4) |
| O2 | 0.4518 (2) | 0.33949 (14) | 0.09103 (11) | 0.0494 (4) |
| O3 | 0.3099 (2) | 0.73376 (13) | 0.36354 (15) | 0.0613 (5) |
| H3A | 0.424333 | 0.742938 | 0.370313 | 0.092* |
| S1 | 0.23759 (8) | 0.01755 (5) | 0.54105 (4) | 0.04174 (15) |
1 Source of material
A mixture of 4-hydroxyaniline (0.55 g, 5 mmol) and phenyl isothiocyanate (0.68 g, 5 mmol) in EtOH (15 mL) was refluxed for 15 min. 3-(2-Bromoacetyl)-2H-chromen-2-one (1.33 g, 5 mmol) was added, and the mixture was refluxed for 4 h. The mixture was left overnight, and the solid formed was filtered, dried, and recrystallized from DMF to give the title heterocycle in 82 % yield, mp 179–181 °C. IR (KBr; cm−1): 3121, 1610, 1517. 1H NMR (δ): 6.56 (s, 1H, thiazolyl), 6.67 (d, 8.6 Hz, 2H, Ar), 6.87 (d, 7.6 Hz, 2H, Ar), 6.98 (t, 7.2 Hz, 1H, Ar), 7.10 (d, 8.6 Hz, 2H, Ar), 7.26–7.36 (m, 4H, Ar), 7.59 (t, 7.2 Hz, 1H, Ar), 7.66 (d, 7.6 Hz, 1H, Ar), 8.15 (s, 1H, Ar), 9.58 (s, exch., 1H, OH). 13C NMR (δ): 100.7, 115.7, 116.7, 118.8, 119.8, 121.5, 123.4, 125.5, 128.9, 129.4, 130.1, 130.5, 133.2, 134.6, 145.0, 152.0, 153.8, 157.3, 158.4, 159.3. Anal. calcd. for C24H16N2O3S (412.46): C, 69.89; H, 3.91; N, 6.79; found: C, 69.93; H, 4.01; N, 6.88 %.
2 Experimental details
The hydrogen atoms were located in the difference Fourier map and refined with idealized geometry using a riding model. The O–H bond distance was set at 0.82 Å with free rotation about the C–O bond and displacement parameter 1.5 times Uiso(O). The C–H distances were set to 0.93 Å and their U(iso) to 1.2 times the Uiso(C). Crystal data, data collection and structure refinement details are summarized in Table 1.
3 Comment
Thiazoles display ample biological activity, and many natural products contain such ring systems [5], [6], [7]. Coumarin is a naturally occurring heterocycle, and has various applications [8, 9]. The design, synthesis, and structure elucidation of heterocycles containing thiazole and coumarin moieties is therefore of general interest. The X-ray crystal structures of other related heterocycles have been reported [10], [11], [12].
The crystal structure of C24H16N2O3S is triclinic, with an asymmetric unit consisting of one molecule (Figure 1) which comprises five planar groups, namely: aniline (A: C1–C6, N1), thiazole (B: C7–C9, N2, S1), benzopyranone (C: C10–C18, O1, O2) and phenol (D: C19–C24, O1). The planes through neighbouring groups in the molecules are twisted in relation to each other, with angles A/B = 63.88(7)°, B/C = 54.59(6)°, C/D = 82.09(5)°, B/D = 86.91(7)°.
In the crystal structure, two O–H⋯N hydrogen bonds (O3⋯N1 = 2.733(2) Å, O3–H3A⋯N1 = 161.5°) link pairs of molecules related by inversion symmetry, with the aniline group accepting a contact from the phenol group. These molecular pairs are also involved in π⋯π contacts between neighbouring like groups, specifically symmetry-related benzopyranone pairs and thiazole pairs. The centroid-to-centroid distances are 4.069 Å for the benzopyranone groups and 3.734 Å for the thiazole groups.
Funding source: Scientific Research, King Saud University
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: We thank the National Research Centre, Cairo, Egypt, and Cardiff University, Cardiff, UK, for their support. G. A. El-Hiti extends his appreciation to the Deanship of Scientific Research, King Saud University, for funding through the Vice Deanship of Scientific Research Chairs, Research Chair of Cornea.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Agilent. CrysAlisPro; Agilent Technologies: Yarnton, England, 2014.Search in Google Scholar
2. Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Search in Google Scholar PubMed
3. Farrugia, L. J. WinGX and ORTEP for Windows: an update. J. Appl. Crystallogr. 2012, 45, 849–854; https://doi.org/10.1107/s0021889812029111.Search in Google Scholar
4. Soft Cambridge. CHEMDRAW Ultra; Cambridge Soft Corporation: Cambridge, Massachusetts, USA, 2001.Search in Google Scholar
5. Arshad, M. F., Alam, A., Alshammari, A. A., Alhazza, M. B., Alzimam, I. M., Alam, M. A., Mustafa, G., Ansari, M. S., Alotaibi, A. M., Alotaibi, A. A., Kumar, S., Asdaq, S. M. B., Imran, M., Deb, P. K., Venugopala, K. N., Thiazole, J. S. A versatile standalone moiety contributing to the development of various drugs and biologically active agents. Molecules 2022, 27, 3994; https://doi.org/10.3390/molecules27133994.Search in Google Scholar PubMed PubMed Central
6. Petrou, A., Fesatidou, M., Geronikaki, A. Thiazole ring-a biologically active scaffold. Molecules 2021, 26, 3166; https://doi.org/10.3390/molecules26113166.Search in Google Scholar PubMed PubMed Central
7. Cascioferro, S., Parrino, B., Carbone, D., Schillaci, D., Giovannetti, E., Cirrincione, G., Diana, P. Thiazoles, their benzofused systems, and thiazolidinone derivatives: versatile and promising tools to combat antibiotic resistance. J. Med. Chem. 2020, 63, 7923–7956; https://doi.org/10.1021/acs.jmedchem.9b01245.Search in Google Scholar PubMed PubMed Central
8. Küpeli Akkol, E., Genç, Y., Karpuz, B., Sobarzo-Sánchez, E., Capasso, R. Coumarins and coumarin-related compounds in pharmacotherapy of cancer. Cancers 2020, 12, 1959; https://doi.org/10.3390/cancers12071959.Search in Google Scholar PubMed PubMed Central
9. Annunziata, F., Pinna, C., Dallavalle, S., Tamborini, L., Pinto, A. An overview of coumarin as a versatile and readily accessible scaffold with broad-ranging biological activities. Int. J. Mol. Sci. 2020, 21, 4618; https://doi.org/10.3390/ijms21134618.Search in Google Scholar PubMed PubMed Central
10. Basheen, M. A., Abdel-Wahab, B. F., Hegazy, A. S., Kariuki, B. M., El-Hiti, G. A. The crystal structure of 4-(4-bromophenyl)-2-(3-(4-bromophenyl)-5-(4-fluorophenyl)-4,5-dihydro- 1H-pyrazol-1-yl)thiazole, C24H16Br2FN3S. Z. Kristallogr. N. Cryst. Struct. 2021, 236, 425–427; https://doi.org/10.1515/ncrs-2020-0605.Search in Google Scholar
11. El-Hiti, G. A., Abdel-Wahab, B. F., Baashen, M., Ghabbour, H. A. Crystal structure of 2-(3-(benzofuran-2-yl)-5-(4-fluorophenyl)-4,5-dihydro-1H-pyrazol-1-yl)-4-(4-chlorophenyl)thiazole, C26H17ClFN3OS. Z. Kristallogr. N. Cryst. Struct. 2016, 231, 911–912; https://doi.org/10.1515/ncrs-2016-0004.Search in Google Scholar
12. Alotaibi, A. A., Abdel-Wahab, B. F., Hegazy, A. S., Kariuki, B. M., El-Hiti, G. A. The crystal structure of 2-(3-(4-bromophenyl)-5-(4-fluorophenyl)-4,5-dihydro-1H-pyrazol-1-yl)-8H-indeno[1,2-d]thiazole, C25H17BrFN3S. Z. Kristallogr. N. Cryst. Struct. 2020, 235, 897–899; https://doi.org/10.1515/ncrs-2020-0088.Search in Google Scholar
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of poly[diaqua-(μ4-3,3′-di(1H-1,2,4-triazol-1-yl)-[1,1′-biphenyl]-4,4′-dicarboxylate-N:N′:O:O′)cadmium(II)], C18H14N6O6Cd
- Crystal structure of (8R,8′S,13S,13′R)-8,8′-bis(hydroxymethyl)-9,9′,10,10′-tetramethoxy-5,5′,6,6′,8,8′,13,13′-octahydro-[13,13′-bi[1,3]dioxolo[4,5-g]isoquinolino[3,2-a]isoquinoline]-7,7′-diium chloride-methanol (1/2), C46H58N2O14Cl2
- The crystal structure of 8-methoxy-2,2-diphenyl-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C29H25BN2O3S
- Crystal structure of aqua-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N″,N‴)copper(II) 5-carboxyisophthalate tetrahydrate, C25H50N4CuO11
- The crystal structure of 1-(naphthalen-2-ylsulfonyl)-2,2-diphenyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ij]quinoline, C31H23BN2O2S
- Crystal structure of iodido-(η6-benzene) (1-(pyridin-2-yl)-N-(p-fluoro-methanamine)-κ2N,Nʹ)ruthenium(II) hexaflourophosphate, (C18H15F7IN2RuP)
- The crystal structure of 1-(3-oxo-1-phenyl-3-(p-tolyl) propylidene)-1,3-dihydro-2H-inden-2-one, C25H20O2
- Crystal structure of tricyclohexyl[4-(4H-1,2,4-triazol-4-yl)-benzoato-κO]tin(IV), C27H39N3O2Sn
- Crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ4N,O,O,O)cadmium(II)]monohydrate, C12H15NO9Cd
- Crystal structure of ethyl 2-((4-(3,5-dimethylisoxazol-4-yl)-2,6-difluorophenyl)amino)benzoate, C20H18F2N2O3
- The crystal structure of 2-(hydroxymethyl)-2-(4H-1,2,4-triazol-4-yl)propane-1,3-diol, C6H11N3O3
- The crystal structure of 1,2-bis(2,4-dinitrophenyl) hydrazine, C12H8N6O8
- Crystal structure of 1-(2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)-1,2-dihydro-4H-benzo[d][1,3]oxazin-4-one, C19H14Cl2N2O3
- The crystal structure of 5-amino-5-oxo-4-(1-oxo-4-(2-oxopyrrolidin-1-yl)isoindolin-2-yl)pentanoic acid, C17H19N3O5
- Crystal structure of N2,N6-bis(2-(((Z)-5-bromo-2-hydroxybenzylidene)amino) phenyl)pyridine-2,6-dicarboxamide, C33H23Br2N5O4
- The crystal structure of (E)-2-methoxy-6-(((5-methyl-1,3,4-thiadiazol-2-yl)imino)methyl)phenol, C11H11N3O2S
- The crystal structure of 3-((tert-butyldiphenylsilyl)methyl)-5,5-diphenyl-6-(p-tolyl) tetrahydro-2H-pyran-2-one, C41H42O2Si
- Crystal structure of 9-fluoro-4-(6-methoxypyridin-3-yl)-5,6-dihydrobenzo[h]quinazolin-2-amine, C18H15FN4O
- The crystal structure of 2-bromo-5-(4-cyanophenoxy)benzyl 1-methyl-1,2,5,6-tetrahydropyridine-3-carboxylate, C21H19BrN2O3
- Crystal structure of 3,3′-(1,4-phenylenebis(methylene))bis(1-isopropyl-1H-imidazol-3-ium) bis(hexafluorophosphate(V)), C10H14F6N2P
- The crystal structure of 2,2-di(thiophen-3-yl)-1-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C24H19BN2O2S3
- Crystal structure of 5-bromo-1-(2-iodobenzoyl)-1H-indole-3-carbaldehyde, C16H9BrINO2
- The crystal structure of monocarbonyl-2-carboxypyridinato-κ2N,O-triphenylphosphine-rhodium(I) acetonitrile solvate, C26H20.50N1.50O3PRh
- Crystal structure of dichlorido-tetrakis(1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ1N)manganese(II), C60H68O4N12Cl10Mn
- Crystal structure of 3-(tert-butyldiphenylsilyl)-1-(2,6-dichlorophenyl)-2,2-diphenylpropan-1-ol, C37H36Cl2OSi
- Crystal structure of langite from Mine du Pradet (France)
- The crystal structure of 5′-(furan-2-yl)-3′-((4-methylphenyl)sulfonamido)-3′,4′,5′,6′-tetrahydro-[1,1′:3′,1″-terphenyl]-4′-carboxylic acid, C30H27NO5S
- Synthesis and crystal structure of bis{2-(((4-acetophenone)imino)methyl)-4-fluorophenolato-κ2N,O}zinc(II), C30H22F2N2O4Zn
- The crystal structure of poly[(tripyridine-κ3N,N′,N″) μ3-(pyridine-3,4-dicarboxylate-κ3N:O:O′) manganese(II)], C22H22N4O8Mn
- The crystal structure of (E)-4-chloro-N′-(1-(4-hydroxyphenyl)propylidene)benzohydrazide, C16H15ClN2O2
- Synthesis and crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino) ethyl)phenyl)imino)methyl)phenolato-κ2N,O}cobalt(II), C40H46CoN4O4
- Crystal structure of tetraaqua-[(1-(carboxymethyl)-1H-pyrazole-3-carboxylato-κ2N,O)cobalt(II)], C6H12CoN2O8
- (6R,7S)-2,3,13-trimethoxy-6,7-dimethyl-5,6,7,8-tetrahydrobenzo[3′,4′]cycloocta [1′,2′:4,5]benzo[1,2-d][1,3]dioxol-1-ol, C22H26O6
- Crystal structure of 2-((2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)amino)benzoic acid, C18H14Cl2N2O3
- Crystal structure of (5aS,6aS,8aR,9R,11aS, 11bS,13R,13aS)-1,1,8a,11a-tetramethyl-9-((S)-1-((S)-5-methyl-6-oxo-3,6-dihydro-2H-pyran-2-yl)ethyl)-3-oxo-1,7,8,8a,9,10,11,11a,11b,12,13,13a-dodecahydro-3H,6H-cyclopenta[5,6]cyclopropa[1,8a]naphtho[2,1-c]oxepin-13-yl acetate, C32H44O6
- Crystal structure of catena-poly[triaqua-(μ2-1-(4-carboxylatophenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylato-O,O′:O″)cobalt(II)], C12H12N2O8Co
- Crystal structure of 3-[(furan-2-ylmethyl)-amino]-2-(2,3,4,5-tetrafluoro-benzoyl)-acrylic acid ethyl ester, C17H13F4NO4
- Crystal structure of methyl 4-(2-ethoxy-2-oxoethoxy)-3-methoxybenzoate, C13H16O6
- Crystal structure of 4-bromo-2-(4-chlorophenyl)-1-methyl-5-(trifluoromethyl)-1H-pyrrole-3-carbonitrile, C13H7BrClF3N2
- The crystal structure of triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ3N,O,O)nickel(II) monohydrate, C12H15NO9Ni
- Crystal structure of dihydroxy(2,4,6-triisopro-pylphenyl)telluronium trifluoromethanesulfonate, C16H25F3O5STe
- The crystal structure of 1-(carboxymethyl)-1H-imidazole 3-oxide
- The crystal structure of 1,3,5-tris(dibromomethyl)benzene, C9H6Br6
- Crystal structure of (Z)-3-(4-methoxyphenyl)-4-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-N-phenylthiazol-2(3H)-imine, C25H21N5OS
- Crystal structure of (Z)-3-(3-(4-hydroxyphenyl)-2-(phenylimino)-2,3-dihydrothiazol-4-yl)-2H-chromen-2-one, C24H16N2O3S
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of poly[diaqua-(μ4-3,3′-di(1H-1,2,4-triazol-1-yl)-[1,1′-biphenyl]-4,4′-dicarboxylate-N:N′:O:O′)cadmium(II)], C18H14N6O6Cd
- Crystal structure of (8R,8′S,13S,13′R)-8,8′-bis(hydroxymethyl)-9,9′,10,10′-tetramethoxy-5,5′,6,6′,8,8′,13,13′-octahydro-[13,13′-bi[1,3]dioxolo[4,5-g]isoquinolino[3,2-a]isoquinoline]-7,7′-diium chloride-methanol (1/2), C46H58N2O14Cl2
- The crystal structure of 8-methoxy-2,2-diphenyl-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C29H25BN2O3S
- Crystal structure of aqua-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N″,N‴)copper(II) 5-carboxyisophthalate tetrahydrate, C25H50N4CuO11
- The crystal structure of 1-(naphthalen-2-ylsulfonyl)-2,2-diphenyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ij]quinoline, C31H23BN2O2S
- Crystal structure of iodido-(η6-benzene) (1-(pyridin-2-yl)-N-(p-fluoro-methanamine)-κ2N,Nʹ)ruthenium(II) hexaflourophosphate, (C18H15F7IN2RuP)
- The crystal structure of 1-(3-oxo-1-phenyl-3-(p-tolyl) propylidene)-1,3-dihydro-2H-inden-2-one, C25H20O2
- Crystal structure of tricyclohexyl[4-(4H-1,2,4-triazol-4-yl)-benzoato-κO]tin(IV), C27H39N3O2Sn
- Crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ4N,O,O,O)cadmium(II)]monohydrate, C12H15NO9Cd
- Crystal structure of ethyl 2-((4-(3,5-dimethylisoxazol-4-yl)-2,6-difluorophenyl)amino)benzoate, C20H18F2N2O3
- The crystal structure of 2-(hydroxymethyl)-2-(4H-1,2,4-triazol-4-yl)propane-1,3-diol, C6H11N3O3
- The crystal structure of 1,2-bis(2,4-dinitrophenyl) hydrazine, C12H8N6O8
- Crystal structure of 1-(2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)-1,2-dihydro-4H-benzo[d][1,3]oxazin-4-one, C19H14Cl2N2O3
- The crystal structure of 5-amino-5-oxo-4-(1-oxo-4-(2-oxopyrrolidin-1-yl)isoindolin-2-yl)pentanoic acid, C17H19N3O5
- Crystal structure of N2,N6-bis(2-(((Z)-5-bromo-2-hydroxybenzylidene)amino) phenyl)pyridine-2,6-dicarboxamide, C33H23Br2N5O4
- The crystal structure of (E)-2-methoxy-6-(((5-methyl-1,3,4-thiadiazol-2-yl)imino)methyl)phenol, C11H11N3O2S
- The crystal structure of 3-((tert-butyldiphenylsilyl)methyl)-5,5-diphenyl-6-(p-tolyl) tetrahydro-2H-pyran-2-one, C41H42O2Si
- Crystal structure of 9-fluoro-4-(6-methoxypyridin-3-yl)-5,6-dihydrobenzo[h]quinazolin-2-amine, C18H15FN4O
- The crystal structure of 2-bromo-5-(4-cyanophenoxy)benzyl 1-methyl-1,2,5,6-tetrahydropyridine-3-carboxylate, C21H19BrN2O3
- Crystal structure of 3,3′-(1,4-phenylenebis(methylene))bis(1-isopropyl-1H-imidazol-3-ium) bis(hexafluorophosphate(V)), C10H14F6N2P
- The crystal structure of 2,2-di(thiophen-3-yl)-1-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C24H19BN2O2S3
- Crystal structure of 5-bromo-1-(2-iodobenzoyl)-1H-indole-3-carbaldehyde, C16H9BrINO2
- The crystal structure of monocarbonyl-2-carboxypyridinato-κ2N,O-triphenylphosphine-rhodium(I) acetonitrile solvate, C26H20.50N1.50O3PRh
- Crystal structure of dichlorido-tetrakis(1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ1N)manganese(II), C60H68O4N12Cl10Mn
- Crystal structure of 3-(tert-butyldiphenylsilyl)-1-(2,6-dichlorophenyl)-2,2-diphenylpropan-1-ol, C37H36Cl2OSi
- Crystal structure of langite from Mine du Pradet (France)
- The crystal structure of 5′-(furan-2-yl)-3′-((4-methylphenyl)sulfonamido)-3′,4′,5′,6′-tetrahydro-[1,1′:3′,1″-terphenyl]-4′-carboxylic acid, C30H27NO5S
- Synthesis and crystal structure of bis{2-(((4-acetophenone)imino)methyl)-4-fluorophenolato-κ2N,O}zinc(II), C30H22F2N2O4Zn
- The crystal structure of poly[(tripyridine-κ3N,N′,N″) μ3-(pyridine-3,4-dicarboxylate-κ3N:O:O′) manganese(II)], C22H22N4O8Mn
- The crystal structure of (E)-4-chloro-N′-(1-(4-hydroxyphenyl)propylidene)benzohydrazide, C16H15ClN2O2
- Synthesis and crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino) ethyl)phenyl)imino)methyl)phenolato-κ2N,O}cobalt(II), C40H46CoN4O4
- Crystal structure of tetraaqua-[(1-(carboxymethyl)-1H-pyrazole-3-carboxylato-κ2N,O)cobalt(II)], C6H12CoN2O8
- (6R,7S)-2,3,13-trimethoxy-6,7-dimethyl-5,6,7,8-tetrahydrobenzo[3′,4′]cycloocta [1′,2′:4,5]benzo[1,2-d][1,3]dioxol-1-ol, C22H26O6
- Crystal structure of 2-((2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)amino)benzoic acid, C18H14Cl2N2O3
- Crystal structure of (5aS,6aS,8aR,9R,11aS, 11bS,13R,13aS)-1,1,8a,11a-tetramethyl-9-((S)-1-((S)-5-methyl-6-oxo-3,6-dihydro-2H-pyran-2-yl)ethyl)-3-oxo-1,7,8,8a,9,10,11,11a,11b,12,13,13a-dodecahydro-3H,6H-cyclopenta[5,6]cyclopropa[1,8a]naphtho[2,1-c]oxepin-13-yl acetate, C32H44O6
- Crystal structure of catena-poly[triaqua-(μ2-1-(4-carboxylatophenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylato-O,O′:O″)cobalt(II)], C12H12N2O8Co
- Crystal structure of 3-[(furan-2-ylmethyl)-amino]-2-(2,3,4,5-tetrafluoro-benzoyl)-acrylic acid ethyl ester, C17H13F4NO4
- Crystal structure of methyl 4-(2-ethoxy-2-oxoethoxy)-3-methoxybenzoate, C13H16O6
- Crystal structure of 4-bromo-2-(4-chlorophenyl)-1-methyl-5-(trifluoromethyl)-1H-pyrrole-3-carbonitrile, C13H7BrClF3N2
- The crystal structure of triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ3N,O,O)nickel(II) monohydrate, C12H15NO9Ni
- Crystal structure of dihydroxy(2,4,6-triisopro-pylphenyl)telluronium trifluoromethanesulfonate, C16H25F3O5STe
- The crystal structure of 1-(carboxymethyl)-1H-imidazole 3-oxide
- The crystal structure of 1,3,5-tris(dibromomethyl)benzene, C9H6Br6
- Crystal structure of (Z)-3-(4-methoxyphenyl)-4-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-N-phenylthiazol-2(3H)-imine, C25H21N5OS
- Crystal structure of (Z)-3-(3-(4-hydroxyphenyl)-2-(phenylimino)-2,3-dihydrothiazol-4-yl)-2H-chromen-2-one, C24H16N2O3S

