Abstract
C20H18F2N2O3, monoclinic, P21/n (no. 14), a = 7.5573(10) Å, b = 14.519(2) Å, c = 16.245(2) Å, β = 98.187(3)°, V = 1764.4(4) Å3, Z = 4, Rgt(F) = 0.0420 wRref(F2) = 0.1142, T = 205 K.
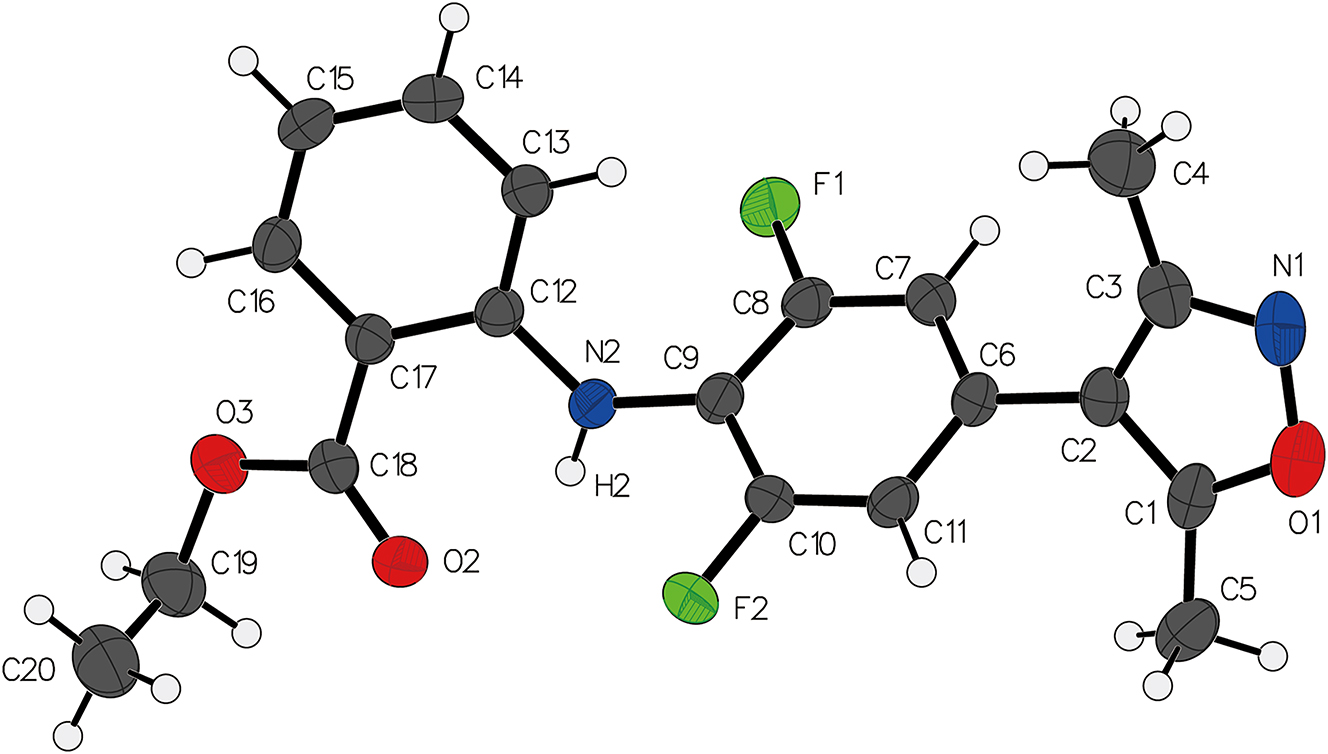
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless needle |
| Size: | 0.18 × 0.11 × 0.05 mm |
| Wavelength: | MoKα radiation (0.71073 Å) |
| μ: | 0.11 mm−1 |
| Diffractometer, scan mode: | Bruker Apex-II, φ and ω |
| θmax, completeness: | 27.5°, >99 % |
| N(hkl)measured, N(hkl)unique, Rint: | 15,609, 4025, 0.039 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2σ(Iobs), 2974 |
| N(param)refined: | 298 |
| Programs: | Bruker [1], Shelx [2, 3], Olex2 [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1 | 0.2935 (2) | 0.80581 (10) | 0.53134 (11) | 0.0371 (4) |
| C2 | 0.3319 (2) | 0.73045 (10) | 0.58256 (10) | 0.0327 (3) |
| C3 | 0.4293 (2) | 0.76722 (11) | 0.65499 (11) | 0.0412 (4) |
| C4 | 0.5159 (3) | 0.72262 (14) | 0.73242 (12) | 0.0536 (5) |
| H4A | 0.424941 | 0.702219 | 0.764865 | 0.080* |
| H4B | 0.594336 | 0.766410 | 0.764596 | 0.080* |
| H4C | 0.585005 | 0.670039 | 0.718477 | 0.080* |
| C5 | 0.1946 (2) | 0.81475 (12) | 0.44590 (11) | 0.0472 (4) |
| H5A | 0.130192 | 0.872723 | 0.440930 | 0.071* |
| H5B | 0.110604 | 0.764217 | 0.435008 | 0.071* |
| H5C | 0.278543 | 0.813159 | 0.405957 | 0.071* |
| C6 | 0.28626 (19) | 0.63283 (9) | 0.56408 (9) | 0.0296 (3) |
| C7 | 0.2203 (2) | 0.57651 (10) | 0.62226 (9) | 0.0327 (3) |
| H7 | 0.199177 | 0.600960 | 0.673540 | 0.039* |
| C8 | 0.1863 (2) | 0.48490 (10) | 0.60434 (9) | 0.0319 (3) |
| C9 | 0.22075 (19) | 0.44286 (9) | 0.53144 (9) | 0.0296 (3) |
| C10 | 0.2770 (2) | 0.50286 (10) | 0.47370 (9) | 0.0307 (3) |
| C11 | 0.3098 (2) | 0.59481 (10) | 0.48769 (9) | 0.0318 (3) |
| H11 | 0.347890 | 0.631852 | 0.446102 | 0.038* |
| C12 | 0.26996 (19) | 0.27538 (9) | 0.55691 (9) | 0.0280 (3) |
| C13 | 0.3653 (2) | 0.28517 (10) | 0.63687 (9) | 0.0325 (3) |
| H13 | 0.384196 | 0.344355 | 0.659897 | 0.039* |
| C14 | 0.4321 (2) | 0.20939 (11) | 0.68256 (10) | 0.0349 (3) |
| H14 | 0.494160 | 0.217623 | 0.736437 | 0.042* |
| C15 | 0.4085 (2) | 0.12110 (11) | 0.64970 (10) | 0.0363 (4) |
| H15 | 0.452531 | 0.069626 | 0.681258 | 0.044* |
| C16 | 0.3193 (2) | 0.11017 (10) | 0.57005 (10) | 0.0339 (3) |
| H16 | 0.305434 | 0.050714 | 0.547146 | 0.041* |
| C17 | 0.2492 (2) | 0.18554 (10) | 0.52266 (9) | 0.0295 (3) |
| C18 | 0.1540 (2) | 0.17082 (10) | 0.43736 (10) | 0.0355 (3) |
| C19Aa | 0.0050 (3) | 0.06043 (14) | 0.34238 (12) | 0.0415 (5) |
| H19Aa | −0.070596 | 0.112649 | 0.321602 | 0.050* |
| H19Ba | −0.072768 | 0.007291 | 0.347210 | 0.050* |
| C19Bb | 0.157 (3) | 0.0582 (15) | 0.3270 (11) | 0.052 (4) |
| H19Cb | 0.231956 | 0.007105 | 0.313141 | 0.063* |
| H19Db | 0.179670 | 0.110817 | 0.292292 | 0.063* |
| C20Aa | 0.1305 (3) | 0.03886 (15) | 0.28244 (13) | 0.0498 (6) |
| H20Aa | 0.063474 | 0.017272 | 0.230660 | 0.075* |
| H20Ba | 0.213250 | −0.008657 | 0.305498 | 0.075* |
| H20Ca | 0.196616 | 0.093895 | 0.272023 | 0.075* |
| C20Bb | −0.028 (3) | 0.0317 (15) | 0.3064 (12) | 0.053 (4) |
| H20Db | −0.053577 | −0.018749 | 0.341994 | 0.079* |
| H20Eb | −0.050344 | 0.012334 | 0.248756 | 0.079* |
| H20Fb | −0.103807 | 0.083778 | 0.314671 | 0.079* |
| F1 | 0.11410 (14) | 0.43314 (6) | 0.66054 (6) | 0.0473 (3) |
| F2 | 0.30249 (14) | 0.46603 (6) | 0.39964 (5) | 0.0447 (3) |
| N1Ac | 0.452 (3) | 0.8603 (9) | 0.6456 (10) | 0.0479 (17) |
| N1Bd | 0.3608 (18) | 0.8846 (11) | 0.5727 (13) | 0.0417 (18) |
| N2 | 0.19246 (19) | 0.34965 (8) | 0.51139 (9) | 0.0350 (3) |
| H2 | 0.160 (2) | 0.3392 (12) | 0.4608 (12) | 0.042* |
| O1Ac | 0.3577 (13) | 0.8811 (7) | 0.5657 (7) | 0.0511 (19) |
| O1Bd | 0.443 (4) | 0.8536 (11) | 0.6524 (12) | 0.052 (3) |
| O2 | 0.11793 (17) | 0.23072 (8) | 0.38560 (7) | 0.0451 (3) |
| O3Aa | 0.1019 (2) | 0.08292 (8) | 0.42402 (8) | 0.0403 (4) |
| O3Bb | 0.210 (2) | 0.0825 (8) | 0.4125 (7) | 0.039 (3) |
-
aOccupancy: 0.911 (4), bOccupancy: 0.089 (4), cOccupancy: 0.580 (14), dOccupancy: 0.420 (14).
1 Source of materials
A mixture of ethyl 2-((4-(2,4-dioxopentan-3-yl)-2,6-difluorophenyl)amino) benzoate (1.88 g, 5 mmol), triethylamine (1.01 g, 10 mmol) and hydroxylamine hydrochloride (0.41 g, 6 mmol) was dissolved in ethanol (10 mL). The mixture was refluxed for 6 h, until the TLC indicated the reaction was completed. The mixture was concentrated. The title compound was separated by silica-gel column chromatography with ethyl acetate-petroleum ether (30 %) gradient solvent system. The target product was obtained as a white solid. Yield: 58.5 %.
2 Experimental details
The crystal structure was solved using the Shelxt program [2] and refined in Shelxl [3]. The Olex2 software package [4] was utilized to visually display the crystal structure.
3 Comment
Diphenylamine and its derivatives are frequently employed as stabilizers in nitrocellulose-based explosives and propellants, in perfumery, as well as antioxidants in the rubber and elastomer industry. It serves as a foundation for multiple derivatives, employed in the production of dyes, pharmaceuticals, photography chemicals, and other specialized applications, making it a widely produced compound in global chemical industries [5]. In previous studies, the presence of intramolecular hydrogen bonding in the crystals of diphenylamine derivatives has been observed [6, 7]. This article presents a newly discovered diphenylamine derivative and provides a detailed analysis of its crystal structure.
The reported compound exhibits a molecular structure whereby all bond lengths, bond angles, and dihedral angles between atoms fall within the range observed in previously reported similar molecular structures [8], [9], [10], [11]. The two substituated phenyl rings exhibit a dihedral angle of 63.8°. Intramolecular hydrogen bonding is observed within the molecule, specifically involving N2–H2⋯O2, with a bond angle of 130.88(12)° and a bond length of 2.0211(16) Å. Furthermore, weak C–H⋯[pi] interactions are formed between the phenyl rings and the methyl and ethyl groups.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: Key laboratory of molecular imaging and drug synthesis of Xianyang City (2021QXNL-PT-0008) and the scientific research plan project of Shaanxi provincial department of education (23JK0328).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. SAINT, APEX2 and SADABS; Bruker AXS Inc.: Madison, WI, USA, 2012.Search in Google Scholar
2. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar PubMed PubMed Central
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar PubMed PubMed Central
4. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
5. Drzyzga, O. Diphenylamine and derivatives in the environment: a review. Chemosphere 2003, 53, 809–818; https://doi.org/10.1016/s0045-6535(03)00613-1.Search in Google Scholar
6. Vinduvahini, M., Roopashree, K. R., Bhattacharya, S., Krishna, K. M., Devaru, V. B. 3-[2-(2,6-Dichloroanilino)benzyl]-4-[(4-methoxybenzylidene) amino]-1H-1,2,4-triazole-5(4H)-thione. Acta Crystallogr. 2011, E67, o2535–o2536; https://doi.org/10.1107/s1600536811034799.Search in Google Scholar
7. López-Mejías, V., Kampf, J. W., Matzger, A. J. Nonamorphism in flufenamic acid and a new record for a polymorphic compound with solved structures. J. Am. Chem. Soc. 2012, 134, 9872–9875; https://doi.org/10.1021/ja302601f.Search in Google Scholar PubMed PubMed Central
8. Bouhlel, A., Curti, C., Khoumeri, O., Vanelle, P. Efficient one-pot double Buchwald–Hartwig coupling reaction on 5-phenyl-4-phenylsulfonyl-2,3-dihydrofuran derivatives. Tetrahedron Lett. 2011, 52, 1919–1923; https://doi.org/10.1016/j.tetlet.2011.02.049.Search in Google Scholar
9. Yang, J.-S., Lin, C.-K., Lahoti, A. M., Tseng, C.-K., Liu, Y.-H., Lee, G.-H., Peng, S.-M. Effect of ground-state twisting on the trans cis photoisomerization and TICT state formation of aminostilbenes. J. Phys. Chem. A 2009, 113, 4868–4877; https://doi.org/10.1021/jp807748t.Search in Google Scholar PubMed
10. Bhattacherjee, D., Thakur, V., Shil, A. K., Das, P. Hypervalent iodine-promoted aromatization of exocyclic β-enaminones for the synthesis of meta-N,N-diarylaminophenols. Adv. Synth. Catal. 2017, 359, 2202–2208; https://doi.org/10.1002/adsc.201700004.Search in Google Scholar
11. Zhoujin, Y., Li, Y., Zhang, M., Parkin, S., Guo, J., Li, T., Yu, F., Long, S. Polymorphism and cocrystal salt formation of 2-((2,6-dichlorophenyl)amino) benzoic acid, harvest of a second form of 2-((2,6-dimethylphenyl)amino) benzoic acid, and isomorphism between the two systems. CrystEngComm 2022, 24, 681–690; https://doi.org/10.1039/d1ce01407b.Search in Google Scholar
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of poly[diaqua-(μ4-3,3′-di(1H-1,2,4-triazol-1-yl)-[1,1′-biphenyl]-4,4′-dicarboxylate-N:N′:O:O′)cadmium(II)], C18H14N6O6Cd
- Crystal structure of (8R,8′S,13S,13′R)-8,8′-bis(hydroxymethyl)-9,9′,10,10′-tetramethoxy-5,5′,6,6′,8,8′,13,13′-octahydro-[13,13′-bi[1,3]dioxolo[4,5-g]isoquinolino[3,2-a]isoquinoline]-7,7′-diium chloride-methanol (1/2), C46H58N2O14Cl2
- The crystal structure of 8-methoxy-2,2-diphenyl-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C29H25BN2O3S
- Crystal structure of aqua-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N″,N‴)copper(II) 5-carboxyisophthalate tetrahydrate, C25H50N4CuO11
- The crystal structure of 1-(naphthalen-2-ylsulfonyl)-2,2-diphenyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ij]quinoline, C31H23BN2O2S
- Crystal structure of iodido-(η6-benzene) (1-(pyridin-2-yl)-N-(p-fluoro-methanamine)-κ2N,Nʹ)ruthenium(II) hexaflourophosphate, (C18H15F7IN2RuP)
- The crystal structure of 1-(3-oxo-1-phenyl-3-(p-tolyl) propylidene)-1,3-dihydro-2H-inden-2-one, C25H20O2
- Crystal structure of tricyclohexyl[4-(4H-1,2,4-triazol-4-yl)-benzoato-κO]tin(IV), C27H39N3O2Sn
- Crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ4N,O,O,O)cadmium(II)]monohydrate, C12H15NO9Cd
- Crystal structure of ethyl 2-((4-(3,5-dimethylisoxazol-4-yl)-2,6-difluorophenyl)amino)benzoate, C20H18F2N2O3
- The crystal structure of 2-(hydroxymethyl)-2-(4H-1,2,4-triazol-4-yl)propane-1,3-diol, C6H11N3O3
- The crystal structure of 1,2-bis(2,4-dinitrophenyl) hydrazine, C12H8N6O8
- Crystal structure of 1-(2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)-1,2-dihydro-4H-benzo[d][1,3]oxazin-4-one, C19H14Cl2N2O3
- The crystal structure of 5-amino-5-oxo-4-(1-oxo-4-(2-oxopyrrolidin-1-yl)isoindolin-2-yl)pentanoic acid, C17H19N3O5
- Crystal structure of N2,N6-bis(2-(((Z)-5-bromo-2-hydroxybenzylidene)amino) phenyl)pyridine-2,6-dicarboxamide, C33H23Br2N5O4
- The crystal structure of (E)-2-methoxy-6-(((5-methyl-1,3,4-thiadiazol-2-yl)imino)methyl)phenol, C11H11N3O2S
- The crystal structure of 3-((tert-butyldiphenylsilyl)methyl)-5,5-diphenyl-6-(p-tolyl) tetrahydro-2H-pyran-2-one, C41H42O2Si
- Crystal structure of 9-fluoro-4-(6-methoxypyridin-3-yl)-5,6-dihydrobenzo[h]quinazolin-2-amine, C18H15FN4O
- The crystal structure of 2-bromo-5-(4-cyanophenoxy)benzyl 1-methyl-1,2,5,6-tetrahydropyridine-3-carboxylate, C21H19BrN2O3
- Crystal structure of 3,3′-(1,4-phenylenebis(methylene))bis(1-isopropyl-1H-imidazol-3-ium) bis(hexafluorophosphate(V)), C10H14F6N2P
- The crystal structure of 2,2-di(thiophen-3-yl)-1-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C24H19BN2O2S3
- Crystal structure of 5-bromo-1-(2-iodobenzoyl)-1H-indole-3-carbaldehyde, C16H9BrINO2
- The crystal structure of monocarbonyl-2-carboxypyridinato-κ2N,O-triphenylphosphine-rhodium(I) acetonitrile solvate, C26H20.50N1.50O3PRh
- Crystal structure of dichlorido-tetrakis(1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ1N)manganese(II), C60H68O4N12Cl10Mn
- Crystal structure of 3-(tert-butyldiphenylsilyl)-1-(2,6-dichlorophenyl)-2,2-diphenylpropan-1-ol, C37H36Cl2OSi
- Crystal structure of langite from Mine du Pradet (France)
- The crystal structure of 5′-(furan-2-yl)-3′-((4-methylphenyl)sulfonamido)-3′,4′,5′,6′-tetrahydro-[1,1′:3′,1″-terphenyl]-4′-carboxylic acid, C30H27NO5S
- Synthesis and crystal structure of bis{2-(((4-acetophenone)imino)methyl)-4-fluorophenolato-κ2N,O}zinc(II), C30H22F2N2O4Zn
- The crystal structure of poly[(tripyridine-κ3N,N′,N″) μ3-(pyridine-3,4-dicarboxylate-κ3N:O:O′) manganese(II)], C22H22N4O8Mn
- The crystal structure of (E)-4-chloro-N′-(1-(4-hydroxyphenyl)propylidene)benzohydrazide, C16H15ClN2O2
- Synthesis and crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino) ethyl)phenyl)imino)methyl)phenolato-κ2N,O}cobalt(II), C40H46CoN4O4
- Crystal structure of tetraaqua-[(1-(carboxymethyl)-1H-pyrazole-3-carboxylato-κ2N,O)cobalt(II)], C6H12CoN2O8
- (6R,7S)-2,3,13-trimethoxy-6,7-dimethyl-5,6,7,8-tetrahydrobenzo[3′,4′]cycloocta [1′,2′:4,5]benzo[1,2-d][1,3]dioxol-1-ol, C22H26O6
- Crystal structure of 2-((2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)amino)benzoic acid, C18H14Cl2N2O3
- Crystal structure of (5aS,6aS,8aR,9R,11aS, 11bS,13R,13aS)-1,1,8a,11a-tetramethyl-9-((S)-1-((S)-5-methyl-6-oxo-3,6-dihydro-2H-pyran-2-yl)ethyl)-3-oxo-1,7,8,8a,9,10,11,11a,11b,12,13,13a-dodecahydro-3H,6H-cyclopenta[5,6]cyclopropa[1,8a]naphtho[2,1-c]oxepin-13-yl acetate, C32H44O6
- Crystal structure of catena-poly[triaqua-(μ2-1-(4-carboxylatophenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylato-O,O′:O″)cobalt(II)], C12H12N2O8Co
- Crystal structure of 3-[(furan-2-ylmethyl)-amino]-2-(2,3,4,5-tetrafluoro-benzoyl)-acrylic acid ethyl ester, C17H13F4NO4
- Crystal structure of methyl 4-(2-ethoxy-2-oxoethoxy)-3-methoxybenzoate, C13H16O6
- Crystal structure of 4-bromo-2-(4-chlorophenyl)-1-methyl-5-(trifluoromethyl)-1H-pyrrole-3-carbonitrile, C13H7BrClF3N2
- The crystal structure of triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ3N,O,O)nickel(II) monohydrate, C12H15NO9Ni
- Crystal structure of dihydroxy(2,4,6-triisopro-pylphenyl)telluronium trifluoromethanesulfonate, C16H25F3O5STe
- The crystal structure of 1-(carboxymethyl)-1H-imidazole 3-oxide
- The crystal structure of 1,3,5-tris(dibromomethyl)benzene, C9H6Br6
- Crystal structure of (Z)-3-(4-methoxyphenyl)-4-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-N-phenylthiazol-2(3H)-imine, C25H21N5OS
- Crystal structure of (Z)-3-(3-(4-hydroxyphenyl)-2-(phenylimino)-2,3-dihydrothiazol-4-yl)-2H-chromen-2-one, C24H16N2O3S
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of poly[diaqua-(μ4-3,3′-di(1H-1,2,4-triazol-1-yl)-[1,1′-biphenyl]-4,4′-dicarboxylate-N:N′:O:O′)cadmium(II)], C18H14N6O6Cd
- Crystal structure of (8R,8′S,13S,13′R)-8,8′-bis(hydroxymethyl)-9,9′,10,10′-tetramethoxy-5,5′,6,6′,8,8′,13,13′-octahydro-[13,13′-bi[1,3]dioxolo[4,5-g]isoquinolino[3,2-a]isoquinoline]-7,7′-diium chloride-methanol (1/2), C46H58N2O14Cl2
- The crystal structure of 8-methoxy-2,2-diphenyl-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C29H25BN2O3S
- Crystal structure of aqua-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N″,N‴)copper(II) 5-carboxyisophthalate tetrahydrate, C25H50N4CuO11
- The crystal structure of 1-(naphthalen-2-ylsulfonyl)-2,2-diphenyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ij]quinoline, C31H23BN2O2S
- Crystal structure of iodido-(η6-benzene) (1-(pyridin-2-yl)-N-(p-fluoro-methanamine)-κ2N,Nʹ)ruthenium(II) hexaflourophosphate, (C18H15F7IN2RuP)
- The crystal structure of 1-(3-oxo-1-phenyl-3-(p-tolyl) propylidene)-1,3-dihydro-2H-inden-2-one, C25H20O2
- Crystal structure of tricyclohexyl[4-(4H-1,2,4-triazol-4-yl)-benzoato-κO]tin(IV), C27H39N3O2Sn
- Crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ4N,O,O,O)cadmium(II)]monohydrate, C12H15NO9Cd
- Crystal structure of ethyl 2-((4-(3,5-dimethylisoxazol-4-yl)-2,6-difluorophenyl)amino)benzoate, C20H18F2N2O3
- The crystal structure of 2-(hydroxymethyl)-2-(4H-1,2,4-triazol-4-yl)propane-1,3-diol, C6H11N3O3
- The crystal structure of 1,2-bis(2,4-dinitrophenyl) hydrazine, C12H8N6O8
- Crystal structure of 1-(2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)-1,2-dihydro-4H-benzo[d][1,3]oxazin-4-one, C19H14Cl2N2O3
- The crystal structure of 5-amino-5-oxo-4-(1-oxo-4-(2-oxopyrrolidin-1-yl)isoindolin-2-yl)pentanoic acid, C17H19N3O5
- Crystal structure of N2,N6-bis(2-(((Z)-5-bromo-2-hydroxybenzylidene)amino) phenyl)pyridine-2,6-dicarboxamide, C33H23Br2N5O4
- The crystal structure of (E)-2-methoxy-6-(((5-methyl-1,3,4-thiadiazol-2-yl)imino)methyl)phenol, C11H11N3O2S
- The crystal structure of 3-((tert-butyldiphenylsilyl)methyl)-5,5-diphenyl-6-(p-tolyl) tetrahydro-2H-pyran-2-one, C41H42O2Si
- Crystal structure of 9-fluoro-4-(6-methoxypyridin-3-yl)-5,6-dihydrobenzo[h]quinazolin-2-amine, C18H15FN4O
- The crystal structure of 2-bromo-5-(4-cyanophenoxy)benzyl 1-methyl-1,2,5,6-tetrahydropyridine-3-carboxylate, C21H19BrN2O3
- Crystal structure of 3,3′-(1,4-phenylenebis(methylene))bis(1-isopropyl-1H-imidazol-3-ium) bis(hexafluorophosphate(V)), C10H14F6N2P
- The crystal structure of 2,2-di(thiophen-3-yl)-1-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C24H19BN2O2S3
- Crystal structure of 5-bromo-1-(2-iodobenzoyl)-1H-indole-3-carbaldehyde, C16H9BrINO2
- The crystal structure of monocarbonyl-2-carboxypyridinato-κ2N,O-triphenylphosphine-rhodium(I) acetonitrile solvate, C26H20.50N1.50O3PRh
- Crystal structure of dichlorido-tetrakis(1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ1N)manganese(II), C60H68O4N12Cl10Mn
- Crystal structure of 3-(tert-butyldiphenylsilyl)-1-(2,6-dichlorophenyl)-2,2-diphenylpropan-1-ol, C37H36Cl2OSi
- Crystal structure of langite from Mine du Pradet (France)
- The crystal structure of 5′-(furan-2-yl)-3′-((4-methylphenyl)sulfonamido)-3′,4′,5′,6′-tetrahydro-[1,1′:3′,1″-terphenyl]-4′-carboxylic acid, C30H27NO5S
- Synthesis and crystal structure of bis{2-(((4-acetophenone)imino)methyl)-4-fluorophenolato-κ2N,O}zinc(II), C30H22F2N2O4Zn
- The crystal structure of poly[(tripyridine-κ3N,N′,N″) μ3-(pyridine-3,4-dicarboxylate-κ3N:O:O′) manganese(II)], C22H22N4O8Mn
- The crystal structure of (E)-4-chloro-N′-(1-(4-hydroxyphenyl)propylidene)benzohydrazide, C16H15ClN2O2
- Synthesis and crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino) ethyl)phenyl)imino)methyl)phenolato-κ2N,O}cobalt(II), C40H46CoN4O4
- Crystal structure of tetraaqua-[(1-(carboxymethyl)-1H-pyrazole-3-carboxylato-κ2N,O)cobalt(II)], C6H12CoN2O8
- (6R,7S)-2,3,13-trimethoxy-6,7-dimethyl-5,6,7,8-tetrahydrobenzo[3′,4′]cycloocta [1′,2′:4,5]benzo[1,2-d][1,3]dioxol-1-ol, C22H26O6
- Crystal structure of 2-((2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)amino)benzoic acid, C18H14Cl2N2O3
- Crystal structure of (5aS,6aS,8aR,9R,11aS, 11bS,13R,13aS)-1,1,8a,11a-tetramethyl-9-((S)-1-((S)-5-methyl-6-oxo-3,6-dihydro-2H-pyran-2-yl)ethyl)-3-oxo-1,7,8,8a,9,10,11,11a,11b,12,13,13a-dodecahydro-3H,6H-cyclopenta[5,6]cyclopropa[1,8a]naphtho[2,1-c]oxepin-13-yl acetate, C32H44O6
- Crystal structure of catena-poly[triaqua-(μ2-1-(4-carboxylatophenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylato-O,O′:O″)cobalt(II)], C12H12N2O8Co
- Crystal structure of 3-[(furan-2-ylmethyl)-amino]-2-(2,3,4,5-tetrafluoro-benzoyl)-acrylic acid ethyl ester, C17H13F4NO4
- Crystal structure of methyl 4-(2-ethoxy-2-oxoethoxy)-3-methoxybenzoate, C13H16O6
- Crystal structure of 4-bromo-2-(4-chlorophenyl)-1-methyl-5-(trifluoromethyl)-1H-pyrrole-3-carbonitrile, C13H7BrClF3N2
- The crystal structure of triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ3N,O,O)nickel(II) monohydrate, C12H15NO9Ni
- Crystal structure of dihydroxy(2,4,6-triisopro-pylphenyl)telluronium trifluoromethanesulfonate, C16H25F3O5STe
- The crystal structure of 1-(carboxymethyl)-1H-imidazole 3-oxide
- The crystal structure of 1,3,5-tris(dibromomethyl)benzene, C9H6Br6
- Crystal structure of (Z)-3-(4-methoxyphenyl)-4-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-N-phenylthiazol-2(3H)-imine, C25H21N5OS
- Crystal structure of (Z)-3-(3-(4-hydroxyphenyl)-2-(phenylimino)-2,3-dihydrothiazol-4-yl)-2H-chromen-2-one, C24H16N2O3S


