Abstract
C25H21N5OS, monoclinic, P21/n (no. 14), a = 12.9303(5) Å, b = 9.1111(4) Å, c = 18.7111(8) Å, β = 97.879(4)°, V = 2183.53(16) Å3, Z = 4, R gt (F) = 0.0487, wR ref (F2) = 0.1327, T = 293(2) K.
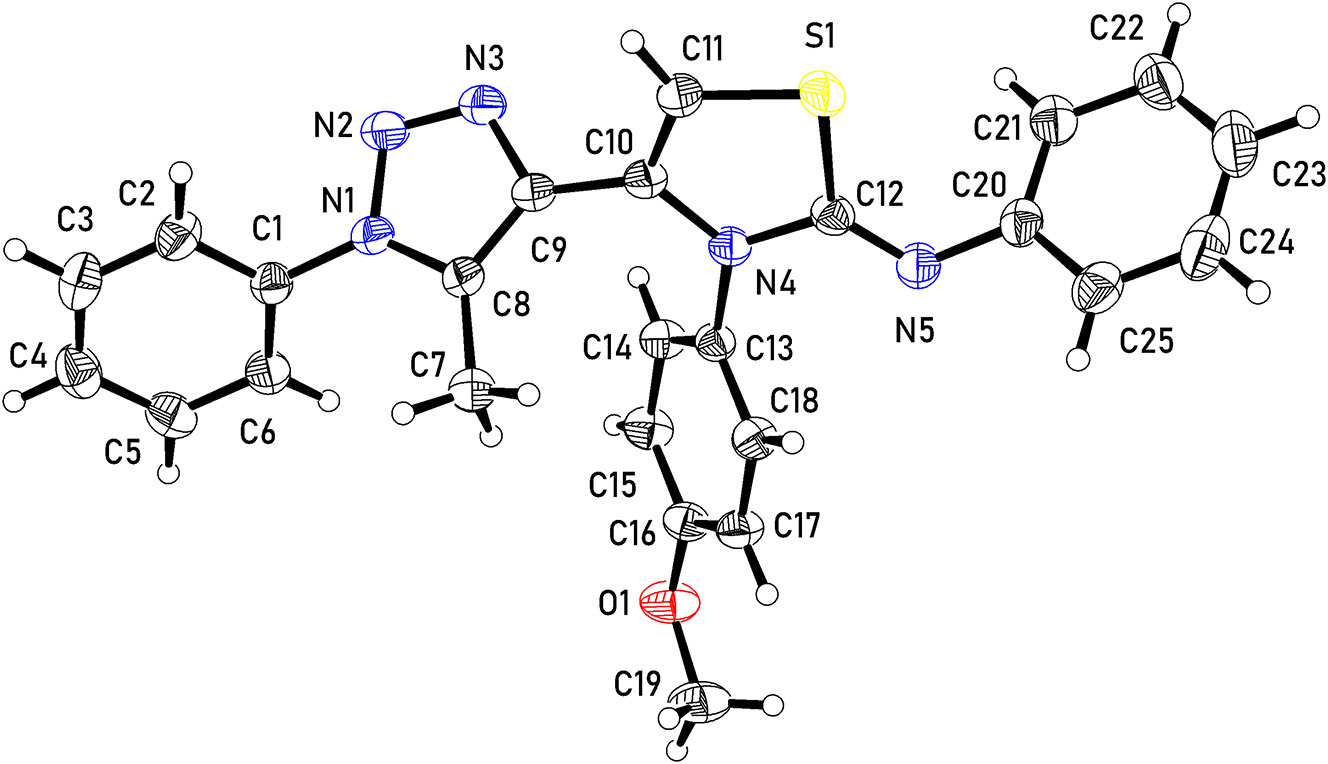
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.17 × 0.10 × 0.10 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 6.0 mm−1 |
| Diffractometer, scan mode: | Bruker APEXII, φ and ω |
| θmax, completeness: | 56.6°, >99 % |
| N(hkl)measured, N(hkl)unique, Rint: | 13,239, 9148, 0.025 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 7183 |
| N(param)refined: | 686 |
| Programs: | CrystAlisPRO [1], SHELX [2], WinGX/ORTEP [3], CHEMDRAW [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1 | 0.03708 (14) | 0.8202 (2) | 0.58167 (10) | 0.0381 (4) |
| C2 | 0.02149 (17) | 0.8323 (2) | 0.65315 (11) | 0.0465 (5) |
| H2 | 0.078034 | 0.840490 | 0.689431 | 0.056* |
| C3 | −0.07936 (18) | 0.8319 (2) | 0.66978 (12) | 0.0527 (5) |
| H3 | −0.090937 | 0.840711 | 0.717563 | 0.063* |
| C4 | −0.16260 (17) | 0.8186 (2) | 0.61607 (14) | 0.0534 (5) |
| H4 | −0.230188 | 0.816764 | 0.627804 | 0.064* |
| C5 | −0.14671 (16) | 0.8080 (3) | 0.54508 (13) | 0.0533 (5) |
| H5 | −0.203530 | 0.799827 | 0.509007 | 0.064* |
| C6 | −0.04606 (15) | 0.8095 (3) | 0.52713 (11) | 0.0472 (5) |
| H6 | −0.034824 | 0.803380 | 0.479181 | 0.057* |
| C7 | 0.14718 (17) | 0.5873 (2) | 0.49655 (13) | 0.0520 (5) |
| H7A | 0.100601 | 0.549008 | 0.527739 | 0.078* |
| H7B | 0.202285 | 0.518070 | 0.493239 | 0.078* |
| H7C | 0.109399 | 0.603970 | 0.449407 | 0.078* |
| C8 | 0.19250 (14) | 0.7281 (2) | 0.52637 (10) | 0.0370 (4) |
| C9 | 0.29014 (14) | 0.7883 (2) | 0.52875 (10) | 0.0370 (4) |
| C10 | 0.38571 (14) | 0.7371 (2) | 0.50277 (10) | 0.0374 (4) |
| C11 | 0.47860 (15) | 0.7321 (3) | 0.54423 (10) | 0.0452 (5) |
| H11 | 0.488089 | 0.753554 | 0.593280 | 0.054* |
| C12 | 0.49111 (13) | 0.6638 (2) | 0.41659 (10) | 0.0360 (4) |
| C13 | 0.30245 (13) | 0.6816 (2) | 0.37593 (9) | 0.0333 (4) |
| C14 | 0.23351 (14) | 0.7963 (2) | 0.35908 (10) | 0.0403 (4) |
| H14 | 0.245273 | 0.886080 | 0.382397 | 0.048* |
| C15 | 0.14671 (15) | 0.7776 (2) | 0.30741 (11) | 0.0438 (5) |
| H15 | 0.099693 | 0.854345 | 0.296742 | 0.053* |
| C16 | 0.13007 (14) | 0.6446 (2) | 0.27167 (10) | 0.0394 (4) |
| C17 | 0.19949 (14) | 0.5302 (2) | 0.28807 (10) | 0.0408 (4) |
| H17 | 0.188671 | 0.440945 | 0.264112 | 0.049* |
| C18 | 0.28517 (14) | 0.5492 (2) | 0.34031 (10) | 0.0384 (4) |
| H18 | 0.331599 | 0.471952 | 0.351557 | 0.046* |
| C19 | 0.01870 (18) | 0.4975 (3) | 0.18771 (13) | 0.0612 (6) |
| H19A | 0.071844 | 0.470723 | 0.158993 | 0.092* |
| H19B | −0.047461 | 0.503426 | 0.157511 | 0.092* |
| H19C | 0.015101 | 0.424652 | 0.224382 | 0.092* |
| C20 | 0.61751 (14) | 0.6008 (2) | 0.34534 (9) | 0.0401 (4) |
| C21 | 0.69189 (16) | 0.7120 (3) | 0.35746 (11) | 0.0470 (5) |
| H21 | 0.673378 | 0.802522 | 0.374875 | 0.056* |
| C22 | 0.79308 (17) | 0.6887 (3) | 0.34376 (13) | 0.0587 (6) |
| H22 | 0.842110 | 0.763633 | 0.351820 | 0.070* |
| C23 | 0.82140 (19) | 0.5553 (4) | 0.31831 (14) | 0.0706 (8) |
| H23 | 0.889682 | 0.539351 | 0.309744 | 0.085* |
| C24 | 0.7486 (2) | 0.4456 (3) | 0.30558 (13) | 0.0670 (7) |
| H24 | 0.767881 | 0.355497 | 0.288194 | 0.080* |
| C25 | 0.64659 (18) | 0.4672 (3) | 0.31826 (11) | 0.0520 (5) |
| H25 | 0.597676 | 0.392473 | 0.308669 | 0.062* |
| N1 | 0.14152 (12) | 0.82403 (18) | 0.56448 (8) | 0.0383 (4) |
| N2 | 0.20444 (13) | 0.9371 (2) | 0.59012 (10) | 0.0481 (4) |
| N3 | 0.29442 (12) | 0.9153 (2) | 0.56804 (9) | 0.0465 (4) |
| N4 | 0.39117 (11) | 0.69774 (17) | 0.43072 (8) | 0.0358 (3) |
| N5 | 0.51235 (12) | 0.6236 (2) | 0.35497 (9) | 0.0436 (4) |
| O1 | 0.04339 (11) | 0.63654 (17) | 0.22080 (8) | 0.0553 (4) |
| S1 | 0.57902 (4) | 0.68154 (7) | 0.49731 (3) | 0.04634 (16) |
1 Source of material
A mixture of 4-methoxyaniline (0.62 g, 5 mmol) and phenyl isothiocyanate (0.68 g, 5 mmol) in anhydrous EtOH (15 ml), was refluxed for 15 min, followed by the addition of 2-bromo-1-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)ethan-1-one (1.2 g, 5 mmol). The mixture was refluxed for 4 h. The solid produced on standing overnight was filtered, dried, and recrystallized from DMF to give the title heterocycle in 80 % yield, mp 179–181 °C. IR (KBr): 3121, 1610, 1517 cm−1. 1H NMR (DMSO-d6): 2.17 (s, 3H, Me), 3.69 (s, 3H, OMe), 6.56 (s, 1H, thiazolyl), 6.88 (d, 8.2 Hz, 2H, Ar), 6.93 (d, 8.2 Hz, 2H, Ar), 7.00 (t, 7.7 Hz, 1H, Ar), 7.23 (d, 9.0 Hz, 2H, Ar), 7.29 (t, 7.7 Hz, 2H, Ar), 7.45–7.58 (m, 5H, Ar). 13C NMR (DMSO-d6): 9.5, 55.8, 100.6, 114.4, 121.5, 122.9, 125.2, 130.1, 130.2, 130.3, 130.5, 130.7, 130.9, 134.2, 135.8, 136.7, 152.1, 158.9, 159.4. Anal. Calcd. for C25H21N5OS (439.54): C, 68.32; H, 4.82; N, 15.93. Found: C, 68.43; H, 4.84; N, 16.08 %.
2 Experimental details
Hydrogen atoms were located in the difference Fourier map. Idealized geometries were subsequently used for the initial positions and a riding model applied during refinement. Methyl C–H bonds were fixed at 0.96 Å, with displacement parameters 1.5 times Uiso(C), and were allowed to spin about the C–C bond. C–H distances were set to 0.93 Å for sp2 hydrogen atoms and their U(iso) set to 1.2 times the Uiso(C). Crystal data, data collection and structure refinement details are summarized in Table 1.
3 Comment
Heterocycles containing the thiazol-2-imine moiety have been found to possess interesting biological activities [5], [6], [7]. In addition, triazoles show a wide range of activities against pathogens [8], [9], [10]. Therefore, synthesizing heterocycles containing N-phenylthiazol-2(3H)-imines and 1,2,3-triazoles has received attention [11, 12]. In previous work, we have reported the X-ray crystal structures of related heterocycles [13, 14].
The crystal structure is monoclinic, with an asymmetric unit comprising one molecule of C25H21N5OS (Figure 1) which is composed of the following five planar groups: phenyl (A: C1–C6), methyltriazole (B: C7–C9, N1–N3), thiazole (C: C10–C12, N4, S1), methoxybenzene (D:C13–C19, O1) and aniline (E: C20–C25, N5). The planes of adjacent groups in the molecule are significantly twisted relative to each other. The twist angles between the groups are: A/B = 55.21(7)°, B/C = 51.60(7)°, C/D = 55.87(7)° D/E = 45.69(5)°.
In the crystal structure, neighbouring molecules are related by inversion symmetry to form pairs with parallel thiazole groups with a C⋯C centroid separated distance of 3.86 Å. The pairs of molecules are stacked to form columns parallel to the b-axis of the crystal. Adjoining columns are linked by C–H⋯O (C11⋯O1 = 3.506(2), C11–H11⋯O1 = 166.1) and C–H⋯N (C19⋯N3 = 3.504(3), C19–H19B⋯N3 = 165.0) interactions.
Funding source: National Research Centre
Funding source: Cardiff University
Funding source: Deanship of Scientific Research, King Saud University
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: We thank the National Research Centre, Cairo, Egypt, and Cardiff University, Cardiff, UK, for their support. G. A. El-Hiti extends his appreciation to the Deanship of Scientific Research, King Saud University, for funding through the Vice Deanship of Scientific Research Chairs, Research Chair of Cornea.
References
1. Agilent CrysAlisPRO; Agilent Technologies: Yarnton, England, 2014.Search in Google Scholar
2. Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Search in Google Scholar PubMed
3. Farrugia, L. J. WinGX and ORTEP for Windows: an update. J. Appl. Crystallogr. 2012, 45, 849–854; https://doi.org/10.1107/s0021889812029111.Search in Google Scholar
4. Cambridge Soft. CHEMDRAW; Cambridge Soft Corporation: Cambridge, Massachusetts, USA, 2001.Search in Google Scholar
5. Sharma, P. C., Bansal, K. K., Sharma, A., Sharma, D., Deep, A. Thiazole-containing compounds as therapeutic targets for cancer therapy. Eur. J. Med. Chem. 2020, 188, 112016; https://doi.org/10.1016/j.ejmech.2019.112016.Search in Google Scholar PubMed
6. Ramachandran, S., Cheriyan, B. V., Aanandhi, M. V. Activities of thiazolidine-4-one and azetidine-2-one derivatives – a review. Res. J. Pharm. Technol. 2021, 14, 4513–4516; https://doi.org/10.52711/0974-360x.2021.00785.Search in Google Scholar
7. Arshad, M. F., Alam, A., Alshammari, A. A., Alhazza, M. B., Alzimam, I. M., Alam, M. A., Mustafa, G., Ansari, M. S., Alotaibi, A. M., Alotaibi, A. A., Kumar, S., Asdaq, S. M. B., Imran, M., Deb, P. K., Venugopala, K. V., Jomah, S. Thiazole: a versatile standalone moiety contributing to the development of various drugs and biologically active agents. Molecules 2022, 27, 3994; https://doi.org/10.3390/molecules27133994.Search in Google Scholar PubMed PubMed Central
8. Varala, R., Bollikolla, H. B., Kurmarayuni, C. M. Synthesis of pharmacological relevant 1,2,3-triazole and its analogues – a review. Curr. Org. Synth. 2021, 18, 101–124; https://doi.org/10.2174/18756271mta54otec0.Search in Google Scholar
9. Kazeminejad, Z., Marzi, M., Shiroudi, A., Kouhpayeh, S. A., Farjam, M., Zarenezhad, E. Novel 1,2,4-triazoles as antifungal agents. Biomed Res. Int. 2022, 2022, 4584846; https://doi.org/10.1155/2022/4584846.Search in Google Scholar PubMed PubMed Central
10. Marzi, M., Farjam, M., Kazeminejad, Z., Shiroudi, A., Kouhpayeh, A., Zarenezhad, E. A recent overview of 1,2,3-triazole-containing hybrids as novel antifungal agents: focusing on synthesis, mechanism of action, and structure-activity relationship (SAR). J. Chem. 2022, 2022, 7884316; https://doi.org/10.1155/2022/7884316.Search in Google Scholar
11. Abdel-Wahab, B. F., Kariuki, B. M., Mohamed, H. A., El-Hiti, G. A. Catalyst-free synthesis of novel 4-(benzofuran-2-yl)-N-phenylthiazol-2(3H)-imines, crystal structure elucidation, and the effect of phenyl substitution on crystal packing. Crystals 2023, 13, 1239; https://doi.org/10.3390/cryst13081239.Search in Google Scholar
12. Abdel-Wahab, B. F., Kariuki, B. M., Mohamed, H. A., Bekheit, M. S., Awad, H. M., El-Hiti, G. A. Synthesis and anticancer activity of 3-(1-aryl-5-methyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazole-4-carbaldehydes. J. Mol. Struct. 2023, 1294, 136528; https://doi.org/10.1016/j.molstruc.2023.136528.Search in Google Scholar
13. Geesi, M. H., Mohamed, H. A., Abdel-Wahab, B. F., Hegazy, A. S., Kariuki, B. M., El-Hiti, G. A. Crystal structure of ethyl 4-amino-5-(5-methyl-1-(4-tolyl)-1H-1,2,3-triazole-4-carbonyl)-2-p̆henylamino)thiophene-3-carboxylate, C24H23N5O3S. Z. Kristallogr. N. Cryst. Struct. 2018, 233, 673–674; https://doi.org/10.1515/ncrs-2018-0002.Search in Google Scholar
14. Basheen, M. A., Abdel-Wahab, B. F., Hegazy, A. S., Kariuki, B. M., El-Hiti, G. A. The crystal structure of 4-(4-bromophenyl)-2-(3-(4-bromophenyl)-5-(4-fluorophenyl)-4,5-dihydro-1H-pyrazol-1-yl)thiazole, C24H16Br2FN3S. Z. Kristallogr. N. Cryst. Struct. 2021, 236, 425–427; https://doi.org/10.1515/ncrs-2020-0605.Search in Google Scholar
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of poly[diaqua-(μ4-3,3′-di(1H-1,2,4-triazol-1-yl)-[1,1′-biphenyl]-4,4′-dicarboxylate-N:N′:O:O′)cadmium(II)], C18H14N6O6Cd
- Crystal structure of (8R,8′S,13S,13′R)-8,8′-bis(hydroxymethyl)-9,9′,10,10′-tetramethoxy-5,5′,6,6′,8,8′,13,13′-octahydro-[13,13′-bi[1,3]dioxolo[4,5-g]isoquinolino[3,2-a]isoquinoline]-7,7′-diium chloride-methanol (1/2), C46H58N2O14Cl2
- The crystal structure of 8-methoxy-2,2-diphenyl-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C29H25BN2O3S
- Crystal structure of aqua-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N″,N‴)copper(II) 5-carboxyisophthalate tetrahydrate, C25H50N4CuO11
- The crystal structure of 1-(naphthalen-2-ylsulfonyl)-2,2-diphenyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ij]quinoline, C31H23BN2O2S
- Crystal structure of iodido-(η6-benzene) (1-(pyridin-2-yl)-N-(p-fluoro-methanamine)-κ2N,Nʹ)ruthenium(II) hexaflourophosphate, (C18H15F7IN2RuP)
- The crystal structure of 1-(3-oxo-1-phenyl-3-(p-tolyl) propylidene)-1,3-dihydro-2H-inden-2-one, C25H20O2
- Crystal structure of tricyclohexyl[4-(4H-1,2,4-triazol-4-yl)-benzoato-κO]tin(IV), C27H39N3O2Sn
- Crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ4N,O,O,O)cadmium(II)]monohydrate, C12H15NO9Cd
- Crystal structure of ethyl 2-((4-(3,5-dimethylisoxazol-4-yl)-2,6-difluorophenyl)amino)benzoate, C20H18F2N2O3
- The crystal structure of 2-(hydroxymethyl)-2-(4H-1,2,4-triazol-4-yl)propane-1,3-diol, C6H11N3O3
- The crystal structure of 1,2-bis(2,4-dinitrophenyl) hydrazine, C12H8N6O8
- Crystal structure of 1-(2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)-1,2-dihydro-4H-benzo[d][1,3]oxazin-4-one, C19H14Cl2N2O3
- The crystal structure of 5-amino-5-oxo-4-(1-oxo-4-(2-oxopyrrolidin-1-yl)isoindolin-2-yl)pentanoic acid, C17H19N3O5
- Crystal structure of N2,N6-bis(2-(((Z)-5-bromo-2-hydroxybenzylidene)amino) phenyl)pyridine-2,6-dicarboxamide, C33H23Br2N5O4
- The crystal structure of (E)-2-methoxy-6-(((5-methyl-1,3,4-thiadiazol-2-yl)imino)methyl)phenol, C11H11N3O2S
- The crystal structure of 3-((tert-butyldiphenylsilyl)methyl)-5,5-diphenyl-6-(p-tolyl) tetrahydro-2H-pyran-2-one, C41H42O2Si
- Crystal structure of 9-fluoro-4-(6-methoxypyridin-3-yl)-5,6-dihydrobenzo[h]quinazolin-2-amine, C18H15FN4O
- The crystal structure of 2-bromo-5-(4-cyanophenoxy)benzyl 1-methyl-1,2,5,6-tetrahydropyridine-3-carboxylate, C21H19BrN2O3
- Crystal structure of 3,3′-(1,4-phenylenebis(methylene))bis(1-isopropyl-1H-imidazol-3-ium) bis(hexafluorophosphate(V)), C10H14F6N2P
- The crystal structure of 2,2-di(thiophen-3-yl)-1-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C24H19BN2O2S3
- Crystal structure of 5-bromo-1-(2-iodobenzoyl)-1H-indole-3-carbaldehyde, C16H9BrINO2
- The crystal structure of monocarbonyl-2-carboxypyridinato-κ2N,O-triphenylphosphine-rhodium(I) acetonitrile solvate, C26H20.50N1.50O3PRh
- Crystal structure of dichlorido-tetrakis(1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ1N)manganese(II), C60H68O4N12Cl10Mn
- Crystal structure of 3-(tert-butyldiphenylsilyl)-1-(2,6-dichlorophenyl)-2,2-diphenylpropan-1-ol, C37H36Cl2OSi
- Crystal structure of langite from Mine du Pradet (France)
- The crystal structure of 5′-(furan-2-yl)-3′-((4-methylphenyl)sulfonamido)-3′,4′,5′,6′-tetrahydro-[1,1′:3′,1″-terphenyl]-4′-carboxylic acid, C30H27NO5S
- Synthesis and crystal structure of bis{2-(((4-acetophenone)imino)methyl)-4-fluorophenolato-κ2N,O}zinc(II), C30H22F2N2O4Zn
- The crystal structure of poly[(tripyridine-κ3N,N′,N″) μ3-(pyridine-3,4-dicarboxylate-κ3N:O:O′) manganese(II)], C22H22N4O8Mn
- The crystal structure of (E)-4-chloro-N′-(1-(4-hydroxyphenyl)propylidene)benzohydrazide, C16H15ClN2O2
- Synthesis and crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino) ethyl)phenyl)imino)methyl)phenolato-κ2N,O}cobalt(II), C40H46CoN4O4
- Crystal structure of tetraaqua-[(1-(carboxymethyl)-1H-pyrazole-3-carboxylato-κ2N,O)cobalt(II)], C6H12CoN2O8
- (6R,7S)-2,3,13-trimethoxy-6,7-dimethyl-5,6,7,8-tetrahydrobenzo[3′,4′]cycloocta [1′,2′:4,5]benzo[1,2-d][1,3]dioxol-1-ol, C22H26O6
- Crystal structure of 2-((2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)amino)benzoic acid, C18H14Cl2N2O3
- Crystal structure of (5aS,6aS,8aR,9R,11aS, 11bS,13R,13aS)-1,1,8a,11a-tetramethyl-9-((S)-1-((S)-5-methyl-6-oxo-3,6-dihydro-2H-pyran-2-yl)ethyl)-3-oxo-1,7,8,8a,9,10,11,11a,11b,12,13,13a-dodecahydro-3H,6H-cyclopenta[5,6]cyclopropa[1,8a]naphtho[2,1-c]oxepin-13-yl acetate, C32H44O6
- Crystal structure of catena-poly[triaqua-(μ2-1-(4-carboxylatophenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylato-O,O′:O″)cobalt(II)], C12H12N2O8Co
- Crystal structure of 3-[(furan-2-ylmethyl)-amino]-2-(2,3,4,5-tetrafluoro-benzoyl)-acrylic acid ethyl ester, C17H13F4NO4
- Crystal structure of methyl 4-(2-ethoxy-2-oxoethoxy)-3-methoxybenzoate, C13H16O6
- Crystal structure of 4-bromo-2-(4-chlorophenyl)-1-methyl-5-(trifluoromethyl)-1H-pyrrole-3-carbonitrile, C13H7BrClF3N2
- The crystal structure of triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ3N,O,O)nickel(II) monohydrate, C12H15NO9Ni
- Crystal structure of dihydroxy(2,4,6-triisopro-pylphenyl)telluronium trifluoromethanesulfonate, C16H25F3O5STe
- The crystal structure of 1-(carboxymethyl)-1H-imidazole 3-oxide
- The crystal structure of 1,3,5-tris(dibromomethyl)benzene, C9H6Br6
- Crystal structure of (Z)-3-(4-methoxyphenyl)-4-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-N-phenylthiazol-2(3H)-imine, C25H21N5OS
- Crystal structure of (Z)-3-(3-(4-hydroxyphenyl)-2-(phenylimino)-2,3-dihydrothiazol-4-yl)-2H-chromen-2-one, C24H16N2O3S
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of poly[diaqua-(μ4-3,3′-di(1H-1,2,4-triazol-1-yl)-[1,1′-biphenyl]-4,4′-dicarboxylate-N:N′:O:O′)cadmium(II)], C18H14N6O6Cd
- Crystal structure of (8R,8′S,13S,13′R)-8,8′-bis(hydroxymethyl)-9,9′,10,10′-tetramethoxy-5,5′,6,6′,8,8′,13,13′-octahydro-[13,13′-bi[1,3]dioxolo[4,5-g]isoquinolino[3,2-a]isoquinoline]-7,7′-diium chloride-methanol (1/2), C46H58N2O14Cl2
- The crystal structure of 8-methoxy-2,2-diphenyl-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C29H25BN2O3S
- Crystal structure of aqua-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N″,N‴)copper(II) 5-carboxyisophthalate tetrahydrate, C25H50N4CuO11
- The crystal structure of 1-(naphthalen-2-ylsulfonyl)-2,2-diphenyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ij]quinoline, C31H23BN2O2S
- Crystal structure of iodido-(η6-benzene) (1-(pyridin-2-yl)-N-(p-fluoro-methanamine)-κ2N,Nʹ)ruthenium(II) hexaflourophosphate, (C18H15F7IN2RuP)
- The crystal structure of 1-(3-oxo-1-phenyl-3-(p-tolyl) propylidene)-1,3-dihydro-2H-inden-2-one, C25H20O2
- Crystal structure of tricyclohexyl[4-(4H-1,2,4-triazol-4-yl)-benzoato-κO]tin(IV), C27H39N3O2Sn
- Crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ4N,O,O,O)cadmium(II)]monohydrate, C12H15NO9Cd
- Crystal structure of ethyl 2-((4-(3,5-dimethylisoxazol-4-yl)-2,6-difluorophenyl)amino)benzoate, C20H18F2N2O3
- The crystal structure of 2-(hydroxymethyl)-2-(4H-1,2,4-triazol-4-yl)propane-1,3-diol, C6H11N3O3
- The crystal structure of 1,2-bis(2,4-dinitrophenyl) hydrazine, C12H8N6O8
- Crystal structure of 1-(2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)-1,2-dihydro-4H-benzo[d][1,3]oxazin-4-one, C19H14Cl2N2O3
- The crystal structure of 5-amino-5-oxo-4-(1-oxo-4-(2-oxopyrrolidin-1-yl)isoindolin-2-yl)pentanoic acid, C17H19N3O5
- Crystal structure of N2,N6-bis(2-(((Z)-5-bromo-2-hydroxybenzylidene)amino) phenyl)pyridine-2,6-dicarboxamide, C33H23Br2N5O4
- The crystal structure of (E)-2-methoxy-6-(((5-methyl-1,3,4-thiadiazol-2-yl)imino)methyl)phenol, C11H11N3O2S
- The crystal structure of 3-((tert-butyldiphenylsilyl)methyl)-5,5-diphenyl-6-(p-tolyl) tetrahydro-2H-pyran-2-one, C41H42O2Si
- Crystal structure of 9-fluoro-4-(6-methoxypyridin-3-yl)-5,6-dihydrobenzo[h]quinazolin-2-amine, C18H15FN4O
- The crystal structure of 2-bromo-5-(4-cyanophenoxy)benzyl 1-methyl-1,2,5,6-tetrahydropyridine-3-carboxylate, C21H19BrN2O3
- Crystal structure of 3,3′-(1,4-phenylenebis(methylene))bis(1-isopropyl-1H-imidazol-3-ium) bis(hexafluorophosphate(V)), C10H14F6N2P
- The crystal structure of 2,2-di(thiophen-3-yl)-1-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C24H19BN2O2S3
- Crystal structure of 5-bromo-1-(2-iodobenzoyl)-1H-indole-3-carbaldehyde, C16H9BrINO2
- The crystal structure of monocarbonyl-2-carboxypyridinato-κ2N,O-triphenylphosphine-rhodium(I) acetonitrile solvate, C26H20.50N1.50O3PRh
- Crystal structure of dichlorido-tetrakis(1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ1N)manganese(II), C60H68O4N12Cl10Mn
- Crystal structure of 3-(tert-butyldiphenylsilyl)-1-(2,6-dichlorophenyl)-2,2-diphenylpropan-1-ol, C37H36Cl2OSi
- Crystal structure of langite from Mine du Pradet (France)
- The crystal structure of 5′-(furan-2-yl)-3′-((4-methylphenyl)sulfonamido)-3′,4′,5′,6′-tetrahydro-[1,1′:3′,1″-terphenyl]-4′-carboxylic acid, C30H27NO5S
- Synthesis and crystal structure of bis{2-(((4-acetophenone)imino)methyl)-4-fluorophenolato-κ2N,O}zinc(II), C30H22F2N2O4Zn
- The crystal structure of poly[(tripyridine-κ3N,N′,N″) μ3-(pyridine-3,4-dicarboxylate-κ3N:O:O′) manganese(II)], C22H22N4O8Mn
- The crystal structure of (E)-4-chloro-N′-(1-(4-hydroxyphenyl)propylidene)benzohydrazide, C16H15ClN2O2
- Synthesis and crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino) ethyl)phenyl)imino)methyl)phenolato-κ2N,O}cobalt(II), C40H46CoN4O4
- Crystal structure of tetraaqua-[(1-(carboxymethyl)-1H-pyrazole-3-carboxylato-κ2N,O)cobalt(II)], C6H12CoN2O8
- (6R,7S)-2,3,13-trimethoxy-6,7-dimethyl-5,6,7,8-tetrahydrobenzo[3′,4′]cycloocta [1′,2′:4,5]benzo[1,2-d][1,3]dioxol-1-ol, C22H26O6
- Crystal structure of 2-((2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)amino)benzoic acid, C18H14Cl2N2O3
- Crystal structure of (5aS,6aS,8aR,9R,11aS, 11bS,13R,13aS)-1,1,8a,11a-tetramethyl-9-((S)-1-((S)-5-methyl-6-oxo-3,6-dihydro-2H-pyran-2-yl)ethyl)-3-oxo-1,7,8,8a,9,10,11,11a,11b,12,13,13a-dodecahydro-3H,6H-cyclopenta[5,6]cyclopropa[1,8a]naphtho[2,1-c]oxepin-13-yl acetate, C32H44O6
- Crystal structure of catena-poly[triaqua-(μ2-1-(4-carboxylatophenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylato-O,O′:O″)cobalt(II)], C12H12N2O8Co
- Crystal structure of 3-[(furan-2-ylmethyl)-amino]-2-(2,3,4,5-tetrafluoro-benzoyl)-acrylic acid ethyl ester, C17H13F4NO4
- Crystal structure of methyl 4-(2-ethoxy-2-oxoethoxy)-3-methoxybenzoate, C13H16O6
- Crystal structure of 4-bromo-2-(4-chlorophenyl)-1-methyl-5-(trifluoromethyl)-1H-pyrrole-3-carbonitrile, C13H7BrClF3N2
- The crystal structure of triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ3N,O,O)nickel(II) monohydrate, C12H15NO9Ni
- Crystal structure of dihydroxy(2,4,6-triisopro-pylphenyl)telluronium trifluoromethanesulfonate, C16H25F3O5STe
- The crystal structure of 1-(carboxymethyl)-1H-imidazole 3-oxide
- The crystal structure of 1,3,5-tris(dibromomethyl)benzene, C9H6Br6
- Crystal structure of (Z)-3-(4-methoxyphenyl)-4-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-N-phenylthiazol-2(3H)-imine, C25H21N5OS
- Crystal structure of (Z)-3-(3-(4-hydroxyphenyl)-2-(phenylimino)-2,3-dihydrothiazol-4-yl)-2H-chromen-2-one, C24H16N2O3S


