Abstract
C21H19BrN2O3, orthorhombic, Pna21 (no. 33) a = 25.412(3) Å, b = 21.329(2) Å, c = 7.0020(8) Å, V = 3795.2(7) Å3, Z = 8, R gt (F) = 0.0390, wR ref (F2) = 0.1009, T = 100(2) K.
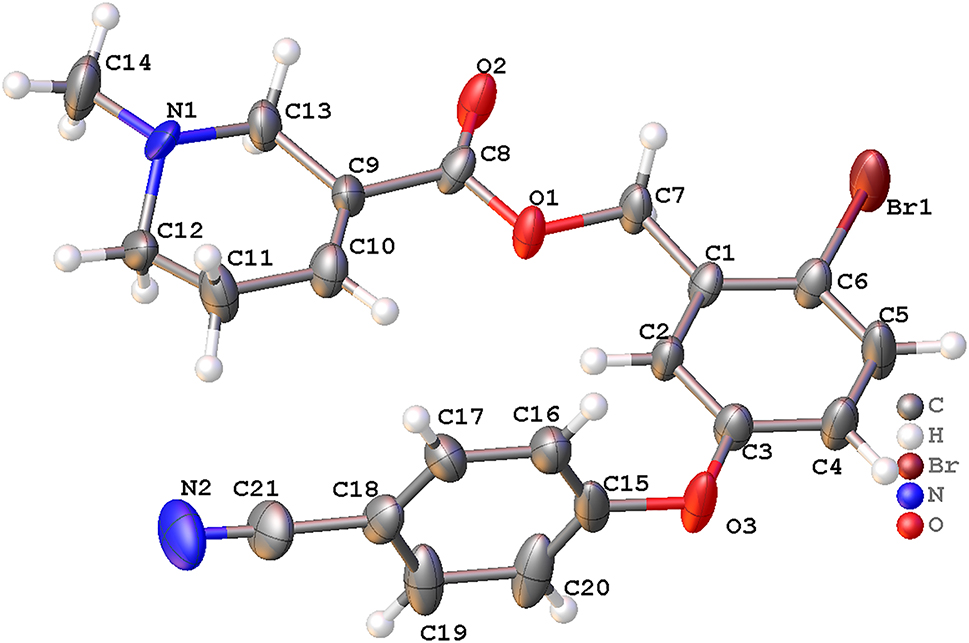
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.12 × 0.12 × 0.10 mm |
| Wavelength: μ: |
Ga Kα radiation (1.34138 Å) 2.08 mm−1 |
| Diffractometer, scan mode: θmax, completeness: |
Bruker Photon III, φ and ω 57.0°, >99 % |
| N(hkl)measured, N(hkl)unique, Rint: | 37012, 7103, 0.051 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 5191 |
| N(param)refined: | 517 |
| Programs: | Bruker [1], Olex2 [2], SHELX [3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| Br1A | 0.47443 (2) | 0.22176 (2) | 0.1711 (2) | 0.04921 (18) |
| Br1 | 0.32938 (2) | 0.76592 (2) | 0.1710 (3) | 0.0575 (2) |
| C1A | 0.37040 (14) | 0.27159 (16) | 0.177 (2) | 0.0334 (10) |
| C1 | 0.22730 (13) | 0.71064 (15) | 0.172 (2) | 0.0297 (8) |
| C2A | 0.33434 (13) | 0.32056 (17) | 0.166 (2) | 0.0386 (11) |
| H2A | 0.298013 | 0.311593 | 0.149057 | 0.046* |
| C2 | 0.19302 (13) | 0.66016 (16) | 0.168 (2) | 0.0367 (9) |
| H2 | 0.156141 | 0.667467 | 0.168047 | 0.044* |
| C3A | 0.35095 (14) | 0.38198 (18) | 0.178 (2) | 0.0388 (11) |
| C3 | 0.21162 (14) | 0.59952 (17) | 0.164 (2) | 0.0381 (10) |
| C4A | 0.40373 (15) | 0.39636 (19) | 0.175 (2) | 0.0525 (15) |
| H4A | 0.415075 | 0.438747 | 0.169061 | 0.063* |
| C4 | 0.26478 (14) | 0.58775 (17) | 0.170 (2) | 0.0491 (12) |
| H4 | 0.277532 | 0.545905 | 0.173970 | 0.059* |
| C5A | 0.43993 (15) | 0.3481 (2) | 0.183 (2) | 0.0519 (17) |
| H5A | 0.476402 | 0.357305 | 0.192793 | 0.062* |
| C5 | 0.29937 (15) | 0.63774 (18) | 0.169 (3) | 0.0579 (15) |
| H5 | 0.336228 | 0.630301 | 0.170228 | 0.069* |
| C6A | 0.42347 (14) | 0.28714 (17) | 0.175 (2) | 0.0385 (10) |
| C6 | 0.28074 (14) | 0.69788 (17) | 0.165 (2) | 0.0371 (10) |
| C7A | 0.35399 (14) | 0.20450 (18) | 0.164 (2) | 0.0425 (11) |
| H7AA | 0.365086 | 0.186688 | 0.039330 | 0.051* |
| H7AB | 0.370486 | 0.179714 | 0.266994 | 0.051* |
| C7 | 0.20793 (13) | 0.77689 (16) | 0.174 (3) | 0.0379 (10) |
| H7A | 0.220042 | 0.798453 | 0.291550 | 0.045* |
| H7B | 0.221654 | 0.799972 | 0.062200 | 0.045* |
| C8A | 0.27617 (15) | 0.14514 (19) | 0.171 (2) | 0.0447 (11) |
| C8 | 0.12785 (14) | 0.83151 (16) | 0.1740 (19) | 0.0364 (10) |
| C9A | 0.21788 (16) | 0.1463 (2) | 0.171 (2) | 0.0513 (12) |
| C9 | 0.06951 (14) | 0.82703 (17) | 0.175 (2) | 0.0387 (10) |
| C10 | 0.04430 (16) | 0.77310 (18) | 0.175 (2) | 0.0440 (12) |
| H10 | 0.063972 | 0.735224 | 0.173244 | 0.053* |
| C10A | 0.19051 (15) | 0.1996 (2) | 0.180 (2) | 0.0540 (16) |
| H10Aa | 0.208882 | 0.238325 | 0.186332 | 0.065* |
| H10Bb | 0.209493 | 0.236646 | 0.209946 | 0.065* |
| C11 | −0.01476 (15) | 0.76990 (19) | 0.177 (3) | 0.0539 (14) |
| H11Ec | −0.027124 | 0.760487 | 0.307767 | 0.065* |
| H11Fc | −0.026779 | 0.735752 | 0.091554 | 0.065* |
| H11Gd | −0.026469 | 0.735618 | 0.262107 | 0.065* |
| H11Hd | −0.028039 | 0.761144 | 0.046584 | 0.065* |
| C11Aa | 0.1304 (4) | 0.1999 (10) | 0.179 (6) | 0.079 (5) |
| H11Aa | 0.117363 | 0.231901 | 0.269494 | 0.094* |
| H11Ba | 0.117553 | 0.211143 | 0.049468 | 0.094* |
| C11Bb | 0.1318 (6) | 0.2065 (19) | 0.145 (10) | 0.079 (5) |
| H11Cb | 0.113340 | 0.219953 | 0.262487 | 0.094* |
| H11Db | 0.124611 | 0.237075 | 0.041899 | 0.094* |
| C12Aa | 0.1093 (7) | 0.1362 (9) | 0.234 (3) | 0.071 (4) |
| H12Aa | 0.116470 | 0.128402 | 0.370962 | 0.086* |
| H12Ba | 0.070757 | 0.135163 | 0.214468 | 0.086* |
| C12c | −0.0382 (6) | 0.8325 (5) | 0.110 (3) | 0.050 (3) |
| H12Ec | −0.031638 | 0.838197 | −0.028345 | 0.061* |
| H12Fc | −0.076746 | 0.832551 | 0.131154 | 0.061* |
| C12′d | −0.0367 (8) | 0.8328 (8) | 0.248 (4) | 0.050 (3) |
| H12Gd | −0.075525 | 0.833576 | 0.235563 | 0.061* |
| H12Hd | −0.027510 | 0.839251 | 0.384032 | 0.061* |
| C12Bb | 0.1153 (13) | 0.1412 (16) | 0.087 (6) | 0.071 (4) |
| H12Cb | 0.127180 | 0.132686 | −0.045256 | 0.086* |
| H12Db | 0.076406 | 0.137982 | 0.089729 | 0.086* |
| C13A | 0.19241 (18) | 0.0832 (2) | 0.150 (2) | 0.0515 (19) |
| H13Aa | 0.205898 | 0.061927 | 0.033980 | 0.062* |
| H13Ba | 0.200474 | 0.056713 | 0.261972 | 0.062* |
| H13Cb | 0.188260 | 0.072667 | 0.012824 | 0.062* |
| H13Db | 0.214307 | 0.050498 | 0.210803 | 0.062* |
| C13 | 0.04194 (15) | 0.88940 (19) | 0.182 (2) | 0.0413 (13) |
| H13Ec | 0.057618 | 0.915224 | 0.284536 | 0.050* |
| H13Fc | 0.047452 | 0.911607 | 0.059190 | 0.050* |
| H13Gd | 0.044411 | 0.906950 | 0.312322 | 0.050* |
| H13Hd | 0.059322 | 0.918990 | 0.092901 | 0.050* |
| C14Aa | 0.1093 (4) | 0.0304 (4) | 0.144 (3) | 0.048 (4) |
| H14Aa | 0.117783 | 0.011950 | 0.268056 | 0.072* |
| H14Ba | 0.124507 | 0.004442 | 0.041963 | 0.072* |
| H14Ca | 0.071060 | 0.032331 | 0.127955 | 0.072* |
| C14 | −0.03988 (17) | 0.9430 (2) | 0.181 (3) | 0.0649 (19) |
| H14Gc | −0.021019 | 0.976177 | 0.249346 | 0.097* |
| H14Hc | −0.076351 | 0.941173 | 0.226070 | 0.097* |
| H14Ic | −0.039518 | 0.952014 | 0.043649 | 0.097* |
| H14Jd | −0.021662 | 0.962384 | 0.289182 | 0.097* |
| H14Kd | −0.076531 | 0.934497 | 0.215928 | 0.097* |
| H14Ld | −0.038981 | 0.971525 | 0.071213 | 0.097* |
| C14Bb | 0.1186 (17) | 0.0257 (15) | 0.227 (10) | 0.18 (2) |
| H14Db | 0.080205 | 0.029793 | 0.230926 | 0.266* |
| H14Eb | 0.130326 | −0.000127 | 0.335043 | 0.266* |
| H14Fb | 0.129149 | 0.005712 | 0.107222 | 0.266* |
| C15 | 0.12504 (15) | 0.55766 (18) | 0.167 (3) | 0.0513 (15) |
| C16 | 0.0989 (4) | 0.5597 (6) | 0.3458 (15) | 0.046 (3) |
| H16 | 0.117738 | 0.555479 | 0.462197 | 0.055* |
| C17 | 0.0451 (4) | 0.5682 (5) | 0.3450 (16) | 0.047 (3) |
| H17 | 0.026738 | 0.569558 | 0.463048 | 0.056* |
| C18 | 0.01841 (16) | 0.5744 (2) | 0.183 (2) | 0.0436 (14) |
| C19 | 0.0445 (4) | 0.5714 (6) | 0.0016 (17) | 0.056 (3) |
| H19 | 0.025599 | 0.575272 | −0.114909 | 0.067* |
| C20 | 0.0997 (5) | 0.5625 (5) | 0.0044 (19) | 0.063 (4) |
| H20 | 0.118539 | 0.559920 | −0.112527 | 0.076* |
| C21 | −0.03796 (17) | 0.5854 (2) | 0.171 (2) | 0.0528 (13) |
| C25 | 0.09983 (16) | 0.39487 (18) | 0.162 (2) | 0.0448 (13) |
| C27 | 0.15644 (15) | 0.40255 (18) | 0.169 (2) | 0.0458 (14) |
| C31 | 0.26323 (16) | 0.41999 (18) | 0.176 (2) | 0.0488 (15) |
| C32 | 0.2381 (4) | 0.4159 (6) | 0.0042 (18) | 0.057 (4) |
| H32 | 0.256422 | 0.419320 | −0.113569 | 0.068* |
| C35 | 0.2369 (4) | 0.4156 (6) | 0.3456 (18) | 0.056 (3) |
| H35 | 0.256347 | 0.417953 | 0.461330 | 0.067* |
| C37 | 0.1828 (4) | 0.4078 (7) | 0.3545 (16) | 0.054 (3) |
| H37 | 0.164234 | 0.406053 | 0.471991 | 0.065* |
| C38 | 0.1830 (4) | 0.4063 (6) | 0.0118 (16) | 0.054 (3) |
| H38 | 0.164528 | 0.402411 | −0.105485 | 0.065* |
| N1Aa | 0.1312 (5) | 0.0935 (5) | 0.133 (2) | 0.061 (4) |
| N1c | −0.0142 (10) | 0.8828 (12) | 0.216 (3) | 0.056 (4) |
| N1′d | −0.0129 (15) | 0.8823 (18) | 0.129 (4) | 0.056 (4) |
| N1Bb | 0.1422 (11) | 0.0867 (12) | 0.240 (5) | 0.061 (4) |
| N2A | 0.05504 (14) | 0.38963 (17) | 0.1795 (19) | 0.0538 (13) |
| N2 | −0.08232 (15) | 0.5944 (2) | 0.166 (2) | 0.0692 (13) |
| O1A | 0.29718 (9) | 0.20253 (12) | 0.1807 (14) | 0.0405 (9) |
| O1 | 0.15120 (9) | 0.77502 (11) | 0.1689 (15) | 0.0389 (7) |
| O2A | 0.30255 (11) | 0.09779 (13) | 0.1744 (16) | 0.0538 (9) |
| O2 | 0.15209 (10) | 0.88021 (12) | 0.1744 (14) | 0.0478 (9) |
| O3A | 0.31702 (10) | 0.43265 (12) | 0.1633 (18) | 0.0549 (10) |
| O3 | 0.17922 (10) | 0.54759 (12) | 0.1630 (19) | 0.0579 (11) |
-
aOccupancy: 0.639(17), bOccupancy: 0.361(17), cOccupancy: 0.58(2), dOccupancy: 0.42(2).
1 Source of materials
All chemicals were of reagent grade and used without further purification. The synthesis of the title compound was regulated in accord to reference [4] with appropriate modification: arecaine (0.282 g, 2 mmol), 4-(4-bromo-3-(hydroxymethyl)phenoxy)benzonitrile (0.608 g, 2 mmol), and 4–dimethylaminopyridine (0.122 g, 1 mmol) were dissolved in DMF (5 mL) initially, and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (0.575 g, 3 mmol) was added to the mixture. The reaction was then stirred overnight at room temperature, monitored by TLC. After confirmation of the completion, a saturated aqueous NaHCO3 (20 mL) was added, and extracted with ethyl acetate (3 times with 15 mL). The combined organics were washed with water and brine, dried over anhydrous Na2SO4, concentrated in vacuo, and purified through column chromatography to obtain the title compound as white solid. Colourless crystals of the title compound were obtained by slow evaporation (n-hexane/acetone) within one weeks.
2 Experimental details
All hydrogen atoms were placed in the calculated positions and constrained to ride on their parent atoms. Using Olex2 [2], the structure was solved with the XM [3] structure solution program using Dual Space and refined with the XL [3].
3 Comment
Arecoline, an alkaloid that acts on the nervous system [5], is from the areca (betel) nut of the areca palm (Areca catechu) endemic to Southeast Asia, the East African seaboard, and the Western Pacific [6]. In addition to being a naturally occurring psychoactive alkaloid, arecoline also has a variety of physiological activities, such as anti-inflammatory [7], anti-atherosclerotic [8], insecticide and antibacterial activity [9], etc., which have been reviewed [10]. In view of a wide range of physiological activities and important significance in drug development of arecoline, research involving single crystal structures of arecoline derivatives is of great significance to reveal the structure-activity relationship.
The title compound crystallizes in the orthorhombic space group Pna21 with two of the title molecules in the asymmetric unit. They show pseudo-symmetry of a mirror plane as discussed before [11]. Each molecule consists of arecoline (methyl 1-methyl-1,2,5,6-tetrahydropyridine-3-carboxylate) and diphenyl ethers (4-(4-bromophenoxy)benzonitrile) (see the Figure). A bromine atom and cyano group are connected to the diphenyl ethers with C(6)–Br(1), C(21)–N(2) bonds, respectively. The C(6)–Br(1), C(21)–N(2), C(3)–O(3) and C(15)–O(3) bond lengths were determined as 1.907(4) Å, 1.144(6) Å, 1.380(4) Å and 1.394(5) Å. All bond lengths and angles are in normal ranges [12, 13]. The dihedral angle between the plane through C(1)–C(6) and the plane through C(15)–C(20) is 91.3(4)∘. The centroid distance between the benzene rings is 4.770(4) Å.
-
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: National Natural Science Foundation of China grant (32260685).
-
Competing interests: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. SAINT, APEX2 and SADABS; Bruker AXS Inc.: Madison, WI, USA, 2012.Search in Google Scholar
2. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Kilian, J., Millard, M., Ozenil, M., Krause, D., Ghaderi, K., Holzer, W., Pichler, V. Synthesis, biological evaluation, and docking studies of antagonistic hydroxylated arecaidine esters targeting mAChRs. Molecules 2022, 27, 3173; https://doi.org/10.3390/molecules27103173.Search in Google Scholar PubMed PubMed Central
5. Myers, A. L. Metabolism of the areca alkaloids-toxic and psychoactive constituents of the areca (betel) nut. Drug Metab. Rev. 2022, 54, 343–360; https://doi.org/10.1080/03602532.2022.2075010.Search in Google Scholar PubMed
6. Volgin, A. D., Bashirzade, A., Amstislavskaya, T. G., Yakovlev, O. A., Demin, K. A., Ho, Y. J., Kalueff, A. V., Shevyrin, V. A., Yan, D., Tang, Z., Wang, J., Wang, M., Alpyshov, E. T., Serikuly, N., Wappler-Guzzetta, E. A., Lakstygal, A. M. DARK classics in chemical neuroscience: arecoline. ACS Chem. Neurosci. 2019, 10, 2176–2185; https://doi.org/10.1021/acschemneuro.8b00711.Search in Google Scholar PubMed
7. Zhang, W., Zhou, S. H., Ling, H. Y., Yao, Q. X., Qi, Z. Q., Wang, G., Hu, B. Arecoline repressed inflammation factor expression of macrophages stimulated by oxidized low density lipoprotein and its mechanism. Chin. J. Arteriosclerosis 2009, 17, 269–272.Search in Google Scholar
8. Duan, Z. B., Wang, H. Regulation effect of arecoline on excess expression of adhesive molecules in endothelial cells injuried with high concentration of D-glucose. Chin. J. Clin. Pharmacol. Ther. 2006, 11, 27–32.Search in Google Scholar
9. Luo, S. S., Zhang, H. D., Liu, X. L., Zhu, L. Study on antimicrobial activity of arecoline from betel nut in vitro. Innovational Edition of Farm Products Processing. 2010, 10, 47–50.Search in Google Scholar
10. Liu, Y. J., Peng, W., Hu, M. B., Xu, M., Wu, C. J. The pharmacology, toxicology and potential applications of arecoline: a review. Pharm. Biol. 2016, 54, 2753–2760; https://doi.org/10.3109/13880209.2016.1160251.Search in Google Scholar PubMed
11. Reiss, G. J., van Megen, M. The twinned crystal structure of [4,4′- bipyridine]-1,1′-diium hexachloridostannate(IV), C10H10N2SnCl6. Z. Kristallogr. N. Cryst. Struct. 2022, 237, 281–284; https://doi.org/10.1515/ncrs-2021-0443.Search in Google Scholar
12. Noland, W. E., Rieger, J. L., Tu, Z. H., Tritch, K. J. A 2:1 co-crystal of 3,5-dibromo-4-cyanobenzoic acid and anthracene. Acta Crystallogr. 2017, E73, 1743–1746; https://doi.org/10.1107/s2056989017014815.Search in Google Scholar PubMed PubMed Central
13. Wu, S., Peng, L., Zhou, P., Zeng, Z. The crystal structure of 1,2-bis((4-methoxyphenyl)ethynyl)benzene, C24H18O2. Z. Kristallogr. N. Cryst. Struct. 2019, 235, 229–231; https://doi.org/10.1515/ncrs-2019-0593.Search in Google Scholar
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of poly[diaqua-(μ4-3,3′-di(1H-1,2,4-triazol-1-yl)-[1,1′-biphenyl]-4,4′-dicarboxylate-N:N′:O:O′)cadmium(II)], C18H14N6O6Cd
- Crystal structure of (8R,8′S,13S,13′R)-8,8′-bis(hydroxymethyl)-9,9′,10,10′-tetramethoxy-5,5′,6,6′,8,8′,13,13′-octahydro-[13,13′-bi[1,3]dioxolo[4,5-g]isoquinolino[3,2-a]isoquinoline]-7,7′-diium chloride-methanol (1/2), C46H58N2O14Cl2
- The crystal structure of 8-methoxy-2,2-diphenyl-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C29H25BN2O3S
- Crystal structure of aqua-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N″,N‴)copper(II) 5-carboxyisophthalate tetrahydrate, C25H50N4CuO11
- The crystal structure of 1-(naphthalen-2-ylsulfonyl)-2,2-diphenyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ij]quinoline, C31H23BN2O2S
- Crystal structure of iodido-(η6-benzene) (1-(pyridin-2-yl)-N-(p-fluoro-methanamine)-κ2N,Nʹ)ruthenium(II) hexaflourophosphate, (C18H15F7IN2RuP)
- The crystal structure of 1-(3-oxo-1-phenyl-3-(p-tolyl) propylidene)-1,3-dihydro-2H-inden-2-one, C25H20O2
- Crystal structure of tricyclohexyl[4-(4H-1,2,4-triazol-4-yl)-benzoato-κO]tin(IV), C27H39N3O2Sn
- Crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ4N,O,O,O)cadmium(II)]monohydrate, C12H15NO9Cd
- Crystal structure of ethyl 2-((4-(3,5-dimethylisoxazol-4-yl)-2,6-difluorophenyl)amino)benzoate, C20H18F2N2O3
- The crystal structure of 2-(hydroxymethyl)-2-(4H-1,2,4-triazol-4-yl)propane-1,3-diol, C6H11N3O3
- The crystal structure of 1,2-bis(2,4-dinitrophenyl) hydrazine, C12H8N6O8
- Crystal structure of 1-(2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)-1,2-dihydro-4H-benzo[d][1,3]oxazin-4-one, C19H14Cl2N2O3
- The crystal structure of 5-amino-5-oxo-4-(1-oxo-4-(2-oxopyrrolidin-1-yl)isoindolin-2-yl)pentanoic acid, C17H19N3O5
- Crystal structure of N2,N6-bis(2-(((Z)-5-bromo-2-hydroxybenzylidene)amino) phenyl)pyridine-2,6-dicarboxamide, C33H23Br2N5O4
- The crystal structure of (E)-2-methoxy-6-(((5-methyl-1,3,4-thiadiazol-2-yl)imino)methyl)phenol, C11H11N3O2S
- The crystal structure of 3-((tert-butyldiphenylsilyl)methyl)-5,5-diphenyl-6-(p-tolyl) tetrahydro-2H-pyran-2-one, C41H42O2Si
- Crystal structure of 9-fluoro-4-(6-methoxypyridin-3-yl)-5,6-dihydrobenzo[h]quinazolin-2-amine, C18H15FN4O
- The crystal structure of 2-bromo-5-(4-cyanophenoxy)benzyl 1-methyl-1,2,5,6-tetrahydropyridine-3-carboxylate, C21H19BrN2O3
- Crystal structure of 3,3′-(1,4-phenylenebis(methylene))bis(1-isopropyl-1H-imidazol-3-ium) bis(hexafluorophosphate(V)), C10H14F6N2P
- The crystal structure of 2,2-di(thiophen-3-yl)-1-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C24H19BN2O2S3
- Crystal structure of 5-bromo-1-(2-iodobenzoyl)-1H-indole-3-carbaldehyde, C16H9BrINO2
- The crystal structure of monocarbonyl-2-carboxypyridinato-κ2N,O-triphenylphosphine-rhodium(I) acetonitrile solvate, C26H20.50N1.50O3PRh
- Crystal structure of dichlorido-tetrakis(1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ1N)manganese(II), C60H68O4N12Cl10Mn
- Crystal structure of 3-(tert-butyldiphenylsilyl)-1-(2,6-dichlorophenyl)-2,2-diphenylpropan-1-ol, C37H36Cl2OSi
- Crystal structure of langite from Mine du Pradet (France)
- The crystal structure of 5′-(furan-2-yl)-3′-((4-methylphenyl)sulfonamido)-3′,4′,5′,6′-tetrahydro-[1,1′:3′,1″-terphenyl]-4′-carboxylic acid, C30H27NO5S
- Synthesis and crystal structure of bis{2-(((4-acetophenone)imino)methyl)-4-fluorophenolato-κ2N,O}zinc(II), C30H22F2N2O4Zn
- The crystal structure of poly[(tripyridine-κ3N,N′,N″) μ3-(pyridine-3,4-dicarboxylate-κ3N:O:O′) manganese(II)], C22H22N4O8Mn
- The crystal structure of (E)-4-chloro-N′-(1-(4-hydroxyphenyl)propylidene)benzohydrazide, C16H15ClN2O2
- Synthesis and crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino) ethyl)phenyl)imino)methyl)phenolato-κ2N,O}cobalt(II), C40H46CoN4O4
- Crystal structure of tetraaqua-[(1-(carboxymethyl)-1H-pyrazole-3-carboxylato-κ2N,O)cobalt(II)], C6H12CoN2O8
- (6R,7S)-2,3,13-trimethoxy-6,7-dimethyl-5,6,7,8-tetrahydrobenzo[3′,4′]cycloocta [1′,2′:4,5]benzo[1,2-d][1,3]dioxol-1-ol, C22H26O6
- Crystal structure of 2-((2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)amino)benzoic acid, C18H14Cl2N2O3
- Crystal structure of (5aS,6aS,8aR,9R,11aS, 11bS,13R,13aS)-1,1,8a,11a-tetramethyl-9-((S)-1-((S)-5-methyl-6-oxo-3,6-dihydro-2H-pyran-2-yl)ethyl)-3-oxo-1,7,8,8a,9,10,11,11a,11b,12,13,13a-dodecahydro-3H,6H-cyclopenta[5,6]cyclopropa[1,8a]naphtho[2,1-c]oxepin-13-yl acetate, C32H44O6
- Crystal structure of catena-poly[triaqua-(μ2-1-(4-carboxylatophenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylato-O,O′:O″)cobalt(II)], C12H12N2O8Co
- Crystal structure of 3-[(furan-2-ylmethyl)-amino]-2-(2,3,4,5-tetrafluoro-benzoyl)-acrylic acid ethyl ester, C17H13F4NO4
- Crystal structure of methyl 4-(2-ethoxy-2-oxoethoxy)-3-methoxybenzoate, C13H16O6
- Crystal structure of 4-bromo-2-(4-chlorophenyl)-1-methyl-5-(trifluoromethyl)-1H-pyrrole-3-carbonitrile, C13H7BrClF3N2
- The crystal structure of triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ3N,O,O)nickel(II) monohydrate, C12H15NO9Ni
- Crystal structure of dihydroxy(2,4,6-triisopro-pylphenyl)telluronium trifluoromethanesulfonate, C16H25F3O5STe
- The crystal structure of 1-(carboxymethyl)-1H-imidazole 3-oxide
- The crystal structure of 1,3,5-tris(dibromomethyl)benzene, C9H6Br6
- Crystal structure of (Z)-3-(4-methoxyphenyl)-4-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-N-phenylthiazol-2(3H)-imine, C25H21N5OS
- Crystal structure of (Z)-3-(3-(4-hydroxyphenyl)-2-(phenylimino)-2,3-dihydrothiazol-4-yl)-2H-chromen-2-one, C24H16N2O3S
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of poly[diaqua-(μ4-3,3′-di(1H-1,2,4-triazol-1-yl)-[1,1′-biphenyl]-4,4′-dicarboxylate-N:N′:O:O′)cadmium(II)], C18H14N6O6Cd
- Crystal structure of (8R,8′S,13S,13′R)-8,8′-bis(hydroxymethyl)-9,9′,10,10′-tetramethoxy-5,5′,6,6′,8,8′,13,13′-octahydro-[13,13′-bi[1,3]dioxolo[4,5-g]isoquinolino[3,2-a]isoquinoline]-7,7′-diium chloride-methanol (1/2), C46H58N2O14Cl2
- The crystal structure of 8-methoxy-2,2-diphenyl-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C29H25BN2O3S
- Crystal structure of aqua-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N″,N‴)copper(II) 5-carboxyisophthalate tetrahydrate, C25H50N4CuO11
- The crystal structure of 1-(naphthalen-2-ylsulfonyl)-2,2-diphenyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ij]quinoline, C31H23BN2O2S
- Crystal structure of iodido-(η6-benzene) (1-(pyridin-2-yl)-N-(p-fluoro-methanamine)-κ2N,Nʹ)ruthenium(II) hexaflourophosphate, (C18H15F7IN2RuP)
- The crystal structure of 1-(3-oxo-1-phenyl-3-(p-tolyl) propylidene)-1,3-dihydro-2H-inden-2-one, C25H20O2
- Crystal structure of tricyclohexyl[4-(4H-1,2,4-triazol-4-yl)-benzoato-κO]tin(IV), C27H39N3O2Sn
- Crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ4N,O,O,O)cadmium(II)]monohydrate, C12H15NO9Cd
- Crystal structure of ethyl 2-((4-(3,5-dimethylisoxazol-4-yl)-2,6-difluorophenyl)amino)benzoate, C20H18F2N2O3
- The crystal structure of 2-(hydroxymethyl)-2-(4H-1,2,4-triazol-4-yl)propane-1,3-diol, C6H11N3O3
- The crystal structure of 1,2-bis(2,4-dinitrophenyl) hydrazine, C12H8N6O8
- Crystal structure of 1-(2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)-1,2-dihydro-4H-benzo[d][1,3]oxazin-4-one, C19H14Cl2N2O3
- The crystal structure of 5-amino-5-oxo-4-(1-oxo-4-(2-oxopyrrolidin-1-yl)isoindolin-2-yl)pentanoic acid, C17H19N3O5
- Crystal structure of N2,N6-bis(2-(((Z)-5-bromo-2-hydroxybenzylidene)amino) phenyl)pyridine-2,6-dicarboxamide, C33H23Br2N5O4
- The crystal structure of (E)-2-methoxy-6-(((5-methyl-1,3,4-thiadiazol-2-yl)imino)methyl)phenol, C11H11N3O2S
- The crystal structure of 3-((tert-butyldiphenylsilyl)methyl)-5,5-diphenyl-6-(p-tolyl) tetrahydro-2H-pyran-2-one, C41H42O2Si
- Crystal structure of 9-fluoro-4-(6-methoxypyridin-3-yl)-5,6-dihydrobenzo[h]quinazolin-2-amine, C18H15FN4O
- The crystal structure of 2-bromo-5-(4-cyanophenoxy)benzyl 1-methyl-1,2,5,6-tetrahydropyridine-3-carboxylate, C21H19BrN2O3
- Crystal structure of 3,3′-(1,4-phenylenebis(methylene))bis(1-isopropyl-1H-imidazol-3-ium) bis(hexafluorophosphate(V)), C10H14F6N2P
- The crystal structure of 2,2-di(thiophen-3-yl)-1-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C24H19BN2O2S3
- Crystal structure of 5-bromo-1-(2-iodobenzoyl)-1H-indole-3-carbaldehyde, C16H9BrINO2
- The crystal structure of monocarbonyl-2-carboxypyridinato-κ2N,O-triphenylphosphine-rhodium(I) acetonitrile solvate, C26H20.50N1.50O3PRh
- Crystal structure of dichlorido-tetrakis(1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ1N)manganese(II), C60H68O4N12Cl10Mn
- Crystal structure of 3-(tert-butyldiphenylsilyl)-1-(2,6-dichlorophenyl)-2,2-diphenylpropan-1-ol, C37H36Cl2OSi
- Crystal structure of langite from Mine du Pradet (France)
- The crystal structure of 5′-(furan-2-yl)-3′-((4-methylphenyl)sulfonamido)-3′,4′,5′,6′-tetrahydro-[1,1′:3′,1″-terphenyl]-4′-carboxylic acid, C30H27NO5S
- Synthesis and crystal structure of bis{2-(((4-acetophenone)imino)methyl)-4-fluorophenolato-κ2N,O}zinc(II), C30H22F2N2O4Zn
- The crystal structure of poly[(tripyridine-κ3N,N′,N″) μ3-(pyridine-3,4-dicarboxylate-κ3N:O:O′) manganese(II)], C22H22N4O8Mn
- The crystal structure of (E)-4-chloro-N′-(1-(4-hydroxyphenyl)propylidene)benzohydrazide, C16H15ClN2O2
- Synthesis and crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino) ethyl)phenyl)imino)methyl)phenolato-κ2N,O}cobalt(II), C40H46CoN4O4
- Crystal structure of tetraaqua-[(1-(carboxymethyl)-1H-pyrazole-3-carboxylato-κ2N,O)cobalt(II)], C6H12CoN2O8
- (6R,7S)-2,3,13-trimethoxy-6,7-dimethyl-5,6,7,8-tetrahydrobenzo[3′,4′]cycloocta [1′,2′:4,5]benzo[1,2-d][1,3]dioxol-1-ol, C22H26O6
- Crystal structure of 2-((2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)amino)benzoic acid, C18H14Cl2N2O3
- Crystal structure of (5aS,6aS,8aR,9R,11aS, 11bS,13R,13aS)-1,1,8a,11a-tetramethyl-9-((S)-1-((S)-5-methyl-6-oxo-3,6-dihydro-2H-pyran-2-yl)ethyl)-3-oxo-1,7,8,8a,9,10,11,11a,11b,12,13,13a-dodecahydro-3H,6H-cyclopenta[5,6]cyclopropa[1,8a]naphtho[2,1-c]oxepin-13-yl acetate, C32H44O6
- Crystal structure of catena-poly[triaqua-(μ2-1-(4-carboxylatophenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylato-O,O′:O″)cobalt(II)], C12H12N2O8Co
- Crystal structure of 3-[(furan-2-ylmethyl)-amino]-2-(2,3,4,5-tetrafluoro-benzoyl)-acrylic acid ethyl ester, C17H13F4NO4
- Crystal structure of methyl 4-(2-ethoxy-2-oxoethoxy)-3-methoxybenzoate, C13H16O6
- Crystal structure of 4-bromo-2-(4-chlorophenyl)-1-methyl-5-(trifluoromethyl)-1H-pyrrole-3-carbonitrile, C13H7BrClF3N2
- The crystal structure of triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ3N,O,O)nickel(II) monohydrate, C12H15NO9Ni
- Crystal structure of dihydroxy(2,4,6-triisopro-pylphenyl)telluronium trifluoromethanesulfonate, C16H25F3O5STe
- The crystal structure of 1-(carboxymethyl)-1H-imidazole 3-oxide
- The crystal structure of 1,3,5-tris(dibromomethyl)benzene, C9H6Br6
- Crystal structure of (Z)-3-(4-methoxyphenyl)-4-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-N-phenylthiazol-2(3H)-imine, C25H21N5OS
- Crystal structure of (Z)-3-(3-(4-hydroxyphenyl)-2-(phenylimino)-2,3-dihydrothiazol-4-yl)-2H-chromen-2-one, C24H16N2O3S


