Abstract
C37H36Cl2OSi, monoclinic P21/n (no. 14), a = 10.4363(14) Å, b = 17.128(2) Å, c = 17.875(3) Å, β = 90.934(2)°, V = 3194.8(8) Å3, Z = 4, R gt (F) = 0.0491, wR ref (F2) = 0.1449, T = 296.15 K.
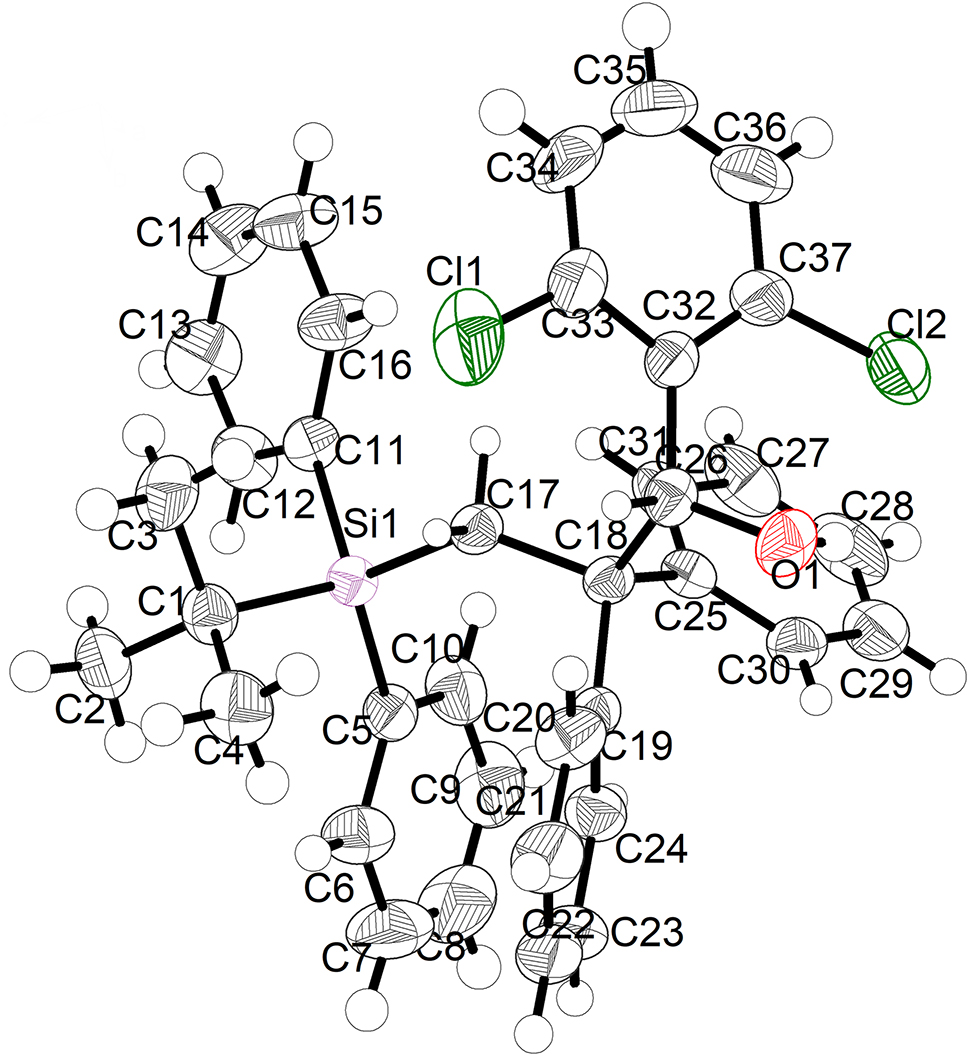
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.12 × 0.11 × 0.08 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.27 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| θmax, completeness: | 27.4°, >99 % |
| N(hkl)measured, N(hkl)unique, Rint: | 18,849, 7173, 0.030 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2σ(Iobs), 4927 |
| N(param)refined: | 382 |
| Programs: | CrysAlisPro [1], SHELX [2, 3], Diamond [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1 | 0.4110 (2) | 0.38280 (13) | 0.89461 (12) | 0.0413 (5) |
| C2 | 0.4680 (3) | 0.37858 (17) | 0.97448 (13) | 0.0568 (7) |
| C3 | 0.2924 (2) | 0.32905 (17) | 0.89086 (16) | 0.0592 (7) |
| C4 | 0.3661 (3) | 0.46710 (16) | 0.87892 (17) | 0.0610 (7) |
| C5 | 0.6962 (2) | 0.39311 (13) | 0.83799 (12) | 0.0387 (5) |
| C6 | 0.7158 (3) | 0.46516 (15) | 0.87236 (15) | 0.0544 (6) |
| C7 | 0.8369 (3) | 0.49780 (19) | 0.8807 (2) | 0.0760 (9) |
| C8 | 0.9423 (3) | 0.4586 (2) | 0.85429 (19) | 0.0818 (10) |
| C9 | 0.9268 (3) | 0.3873 (2) | 0.82068 (17) | 0.0722 (9) |
| C10 | 0.8053 (2) | 0.35490 (17) | 0.81292 (14) | 0.0529 (6) |
| C11 | 0.5509 (2) | 0.23854 (12) | 0.83889 (12) | 0.0361 (5) |
| C12 | 0.6332 (2) | 0.20960 (14) | 0.89511 (14) | 0.0482 (6) |
| C13 | 0.6387 (3) | 0.13056 (16) | 0.91247 (17) | 0.0609 (7) |
| C14 | 0.5637 (3) | 0.07835 (15) | 0.87313 (18) | 0.0643 (8) |
| C15 | 0.4824 (3) | 0.10414 (15) | 0.81750 (18) | 0.0636 (8) |
| C16 | 0.4756 (3) | 0.18340 (14) | 0.80053 (15) | 0.0518 (6) |
| C17 | 0.4507 (2) | 0.36066 (13) | 0.72768 (12) | 0.0345 (5) |
| C18 | 0.49834 (19) | 0.40564 (11) | 0.65783 (11) | 0.0315 (4) |
| C19 | 0.5053 (2) | 0.49315 (12) | 0.67961 (11) | 0.0354 (5) |
| C20 | 0.3962 (2) | 0.54074 (14) | 0.67906 (15) | 0.0514 (6) |
| C21 | 0.4019 (3) | 0.61707 (15) | 0.70533 (17) | 0.0622 (7) |
| C22 | 0.5156 (3) | 0.64795 (15) | 0.73170 (16) | 0.0615 (8) |
| C23 | 0.6237 (3) | 0.60274 (14) | 0.73229 (15) | 0.0554 (7) |
| C24 | 0.6182 (2) | 0.52604 (13) | 0.70675 (13) | 0.0440 (5) |
| C25 | 0.62576 (19) | 0.37517 (12) | 0.62846 (11) | 0.0330 (4) |
| C26 | 0.6687 (2) | 0.29965 (13) | 0.64399 (13) | 0.0440 (5) |
| C27 | 0.7802 (3) | 0.27077 (17) | 0.61177 (15) | 0.0595 (7) |
| C28 | 0.8491 (3) | 0.31643 (19) | 0.56355 (17) | 0.0644 (8) |
| C29 | 0.8086 (2) | 0.39063 (17) | 0.54715 (15) | 0.0554 (7) |
| C30 | 0.6988 (2) | 0.42010 (13) | 0.57962 (13) | 0.0416 (5) |
| C31 | 0.3906 (2) | 0.39440 (13) | 0.59428 (11) | 0.0373 (5) |
| C32 | 0.3595 (2) | 0.30944 (14) | 0.57629 (12) | 0.0404 (5) |
| C33 | 0.2567 (3) | 0.27159 (18) | 0.61096 (14) | 0.0606 (8) |
| C34 | 0.2315 (4) | 0.1923 (2) | 0.60193 (19) | 0.0885 (13) |
| C35 | 0.3063 (5) | 0.1491 (2) | 0.5555 (2) | 0.0947 (13) |
| C36 | 0.4003 (4) | 0.18283 (17) | 0.51649 (18) | 0.0711 (9) |
| C37 | 0.4253 (2) | 0.26193 (15) | 0.52568 (13) | 0.0481 (6) |
| Cl1 | 0.14991 (7) | 0.32299 (7) | 0.66617 (5) | 0.0914 (3) |
| Cl2 | 0.54013 (7) | 0.29926 (5) | 0.46575 (4) | 0.0664 (2) |
| H00A | 0.312120 | 0.415468 | 0.615897 | 0.045* |
| H00D | 0.673162 | 0.470878 | 0.568640 | 0.050* |
| H00F | 0.622512 | 0.268122 | 0.676217 | 0.053* |
| H00G | 0.692384 | 0.495976 | 0.707931 | 0.053* |
| H00H | 0.685547 | 0.244241 | 0.921510 | 0.058* |
| H00I | 0.796519 | 0.306244 | 0.790320 | 0.063* |
| H00K | 0.419719 | 0.200133 | 0.762789 | 0.062* |
| H00L | 0.645447 | 0.492285 | 0.890302 | 0.065* |
| H00M | 0.318714 | 0.520987 | 0.660866 | 0.062* |
| H00N | 0.701018 | 0.623307 | 0.749786 | 0.067* |
| H00O | 0.854773 | 0.421340 | 0.514199 | 0.067* |
| H00P | 0.518915 | 0.699135 | 0.748991 | 0.074* |
| H00Q | 0.328167 | 0.647547 | 0.705064 | 0.075* |
| H00E | 0.256822 | 0.329467 | 0.840988 | 0.089* |
| H00J | 0.229378 | 0.347467 | 0.925211 | 0.089* |
| H00R | 0.317086 | 0.276790 | 0.904061 | 0.089* |
| H00T | 0.807922 | 0.220452 | 0.622975 | 0.071* |
| H00U | 0.923164 | 0.296936 | 0.542108 | 0.077* |
| H00S | 0.498778 | 0.326670 | 0.984133 | 0.085* |
| H00V | 0.403085 | 0.391529 | 1.009837 | 0.085* |
| H00W | 0.537709 | 0.414899 | 0.979276 | 0.085* |
| H00X | 0.693080 | 0.113025 | 0.950636 | 0.073* |
| H | 0.308469 | 0.483325 | 0.917241 | 0.092* |
| HA | 0.322897 | 0.469177 | 0.831140 | 0.092* |
| H00Y | 0.439017 | 0.501258 | 0.878629 | 0.092* |
| H00Z | 0.568105 | 0.025385 | 0.884314 | 0.077* |
| H00 | 0.431712 | 0.068681 | 0.791019 | 0.076* |
| H00B | 0.366 (2) | 0.3832 (13) | 0.7388 (12) | 0.040 (6)* |
| H00C | 0.433 (2) | 0.3072 (14) | 0.7114 (13) | 0.045 (7)* |
| H004 | 0.479602 | 0.427307 | 0.511119 | 0.077* |
| H010 | 0.997759 | 0.360589 | 0.803019 | 0.087* |
| H011 | 0.846969 | 0.545990 | 0.904040 | 0.091* |
| H012 | 0.448356 | 0.153344 | 0.483470 | 0.085* |
| H013 | 0.164485 | 0.168925 | 0.627263 | 0.106* |
| H014 | 1.023538 | 0.480501 | 0.859271 | 0.098* |
| H015 | 0.291980 | 0.095749 | 0.550824 | 0.114* |
| O1 | 0.41224 (17) | 0.44062 (10) | 0.53036 (9) | 0.0514 (4) |
| Si1 | 0.53266 (5) | 0.34733 (3) | 0.82295 (3) | 0.03081 (14) |
1 Source of materials
An improved synthesis was performed which was similar to the previously report [5], [6], [7], [8]. To the solution of 2,6-dichlorobenzaldehyde (1.75 g, 10.0 mmol) in anhydrous 1,4-dioxane (100 mL) was added tert-butyldiphenylsilanecarboxylic acid (4.26 g, 15.0 mmol), 1,1-diphenylethylene (2.70 g, 15.0 mmol), NaHCO3 (0.84 g, 10.0 mmol) and organic photo catalyst 4CzIPN (394 mg, 0.5 mmol) under argon atmosphere. After addition, the reaction mixture was irradiated under 440 nm Kessil light for 12 h. TLC detection showed the reaction was finished. After simple filtration by silicon gel, the solvent was removed in vacuo. The obtained residue was dissolved in dichloromethane (100 mL), washed with water (50 mL × 2), brine (50 mL × 2), and dried over anhydrous MgSO4. After filtration, the solvent was evaporated in vacuo. The obtained residue was purified by column chromatography to afford white solid of 3-(tert-butyldiphenylsilyl)-1-(2,6-dichlorophenyl)-2,2-diphenylpropan-1-ol (4.38 g, 72 %), which was further purified by recrystallization from hexane and ethyl acetate. Crystals were obtained by slow evaporation from the solution at 268–270 K.
2 Experimental details
Carbon bound hydrogen atoms were included using riding models. For the OH group additionally a rotation about the C–O bond was allowed.
3 Comment
The 3-(tert-butyldiphenylsilyl)-1-(2,6-dichlorophenyl)-2,2-diphenylpropan-1-ol is a product formed by the reductive radical polar crossover (RPC)-type reaction [9], in which the anion is generated from single-electron reduction. RPC reactions have been proven useful in the formation of multiple bonds in a single transformation, including a multicomponent reaction (MCR) manifold [10]. In this paper, a silane radical was produced through single electron oxidation and decarboxylation of tert-butyldiphenylsilanecarboxylic acid anion, which then was added to the olefin to form carbon-centered radical. Next, carbon anion was achieved after single-electron reduction with low-valence organic photo catalyst 4CzIPN. Finally, carbon anion can then be added to aldehyde to provide the 3-silyl-propan-1-ol derivative. Different from Nozaki–Hiyama-Kishi type reaction [11], multicomponent strategy of reductive RPC reactions without alkyl metal species was rarely reported [8], especially with silane radical. Crystals of 3-(tert-butyl diphenylsilyl)-1-(2,6-dichlorophenyl)-2,2-diphenylpropan-1-ol were obtained by continuous adjustment of crystallization conditions, which revealed the specific structure of the multicomponent RPC reaction product with silanecarboxylic acid for the first time. In the meantime, this result also provides direct structural proof for the structure of the previous report [8], which is very significant for further understanding 3-silyl-propan-1-ol derivative and multicomponent RPC reaction. The title structure (see the Figure) was reported for the first time in this paper. This crystal structure is a racemic alcohol with a chiral carbon atom (C31). The propyl alcohol was linked to a 2,6-dichlorophenyl at C31, two phenyl at C18, and a silyl group at C17. The bond lengths and angles are in the expected ranges [12, 13].
Funding source: Nanchang Normal University
Award Identifier / Grant number: NSBSJJ2020009
Funding source: Ph.D. Programs Foundation of Jiangxi Science and Technology Normal University
Funding source: Science and Technology project of Jiangxi Provincial Education Office
Award Identifier / Grant number: GJJ2201329
-
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work was supported by from the Nanchang Normal University (NSBSJJ2020009), the Ph.D. Programs Foundation of Jiangxi Science and Technology Normal University,the Science and Technology project of Jiangxi Provincial Education Office (GJJ2201329).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Oxford Diffraction Ltd. CrysAlisPRO: Abingdon, Oxfordshire, England, 2006.Suche in Google Scholar
2. Sheldrick, G. M. Shelxtl – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8.10.1107/S2053273314026370Suche in Google Scholar PubMed PubMed Central
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar
4. Brandenburg, K. DIAMOND. Visual Crystal Structure Information System. Ver. 4.0; Crystal Impact: Bonn, Germany, 2015.Suche in Google Scholar
5. Venditto, N. J., Boerth, J. A. Photoredox-catalyzed multi-component synthesis of functionalized γ-amino butyric acids via reductive radical polar crossover. Org. Lett. 2023, 25, 3429–3434; https://doi.org/10.1021/acs.orglett.3c00991.Suche in Google Scholar PubMed
6. Cheng, Y.-Y., Hou, H.-Y., Liu, Y., Yu, J.-X., Chen, B., Tung, C.-H., Wu, L. α–Acylation of alkenes by a single photocatalyst. Angew. Chem. Int. Ed. 2022, 61, e202208831; https://doi.org/10.1002/anie.202208831.Suche in Google Scholar PubMed
7. Bao, Q.-F., Li, M., Xia, Y., Wang, Y.-Z., Zhou, Z.-Z., Liang, Y.-M. Visible-light-mediated decarboxylative radical addition bifunctionalization cascade for the production of 1,4-amino alcohols. Org. Lett. 2021, 23, 1107–1112; https://doi.org/10.1021/acs.orglett.1c00034.Suche in Google Scholar PubMed
8. Hou, J., Ee, A., Cao, H., Ong, H.-W., Xu, J.-H., Wu, J. Visible-light-mediated metal-free difunctionalization of alkenes with CO2 and silanes or C(sp3)–H alkanes. Angew. Chem. Int. Ed. 2018, 57, 17220–17224; https://doi.org/10.1002/anie.201811266.Suche in Google Scholar PubMed
9. Pitzer, L., Schwarz, J. L., Glorius, F. Reductive radical-polar crossover: traditional electrophiles in modern radical reactions. Chem. Sci. 2019, 10, 8285–8291; https://doi.org/10.1039/c9sc03359a.Suche in Google Scholar PubMed PubMed Central
10. Sharma, S., Singh, J., Sharma, A. Visible light assisted radical-polar/polar-radical crossover reactions in organic synthesis. Adv. Synth. Catal. 2021, 363, 3146–3169; https://doi.org/10.1002/adsc.202100205.Suche in Google Scholar
11. Schwarz, J. L., Schäfers, F., Tlahuext-Aca, A., Lückemeier, L., Glorius, F. Diastereoselective allylation of aldehydes by dual photoredox and chromium catalysis. J. Am. Chem. Soc. 2018, 140, 12705–12709; https://doi.org/10.1021/jacs.8b08052.Suche in Google Scholar PubMed
12. Bauer, J. O., The crystal structure of adamantylmethoxydiphenylsilane, C23H28OSi. Z. Krist. - New Cryst. Struct. 2021, 236, 1117–1120; https://doi.org/10.1039/c8sc05677c.Suche in Google Scholar PubMed PubMed Central
13. Lei, Z., Xue, F., Wang, B., Wang, S., Xia, Y., Zhang, Y., Jin, W., Liu, C., CSD Communication, 2023.Suche in Google Scholar
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of poly[diaqua-(μ4-3,3′-di(1H-1,2,4-triazol-1-yl)-[1,1′-biphenyl]-4,4′-dicarboxylate-N:N′:O:O′)cadmium(II)], C18H14N6O6Cd
- Crystal structure of (8R,8′S,13S,13′R)-8,8′-bis(hydroxymethyl)-9,9′,10,10′-tetramethoxy-5,5′,6,6′,8,8′,13,13′-octahydro-[13,13′-bi[1,3]dioxolo[4,5-g]isoquinolino[3,2-a]isoquinoline]-7,7′-diium chloride-methanol (1/2), C46H58N2O14Cl2
- The crystal structure of 8-methoxy-2,2-diphenyl-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C29H25BN2O3S
- Crystal structure of aqua-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N″,N‴)copper(II) 5-carboxyisophthalate tetrahydrate, C25H50N4CuO11
- The crystal structure of 1-(naphthalen-2-ylsulfonyl)-2,2-diphenyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ij]quinoline, C31H23BN2O2S
- Crystal structure of iodido-(η6-benzene) (1-(pyridin-2-yl)-N-(p-fluoro-methanamine)-κ2N,Nʹ)ruthenium(II) hexaflourophosphate, (C18H15F7IN2RuP)
- The crystal structure of 1-(3-oxo-1-phenyl-3-(p-tolyl) propylidene)-1,3-dihydro-2H-inden-2-one, C25H20O2
- Crystal structure of tricyclohexyl[4-(4H-1,2,4-triazol-4-yl)-benzoato-κO]tin(IV), C27H39N3O2Sn
- Crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ4N,O,O,O)cadmium(II)]monohydrate, C12H15NO9Cd
- Crystal structure of ethyl 2-((4-(3,5-dimethylisoxazol-4-yl)-2,6-difluorophenyl)amino)benzoate, C20H18F2N2O3
- The crystal structure of 2-(hydroxymethyl)-2-(4H-1,2,4-triazol-4-yl)propane-1,3-diol, C6H11N3O3
- The crystal structure of 1,2-bis(2,4-dinitrophenyl) hydrazine, C12H8N6O8
- Crystal structure of 1-(2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)-1,2-dihydro-4H-benzo[d][1,3]oxazin-4-one, C19H14Cl2N2O3
- The crystal structure of 5-amino-5-oxo-4-(1-oxo-4-(2-oxopyrrolidin-1-yl)isoindolin-2-yl)pentanoic acid, C17H19N3O5
- Crystal structure of N2,N6-bis(2-(((Z)-5-bromo-2-hydroxybenzylidene)amino) phenyl)pyridine-2,6-dicarboxamide, C33H23Br2N5O4
- The crystal structure of (E)-2-methoxy-6-(((5-methyl-1,3,4-thiadiazol-2-yl)imino)methyl)phenol, C11H11N3O2S
- The crystal structure of 3-((tert-butyldiphenylsilyl)methyl)-5,5-diphenyl-6-(p-tolyl) tetrahydro-2H-pyran-2-one, C41H42O2Si
- Crystal structure of 9-fluoro-4-(6-methoxypyridin-3-yl)-5,6-dihydrobenzo[h]quinazolin-2-amine, C18H15FN4O
- The crystal structure of 2-bromo-5-(4-cyanophenoxy)benzyl 1-methyl-1,2,5,6-tetrahydropyridine-3-carboxylate, C21H19BrN2O3
- Crystal structure of 3,3′-(1,4-phenylenebis(methylene))bis(1-isopropyl-1H-imidazol-3-ium) bis(hexafluorophosphate(V)), C10H14F6N2P
- The crystal structure of 2,2-di(thiophen-3-yl)-1-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C24H19BN2O2S3
- Crystal structure of 5-bromo-1-(2-iodobenzoyl)-1H-indole-3-carbaldehyde, C16H9BrINO2
- The crystal structure of monocarbonyl-2-carboxypyridinato-κ2N,O-triphenylphosphine-rhodium(I) acetonitrile solvate, C26H20.50N1.50O3PRh
- Crystal structure of dichlorido-tetrakis(1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ1N)manganese(II), C60H68O4N12Cl10Mn
- Crystal structure of 3-(tert-butyldiphenylsilyl)-1-(2,6-dichlorophenyl)-2,2-diphenylpropan-1-ol, C37H36Cl2OSi
- Crystal structure of langite from Mine du Pradet (France)
- The crystal structure of 5′-(furan-2-yl)-3′-((4-methylphenyl)sulfonamido)-3′,4′,5′,6′-tetrahydro-[1,1′:3′,1″-terphenyl]-4′-carboxylic acid, C30H27NO5S
- Synthesis and crystal structure of bis{2-(((4-acetophenone)imino)methyl)-4-fluorophenolato-κ2N,O}zinc(II), C30H22F2N2O4Zn
- The crystal structure of poly[(tripyridine-κ3N,N′,N″) μ3-(pyridine-3,4-dicarboxylate-κ3N:O:O′) manganese(II)], C22H22N4O8Mn
- The crystal structure of (E)-4-chloro-N′-(1-(4-hydroxyphenyl)propylidene)benzohydrazide, C16H15ClN2O2
- Synthesis and crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino) ethyl)phenyl)imino)methyl)phenolato-κ2N,O}cobalt(II), C40H46CoN4O4
- Crystal structure of tetraaqua-[(1-(carboxymethyl)-1H-pyrazole-3-carboxylato-κ2N,O)cobalt(II)], C6H12CoN2O8
- (6R,7S)-2,3,13-trimethoxy-6,7-dimethyl-5,6,7,8-tetrahydrobenzo[3′,4′]cycloocta [1′,2′:4,5]benzo[1,2-d][1,3]dioxol-1-ol, C22H26O6
- Crystal structure of 2-((2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)amino)benzoic acid, C18H14Cl2N2O3
- Crystal structure of (5aS,6aS,8aR,9R,11aS, 11bS,13R,13aS)-1,1,8a,11a-tetramethyl-9-((S)-1-((S)-5-methyl-6-oxo-3,6-dihydro-2H-pyran-2-yl)ethyl)-3-oxo-1,7,8,8a,9,10,11,11a,11b,12,13,13a-dodecahydro-3H,6H-cyclopenta[5,6]cyclopropa[1,8a]naphtho[2,1-c]oxepin-13-yl acetate, C32H44O6
- Crystal structure of catena-poly[triaqua-(μ2-1-(4-carboxylatophenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylato-O,O′:O″)cobalt(II)], C12H12N2O8Co
- Crystal structure of 3-[(furan-2-ylmethyl)-amino]-2-(2,3,4,5-tetrafluoro-benzoyl)-acrylic acid ethyl ester, C17H13F4NO4
- Crystal structure of methyl 4-(2-ethoxy-2-oxoethoxy)-3-methoxybenzoate, C13H16O6
- Crystal structure of 4-bromo-2-(4-chlorophenyl)-1-methyl-5-(trifluoromethyl)-1H-pyrrole-3-carbonitrile, C13H7BrClF3N2
- The crystal structure of triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ3N,O,O)nickel(II) monohydrate, C12H15NO9Ni
- Crystal structure of dihydroxy(2,4,6-triisopro-pylphenyl)telluronium trifluoromethanesulfonate, C16H25F3O5STe
- The crystal structure of 1-(carboxymethyl)-1H-imidazole 3-oxide
- The crystal structure of 1,3,5-tris(dibromomethyl)benzene, C9H6Br6
- Crystal structure of (Z)-3-(4-methoxyphenyl)-4-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-N-phenylthiazol-2(3H)-imine, C25H21N5OS
- Crystal structure of (Z)-3-(3-(4-hydroxyphenyl)-2-(phenylimino)-2,3-dihydrothiazol-4-yl)-2H-chromen-2-one, C24H16N2O3S
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of poly[diaqua-(μ4-3,3′-di(1H-1,2,4-triazol-1-yl)-[1,1′-biphenyl]-4,4′-dicarboxylate-N:N′:O:O′)cadmium(II)], C18H14N6O6Cd
- Crystal structure of (8R,8′S,13S,13′R)-8,8′-bis(hydroxymethyl)-9,9′,10,10′-tetramethoxy-5,5′,6,6′,8,8′,13,13′-octahydro-[13,13′-bi[1,3]dioxolo[4,5-g]isoquinolino[3,2-a]isoquinoline]-7,7′-diium chloride-methanol (1/2), C46H58N2O14Cl2
- The crystal structure of 8-methoxy-2,2-diphenyl-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C29H25BN2O3S
- Crystal structure of aqua-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N″,N‴)copper(II) 5-carboxyisophthalate tetrahydrate, C25H50N4CuO11
- The crystal structure of 1-(naphthalen-2-ylsulfonyl)-2,2-diphenyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ij]quinoline, C31H23BN2O2S
- Crystal structure of iodido-(η6-benzene) (1-(pyridin-2-yl)-N-(p-fluoro-methanamine)-κ2N,Nʹ)ruthenium(II) hexaflourophosphate, (C18H15F7IN2RuP)
- The crystal structure of 1-(3-oxo-1-phenyl-3-(p-tolyl) propylidene)-1,3-dihydro-2H-inden-2-one, C25H20O2
- Crystal structure of tricyclohexyl[4-(4H-1,2,4-triazol-4-yl)-benzoato-κO]tin(IV), C27H39N3O2Sn
- Crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ4N,O,O,O)cadmium(II)]monohydrate, C12H15NO9Cd
- Crystal structure of ethyl 2-((4-(3,5-dimethylisoxazol-4-yl)-2,6-difluorophenyl)amino)benzoate, C20H18F2N2O3
- The crystal structure of 2-(hydroxymethyl)-2-(4H-1,2,4-triazol-4-yl)propane-1,3-diol, C6H11N3O3
- The crystal structure of 1,2-bis(2,4-dinitrophenyl) hydrazine, C12H8N6O8
- Crystal structure of 1-(2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)-1,2-dihydro-4H-benzo[d][1,3]oxazin-4-one, C19H14Cl2N2O3
- The crystal structure of 5-amino-5-oxo-4-(1-oxo-4-(2-oxopyrrolidin-1-yl)isoindolin-2-yl)pentanoic acid, C17H19N3O5
- Crystal structure of N2,N6-bis(2-(((Z)-5-bromo-2-hydroxybenzylidene)amino) phenyl)pyridine-2,6-dicarboxamide, C33H23Br2N5O4
- The crystal structure of (E)-2-methoxy-6-(((5-methyl-1,3,4-thiadiazol-2-yl)imino)methyl)phenol, C11H11N3O2S
- The crystal structure of 3-((tert-butyldiphenylsilyl)methyl)-5,5-diphenyl-6-(p-tolyl) tetrahydro-2H-pyran-2-one, C41H42O2Si
- Crystal structure of 9-fluoro-4-(6-methoxypyridin-3-yl)-5,6-dihydrobenzo[h]quinazolin-2-amine, C18H15FN4O
- The crystal structure of 2-bromo-5-(4-cyanophenoxy)benzyl 1-methyl-1,2,5,6-tetrahydropyridine-3-carboxylate, C21H19BrN2O3
- Crystal structure of 3,3′-(1,4-phenylenebis(methylene))bis(1-isopropyl-1H-imidazol-3-ium) bis(hexafluorophosphate(V)), C10H14F6N2P
- The crystal structure of 2,2-di(thiophen-3-yl)-1-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C24H19BN2O2S3
- Crystal structure of 5-bromo-1-(2-iodobenzoyl)-1H-indole-3-carbaldehyde, C16H9BrINO2
- The crystal structure of monocarbonyl-2-carboxypyridinato-κ2N,O-triphenylphosphine-rhodium(I) acetonitrile solvate, C26H20.50N1.50O3PRh
- Crystal structure of dichlorido-tetrakis(1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ1N)manganese(II), C60H68O4N12Cl10Mn
- Crystal structure of 3-(tert-butyldiphenylsilyl)-1-(2,6-dichlorophenyl)-2,2-diphenylpropan-1-ol, C37H36Cl2OSi
- Crystal structure of langite from Mine du Pradet (France)
- The crystal structure of 5′-(furan-2-yl)-3′-((4-methylphenyl)sulfonamido)-3′,4′,5′,6′-tetrahydro-[1,1′:3′,1″-terphenyl]-4′-carboxylic acid, C30H27NO5S
- Synthesis and crystal structure of bis{2-(((4-acetophenone)imino)methyl)-4-fluorophenolato-κ2N,O}zinc(II), C30H22F2N2O4Zn
- The crystal structure of poly[(tripyridine-κ3N,N′,N″) μ3-(pyridine-3,4-dicarboxylate-κ3N:O:O′) manganese(II)], C22H22N4O8Mn
- The crystal structure of (E)-4-chloro-N′-(1-(4-hydroxyphenyl)propylidene)benzohydrazide, C16H15ClN2O2
- Synthesis and crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino) ethyl)phenyl)imino)methyl)phenolato-κ2N,O}cobalt(II), C40H46CoN4O4
- Crystal structure of tetraaqua-[(1-(carboxymethyl)-1H-pyrazole-3-carboxylato-κ2N,O)cobalt(II)], C6H12CoN2O8
- (6R,7S)-2,3,13-trimethoxy-6,7-dimethyl-5,6,7,8-tetrahydrobenzo[3′,4′]cycloocta [1′,2′:4,5]benzo[1,2-d][1,3]dioxol-1-ol, C22H26O6
- Crystal structure of 2-((2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)amino)benzoic acid, C18H14Cl2N2O3
- Crystal structure of (5aS,6aS,8aR,9R,11aS, 11bS,13R,13aS)-1,1,8a,11a-tetramethyl-9-((S)-1-((S)-5-methyl-6-oxo-3,6-dihydro-2H-pyran-2-yl)ethyl)-3-oxo-1,7,8,8a,9,10,11,11a,11b,12,13,13a-dodecahydro-3H,6H-cyclopenta[5,6]cyclopropa[1,8a]naphtho[2,1-c]oxepin-13-yl acetate, C32H44O6
- Crystal structure of catena-poly[triaqua-(μ2-1-(4-carboxylatophenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylato-O,O′:O″)cobalt(II)], C12H12N2O8Co
- Crystal structure of 3-[(furan-2-ylmethyl)-amino]-2-(2,3,4,5-tetrafluoro-benzoyl)-acrylic acid ethyl ester, C17H13F4NO4
- Crystal structure of methyl 4-(2-ethoxy-2-oxoethoxy)-3-methoxybenzoate, C13H16O6
- Crystal structure of 4-bromo-2-(4-chlorophenyl)-1-methyl-5-(trifluoromethyl)-1H-pyrrole-3-carbonitrile, C13H7BrClF3N2
- The crystal structure of triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ3N,O,O)nickel(II) monohydrate, C12H15NO9Ni
- Crystal structure of dihydroxy(2,4,6-triisopro-pylphenyl)telluronium trifluoromethanesulfonate, C16H25F3O5STe
- The crystal structure of 1-(carboxymethyl)-1H-imidazole 3-oxide
- The crystal structure of 1,3,5-tris(dibromomethyl)benzene, C9H6Br6
- Crystal structure of (Z)-3-(4-methoxyphenyl)-4-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-N-phenylthiazol-2(3H)-imine, C25H21N5OS
- Crystal structure of (Z)-3-(3-(4-hydroxyphenyl)-2-(phenylimino)-2,3-dihydrothiazol-4-yl)-2H-chromen-2-one, C24H16N2O3S

