Abstract
C27H39N3O2Sn, monoclinic, P21/c (no. 14), a = 10.3317(6) Å, b = 15.8067(9) Å, c = 16.5497(9) Å, β = 90.5380(10)°, V = 2702.6(3) Å3, Z = 4, Rgt(F) = 0.0370, wRref(F2) = 0.0954, T = 296(2) K.
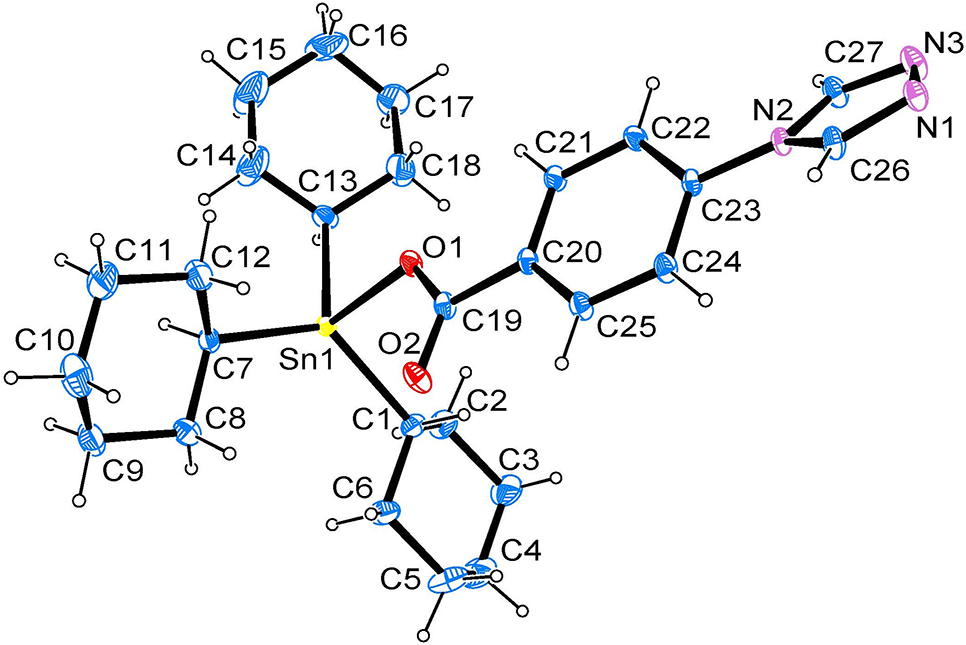
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colorless lump |
| Size: | 0.20 × 0.19 × 0.18 mm |
| Wavelength: | MoKα radiation (0.71073 Å) |
| μ: | 0.97 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| θmax, completeness: | 26.4°, >99 % |
| N(hkl)measured, N(hkl)unique, Rint: | 30,612, 5552, 0.016 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2σ(Iobs), 5311 |
| N(param)refined: | 299 |
| Programs: | Bruker [1], Shelx [2, 3], WinGX/Ortep [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| Sn1 | 0.25939 (2) | 0.21054 (2) | 0.02219 (2) | 0.03203 (9) |
| C1 | 0.2663 (3) | 0.3468 (2) | 0.0329 (2) | 0.0447 (8) |
| H1 | 0.339761 | 0.358251 | 0.069176 | 0.054* |
| O1 | 0.4246 (2) | 0.19177 (16) | 0.10000 (14) | 0.0420 (5) |
| O2 | 0.5619 (2) | 0.2620 (2) | 0.02118 (15) | 0.0533 (6) |
| C2 | 0.1505 (5) | 0.3859 (3) | 0.0740 (3) | 0.0649 (11) |
| H2A | 0.138742 | 0.359771 | 0.126453 | 0.078* |
| H2B | 0.073205 | 0.375544 | 0.041784 | 0.078* |
| C6 | 0.2990 (5) | 0.3912 (3) | −0.0452 (3) | 0.0669 (12) |
| H6A | 0.232310 | 0.379055 | −0.085154 | 0.080* |
| H6B | 0.380261 | 0.369186 | −0.065302 | 0.080* |
| N1 | 1.0393 (3) | 0.2688 (2) | 0.43958 (18) | 0.0454 (7) |
| C7 | 0.3145 (3) | 0.1415 (2) | −0.0837 (2) | 0.0432 (7) |
| H7 | 0.236560 | 0.110620 | −0.100554 | 0.052* |
| C12 | 0.4159 (5) | 0.0737 (3) | −0.0674 (3) | 0.0668 (12) |
| H12A | 0.387663 | 0.037809 | −0.023373 | 0.080* |
| H12B | 0.496730 | 0.100248 | −0.051165 | 0.080* |
| C11 | 0.4378 (7) | 0.0198 (3) | −0.1425 (3) | 0.0919 (18) |
| H11A | 0.507753 | −0.019686 | −0.131855 | 0.110* |
| H11B | 0.360212 | −0.012769 | −0.153939 | 0.110* |
| C10 | 0.4703 (6) | 0.0720 (4) | −0.2148 (3) | 0.0951 (19) |
| H10A | 0.554286 | 0.098135 | −0.206533 | 0.114* |
| H10B | 0.475849 | 0.035455 | −0.261781 | 0.114* |
| C9 | 0.3717 (5) | 0.1392 (3) | −0.2306 (3) | 0.0723 (13) |
| H9A | 0.290456 | 0.113014 | −0.246415 | 0.087* |
| H9B | 0.400171 | 0.174551 | −0.274874 | 0.087* |
| C8 | 0.3506 (4) | 0.1943 (3) | −0.1553 (2) | 0.0535 (9) |
| H8A | 0.429229 | 0.225517 | −0.143122 | 0.064* |
| H8B | 0.282320 | 0.234978 | −0.166386 | 0.064* |
| C13 | 0.1399 (4) | 0.1365 (3) | 0.1016 (2) | 0.0549 (9) |
| H13 | 0.053142 | 0.160816 | 0.095097 | 0.066* |
| C14 | 0.1259 (10) | 0.0496 (4) | 0.0742 (4) | 0.160 (3) |
| H14A | 0.211128 | 0.024270 | 0.070552 | 0.192* |
| H14B | 0.087983 | 0.049820 | 0.020316 | 0.192* |
| C15 | 0.0424 (10) | −0.0048 (5) | 0.1290 (4) | 0.177 (3) |
| H15A | −0.047564 | 0.010579 | 0.120265 | 0.212* |
| H15B | 0.052024 | −0.063498 | 0.112890 | 0.212* |
| C16 | 0.0718 (9) | 0.0018 (4) | 0.2159 (4) | 0.134 (2) |
| H16A | 0.005966 | −0.027511 | 0.246482 | 0.161* |
| H16B | 0.154610 | −0.024623 | 0.227489 | 0.161* |
| C17 | 0.0760 (7) | 0.0899 (4) | 0.2398 (3) | 0.1064 (19) |
| H17A | 0.103583 | 0.093434 | 0.295890 | 0.128* |
| H17B | −0.010508 | 0.113445 | 0.235798 | 0.128* |
| C18 | 0.1671 (7) | 0.1426 (4) | 0.1884 (3) | 0.1012 (18) |
| H18A | 0.160640 | 0.201317 | 0.204736 | 0.121* |
| H18B | 0.255302 | 0.124204 | 0.198629 | 0.121* |
| C3 | 0.1700 (6) | 0.4820 (3) | 0.0846 (4) | 0.0852 (16) |
| H3A | 0.091993 | 0.506405 | 0.106965 | 0.102* |
| H3B | 0.240168 | 0.491776 | 0.122827 | 0.102* |
| C4 | 0.1999 (7) | 0.5249 (3) | 0.0077 (4) | 0.0973 (19) |
| H4A | 0.124308 | 0.522668 | −0.027436 | 0.117* |
| H4B | 0.218802 | 0.583958 | 0.018530 | 0.117* |
| C5 | 0.3105 (6) | 0.4869 (3) | −0.0345 (4) | 0.0921 (18) |
| H5A | 0.389130 | 0.499251 | −0.004276 | 0.111* |
| H5B | 0.318083 | 0.512940 | −0.087288 | 0.111* |
| C19 | 0.5337 (3) | 0.2290 (2) | 0.0854 (2) | 0.0378 (7) |
| C20 | 0.6258 (3) | 0.2314 (2) | 0.15666 (19) | 0.0354 (6) |
| C21 | 0.5963 (3) | 0.1905 (2) | 0.2283 (2) | 0.0405 (7) |
| H21 | 0.520875 | 0.158523 | 0.231513 | 0.049* |
| C22 | 0.6777 (3) | 0.1965 (2) | 0.2952 (2) | 0.0426 (8) |
| H22 | 0.657773 | 0.168502 | 0.342951 | 0.051* |
| C23 | 0.7891 (3) | 0.2450 (2) | 0.28989 (19) | 0.0349 (6) |
| C24 | 0.8206 (3) | 0.2858 (2) | 0.2187 (2) | 0.0437 (8) |
| H24 | 0.895536 | 0.318204 | 0.215589 | 0.052* |
| C25 | 0.7393 (3) | 0.2778 (2) | 0.1520 (2) | 0.0430 (8) |
| H25 | 0.761216 | 0.303799 | 0.103596 | 0.052* |
| N2 | 0.8698 (2) | 0.25446 (18) | 0.36022 (16) | 0.0359 (6) |
| C27 | 0.8318 (3) | 0.2574 (3) | 0.4385 (2) | 0.0470 (8) |
| H27 | 0.746327 | 0.253900 | 0.455224 | 0.056* |
| N3 | 0.9303 (3) | 0.2658 (3) | 0.48687 (19) | 0.0550 (8) |
| C26 | 1.0007 (3) | 0.2624 (2) | 0.3645 (2) | 0.0423 (7) |
| H26 | 1.055193 | 0.263108 | 0.320125 | 0.051* |
1 Source of material
All chemicals were purchased from commercial sources and used as received without further purification. An amount of 1 mmol tricyclohexyltin hydroxide and 1 mmol 4-(4H-1,2,4-triazol-4-yl)benzoic acid were added in CH3OH (25 mL) and refluxed with agitating for 12 h. The reaction solution was filtered. Finally, the title crystal was precipitated by controlling solvent volatilization.
2 Experimental details
All H-atoms bonded to C atoms were placed geometrically and refined using a riding model with common isotropic displacement factors Uiso(H) = 1.2 or 1.5 Ueq(parent C-atom).
3 Comment
Organotin carboxylates, a class of organometallic compounds, have drawn considerable interest due to their diverse structures and significant biological activities [5, 6]. As these have shown higher apoptosis inducing character compared to the platinum complexes, both in vivo and in vitro, organotin carboxylates are found be the most promising alternatives [7, 8]. It is found that the activity and structure of organotin carboxylates are related. Therefore, the investigation on the crystal structures of organotin carboxylates has received wide attention [9, 10]. In this context, we have crystallized the title compound.
In the molecular structure (Figure), the Sn(IV) ion is four-coordinated by one oxygen atom of one carboxyl group and three carbon atoms of three cyclohexyl groups. Bond lengths and angles are all in the expected ranges [11–13]. The C–Sn–C angles and C–Sn–O angles are ranging from 112.29(15)° to 124.37(14)° and 90.93(12)° to 101.76(11)°, respectively. The bond lengths of Sn–C are ranging from 2.146(3) to 2.162(4) Å, and Sn1–O1 is 2.149(2) Å.
-
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work was supported by College Students Innovation and Entrepreneurship Training Program of Hunan Province General Project, College Students Innovation and Entrepreneurship Training Program of National General Project.
-
Competing interests: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. SAINT, APEX2 and SADABS; Bruker AXS Inc.: Madison, WI, USA, 2012.Search in Google Scholar
2. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar PubMed PubMed Central
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Farrugia, L. J. WINGX and ORTEP for Windows: an update. J. Appl. Crystallogr. 2012, 45, 849–854; https://doi.org/10.1107/s0021889812029111.Search in Google Scholar
5. Du, X.-M., Ma, J.-W., Li, Q.-L., Cheng, S., Cui, Y., Ma, C.-L. Syntheses, structures, and the in vitro anticancer mechanism of triorganotin(IV) complexes based on 4,4′-stilbenedicarboxylic acid. Appl. Organomet. Chem. 2023, 37, e7016; https://doi.org/10.1002/aoc.7016.Search in Google Scholar
6. Amir, M. K., Khan, S., Rehman, Z., Shah, A., Butler, I. S. Anticancer activity of organotin(IV) carboxylates. Inorg. Chim. Acta 2014, 423, 14–25; https://doi.org/10.1016/j.ica.2014.07.053.Search in Google Scholar
7. Muhammad, N., Ahmad, M., Sirajuddin, M., Ali, Z., Tumanov, N., Wouters, J., Chafik, A., Solak, K., Mavi, A., Muhammad, S., Shujah, S., Ali, S., Al-Sehemi, A. G. Synthesis, characterization, biological activity and molecular docking studies of novel organotin(IV) carboxylates. Front. Pharmacol 2022, 13, 864336–864352; https://doi.org/10.3389/fphar.2022.864336.Search in Google Scholar PubMed PubMed Central
8. Butt, A. F., Ahmed, M. N., Bhatti, M. H., Choudhary, M. A., Ayub, K., Tahir, M. N., Mahmood, T. Synthesis, structural properties, DFT studies, antimicrobial activities and DNA binding interactions of two newly synthesized organotin(IV) carboxylates. J. Mol. Struct. 2019, 1191, 291–300; https://doi.org/10.1016/j.molstruc.2019.04.066.Search in Google Scholar
9. Sun, J. The crystal structure of the cocrystal di-μ2-chlorido-octamethyl-di-μ3-oxido-bis(2,3,4,5-tetrafluorobenzoato-κ2O,O′)tetratin(IV) – octamethyl-di-μ3-oxido-bis(μ2-2,3,4,5-tetrafluorobenzoato-κ2O:O′)-bis(μ2-2,3,4,5-tetrafluorobenzoato-κ2O:O; O′)tetratin(IV) C58H54Cl2F24O16Sn8. Z. Kristallogr. – New Cryst. Struct. 2021, 236, 1231–1233; https://doi.org/10.1515/ncrs-2021-0288.Search in Google Scholar
10. Ding, R.-F., Wang, Q.-B., Wen, X.-M. Crystal structure of bis(μ3-oxo)-bis[μ2-2-(2-formylphenoxy)acetato-O, O′]-bis[μ2-2-(2-formylphenoxy)acetato-O,O′]-octakis(n-butyl)tetratin(IV), Sn4O2(C9H7O4)4(C4H9)8. Z. Kristallogr. – New Cryst. Struct. 2011, 226, 31–32; https://doi.org/10.1524/ncrs.2011.0016.Search in Google Scholar
11. Liu, X., Zhang, F.-X., He, L.-F., Li, D.-W., Zeng, W.-H., Jiang, S.-Y., He, X., Sheng, L.-B., Zhu, X.-M. Synthesis, structure and antitumor activity of two tris(o-bromobenzyl)tin carboxylates. Chin. J. Inorg. Chem. 2022, 38, 46–52.Search in Google Scholar
12. Song, X., Cahill, C., Eng, G. Crystal structure of tricyclohexyltin 4-hydroxybenzoate. Main Group Met. Chem. 2002, 25, 703–704; https://doi.org/10.1515/mgmc.2002.25.11.703.Search in Google Scholar
13. Danish, M., Tahir, M. N., Ahmad, N., Ali, S., Badshah, A. Tricyclohexyl[2-(2,3-dimethylanilino)benzoato-κO]tin(IV). Acta Crystallogr. 2009, E65, m1614; https://doi.org/10.1107/s1600536809048314.Search in Google Scholar
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of poly[diaqua-(μ4-3,3′-di(1H-1,2,4-triazol-1-yl)-[1,1′-biphenyl]-4,4′-dicarboxylate-N:N′:O:O′)cadmium(II)], C18H14N6O6Cd
- Crystal structure of (8R,8′S,13S,13′R)-8,8′-bis(hydroxymethyl)-9,9′,10,10′-tetramethoxy-5,5′,6,6′,8,8′,13,13′-octahydro-[13,13′-bi[1,3]dioxolo[4,5-g]isoquinolino[3,2-a]isoquinoline]-7,7′-diium chloride-methanol (1/2), C46H58N2O14Cl2
- The crystal structure of 8-methoxy-2,2-diphenyl-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C29H25BN2O3S
- Crystal structure of aqua-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N″,N‴)copper(II) 5-carboxyisophthalate tetrahydrate, C25H50N4CuO11
- The crystal structure of 1-(naphthalen-2-ylsulfonyl)-2,2-diphenyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ij]quinoline, C31H23BN2O2S
- Crystal structure of iodido-(η6-benzene) (1-(pyridin-2-yl)-N-(p-fluoro-methanamine)-κ2N,Nʹ)ruthenium(II) hexaflourophosphate, (C18H15F7IN2RuP)
- The crystal structure of 1-(3-oxo-1-phenyl-3-(p-tolyl) propylidene)-1,3-dihydro-2H-inden-2-one, C25H20O2
- Crystal structure of tricyclohexyl[4-(4H-1,2,4-triazol-4-yl)-benzoato-κO]tin(IV), C27H39N3O2Sn
- Crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ4N,O,O,O)cadmium(II)]monohydrate, C12H15NO9Cd
- Crystal structure of ethyl 2-((4-(3,5-dimethylisoxazol-4-yl)-2,6-difluorophenyl)amino)benzoate, C20H18F2N2O3
- The crystal structure of 2-(hydroxymethyl)-2-(4H-1,2,4-triazol-4-yl)propane-1,3-diol, C6H11N3O3
- The crystal structure of 1,2-bis(2,4-dinitrophenyl) hydrazine, C12H8N6O8
- Crystal structure of 1-(2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)-1,2-dihydro-4H-benzo[d][1,3]oxazin-4-one, C19H14Cl2N2O3
- The crystal structure of 5-amino-5-oxo-4-(1-oxo-4-(2-oxopyrrolidin-1-yl)isoindolin-2-yl)pentanoic acid, C17H19N3O5
- Crystal structure of N2,N6-bis(2-(((Z)-5-bromo-2-hydroxybenzylidene)amino) phenyl)pyridine-2,6-dicarboxamide, C33H23Br2N5O4
- The crystal structure of (E)-2-methoxy-6-(((5-methyl-1,3,4-thiadiazol-2-yl)imino)methyl)phenol, C11H11N3O2S
- The crystal structure of 3-((tert-butyldiphenylsilyl)methyl)-5,5-diphenyl-6-(p-tolyl) tetrahydro-2H-pyran-2-one, C41H42O2Si
- Crystal structure of 9-fluoro-4-(6-methoxypyridin-3-yl)-5,6-dihydrobenzo[h]quinazolin-2-amine, C18H15FN4O
- The crystal structure of 2-bromo-5-(4-cyanophenoxy)benzyl 1-methyl-1,2,5,6-tetrahydropyridine-3-carboxylate, C21H19BrN2O3
- Crystal structure of 3,3′-(1,4-phenylenebis(methylene))bis(1-isopropyl-1H-imidazol-3-ium) bis(hexafluorophosphate(V)), C10H14F6N2P
- The crystal structure of 2,2-di(thiophen-3-yl)-1-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C24H19BN2O2S3
- Crystal structure of 5-bromo-1-(2-iodobenzoyl)-1H-indole-3-carbaldehyde, C16H9BrINO2
- The crystal structure of monocarbonyl-2-carboxypyridinato-κ2N,O-triphenylphosphine-rhodium(I) acetonitrile solvate, C26H20.50N1.50O3PRh
- Crystal structure of dichlorido-tetrakis(1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ1N)manganese(II), C60H68O4N12Cl10Mn
- Crystal structure of 3-(tert-butyldiphenylsilyl)-1-(2,6-dichlorophenyl)-2,2-diphenylpropan-1-ol, C37H36Cl2OSi
- Crystal structure of langite from Mine du Pradet (France)
- The crystal structure of 5′-(furan-2-yl)-3′-((4-methylphenyl)sulfonamido)-3′,4′,5′,6′-tetrahydro-[1,1′:3′,1″-terphenyl]-4′-carboxylic acid, C30H27NO5S
- Synthesis and crystal structure of bis{2-(((4-acetophenone)imino)methyl)-4-fluorophenolato-κ2N,O}zinc(II), C30H22F2N2O4Zn
- The crystal structure of poly[(tripyridine-κ3N,N′,N″) μ3-(pyridine-3,4-dicarboxylate-κ3N:O:O′) manganese(II)], C22H22N4O8Mn
- The crystal structure of (E)-4-chloro-N′-(1-(4-hydroxyphenyl)propylidene)benzohydrazide, C16H15ClN2O2
- Synthesis and crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino) ethyl)phenyl)imino)methyl)phenolato-κ2N,O}cobalt(II), C40H46CoN4O4
- Crystal structure of tetraaqua-[(1-(carboxymethyl)-1H-pyrazole-3-carboxylato-κ2N,O)cobalt(II)], C6H12CoN2O8
- (6R,7S)-2,3,13-trimethoxy-6,7-dimethyl-5,6,7,8-tetrahydrobenzo[3′,4′]cycloocta [1′,2′:4,5]benzo[1,2-d][1,3]dioxol-1-ol, C22H26O6
- Crystal structure of 2-((2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)amino)benzoic acid, C18H14Cl2N2O3
- Crystal structure of (5aS,6aS,8aR,9R,11aS, 11bS,13R,13aS)-1,1,8a,11a-tetramethyl-9-((S)-1-((S)-5-methyl-6-oxo-3,6-dihydro-2H-pyran-2-yl)ethyl)-3-oxo-1,7,8,8a,9,10,11,11a,11b,12,13,13a-dodecahydro-3H,6H-cyclopenta[5,6]cyclopropa[1,8a]naphtho[2,1-c]oxepin-13-yl acetate, C32H44O6
- Crystal structure of catena-poly[triaqua-(μ2-1-(4-carboxylatophenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylato-O,O′:O″)cobalt(II)], C12H12N2O8Co
- Crystal structure of 3-[(furan-2-ylmethyl)-amino]-2-(2,3,4,5-tetrafluoro-benzoyl)-acrylic acid ethyl ester, C17H13F4NO4
- Crystal structure of methyl 4-(2-ethoxy-2-oxoethoxy)-3-methoxybenzoate, C13H16O6
- Crystal structure of 4-bromo-2-(4-chlorophenyl)-1-methyl-5-(trifluoromethyl)-1H-pyrrole-3-carbonitrile, C13H7BrClF3N2
- The crystal structure of triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ3N,O,O)nickel(II) monohydrate, C12H15NO9Ni
- Crystal structure of dihydroxy(2,4,6-triisopro-pylphenyl)telluronium trifluoromethanesulfonate, C16H25F3O5STe
- The crystal structure of 1-(carboxymethyl)-1H-imidazole 3-oxide
- The crystal structure of 1,3,5-tris(dibromomethyl)benzene, C9H6Br6
- Crystal structure of (Z)-3-(4-methoxyphenyl)-4-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-N-phenylthiazol-2(3H)-imine, C25H21N5OS
- Crystal structure of (Z)-3-(3-(4-hydroxyphenyl)-2-(phenylimino)-2,3-dihydrothiazol-4-yl)-2H-chromen-2-one, C24H16N2O3S
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of poly[diaqua-(μ4-3,3′-di(1H-1,2,4-triazol-1-yl)-[1,1′-biphenyl]-4,4′-dicarboxylate-N:N′:O:O′)cadmium(II)], C18H14N6O6Cd
- Crystal structure of (8R,8′S,13S,13′R)-8,8′-bis(hydroxymethyl)-9,9′,10,10′-tetramethoxy-5,5′,6,6′,8,8′,13,13′-octahydro-[13,13′-bi[1,3]dioxolo[4,5-g]isoquinolino[3,2-a]isoquinoline]-7,7′-diium chloride-methanol (1/2), C46H58N2O14Cl2
- The crystal structure of 8-methoxy-2,2-diphenyl-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C29H25BN2O3S
- Crystal structure of aqua-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N″,N‴)copper(II) 5-carboxyisophthalate tetrahydrate, C25H50N4CuO11
- The crystal structure of 1-(naphthalen-2-ylsulfonyl)-2,2-diphenyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ij]quinoline, C31H23BN2O2S
- Crystal structure of iodido-(η6-benzene) (1-(pyridin-2-yl)-N-(p-fluoro-methanamine)-κ2N,Nʹ)ruthenium(II) hexaflourophosphate, (C18H15F7IN2RuP)
- The crystal structure of 1-(3-oxo-1-phenyl-3-(p-tolyl) propylidene)-1,3-dihydro-2H-inden-2-one, C25H20O2
- Crystal structure of tricyclohexyl[4-(4H-1,2,4-triazol-4-yl)-benzoato-κO]tin(IV), C27H39N3O2Sn
- Crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ4N,O,O,O)cadmium(II)]monohydrate, C12H15NO9Cd
- Crystal structure of ethyl 2-((4-(3,5-dimethylisoxazol-4-yl)-2,6-difluorophenyl)amino)benzoate, C20H18F2N2O3
- The crystal structure of 2-(hydroxymethyl)-2-(4H-1,2,4-triazol-4-yl)propane-1,3-diol, C6H11N3O3
- The crystal structure of 1,2-bis(2,4-dinitrophenyl) hydrazine, C12H8N6O8
- Crystal structure of 1-(2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)-1,2-dihydro-4H-benzo[d][1,3]oxazin-4-one, C19H14Cl2N2O3
- The crystal structure of 5-amino-5-oxo-4-(1-oxo-4-(2-oxopyrrolidin-1-yl)isoindolin-2-yl)pentanoic acid, C17H19N3O5
- Crystal structure of N2,N6-bis(2-(((Z)-5-bromo-2-hydroxybenzylidene)amino) phenyl)pyridine-2,6-dicarboxamide, C33H23Br2N5O4
- The crystal structure of (E)-2-methoxy-6-(((5-methyl-1,3,4-thiadiazol-2-yl)imino)methyl)phenol, C11H11N3O2S
- The crystal structure of 3-((tert-butyldiphenylsilyl)methyl)-5,5-diphenyl-6-(p-tolyl) tetrahydro-2H-pyran-2-one, C41H42O2Si
- Crystal structure of 9-fluoro-4-(6-methoxypyridin-3-yl)-5,6-dihydrobenzo[h]quinazolin-2-amine, C18H15FN4O
- The crystal structure of 2-bromo-5-(4-cyanophenoxy)benzyl 1-methyl-1,2,5,6-tetrahydropyridine-3-carboxylate, C21H19BrN2O3
- Crystal structure of 3,3′-(1,4-phenylenebis(methylene))bis(1-isopropyl-1H-imidazol-3-ium) bis(hexafluorophosphate(V)), C10H14F6N2P
- The crystal structure of 2,2-di(thiophen-3-yl)-1-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C24H19BN2O2S3
- Crystal structure of 5-bromo-1-(2-iodobenzoyl)-1H-indole-3-carbaldehyde, C16H9BrINO2
- The crystal structure of monocarbonyl-2-carboxypyridinato-κ2N,O-triphenylphosphine-rhodium(I) acetonitrile solvate, C26H20.50N1.50O3PRh
- Crystal structure of dichlorido-tetrakis(1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ1N)manganese(II), C60H68O4N12Cl10Mn
- Crystal structure of 3-(tert-butyldiphenylsilyl)-1-(2,6-dichlorophenyl)-2,2-diphenylpropan-1-ol, C37H36Cl2OSi
- Crystal structure of langite from Mine du Pradet (France)
- The crystal structure of 5′-(furan-2-yl)-3′-((4-methylphenyl)sulfonamido)-3′,4′,5′,6′-tetrahydro-[1,1′:3′,1″-terphenyl]-4′-carboxylic acid, C30H27NO5S
- Synthesis and crystal structure of bis{2-(((4-acetophenone)imino)methyl)-4-fluorophenolato-κ2N,O}zinc(II), C30H22F2N2O4Zn
- The crystal structure of poly[(tripyridine-κ3N,N′,N″) μ3-(pyridine-3,4-dicarboxylate-κ3N:O:O′) manganese(II)], C22H22N4O8Mn
- The crystal structure of (E)-4-chloro-N′-(1-(4-hydroxyphenyl)propylidene)benzohydrazide, C16H15ClN2O2
- Synthesis and crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino) ethyl)phenyl)imino)methyl)phenolato-κ2N,O}cobalt(II), C40H46CoN4O4
- Crystal structure of tetraaqua-[(1-(carboxymethyl)-1H-pyrazole-3-carboxylato-κ2N,O)cobalt(II)], C6H12CoN2O8
- (6R,7S)-2,3,13-trimethoxy-6,7-dimethyl-5,6,7,8-tetrahydrobenzo[3′,4′]cycloocta [1′,2′:4,5]benzo[1,2-d][1,3]dioxol-1-ol, C22H26O6
- Crystal structure of 2-((2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)amino)benzoic acid, C18H14Cl2N2O3
- Crystal structure of (5aS,6aS,8aR,9R,11aS, 11bS,13R,13aS)-1,1,8a,11a-tetramethyl-9-((S)-1-((S)-5-methyl-6-oxo-3,6-dihydro-2H-pyran-2-yl)ethyl)-3-oxo-1,7,8,8a,9,10,11,11a,11b,12,13,13a-dodecahydro-3H,6H-cyclopenta[5,6]cyclopropa[1,8a]naphtho[2,1-c]oxepin-13-yl acetate, C32H44O6
- Crystal structure of catena-poly[triaqua-(μ2-1-(4-carboxylatophenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylato-O,O′:O″)cobalt(II)], C12H12N2O8Co
- Crystal structure of 3-[(furan-2-ylmethyl)-amino]-2-(2,3,4,5-tetrafluoro-benzoyl)-acrylic acid ethyl ester, C17H13F4NO4
- Crystal structure of methyl 4-(2-ethoxy-2-oxoethoxy)-3-methoxybenzoate, C13H16O6
- Crystal structure of 4-bromo-2-(4-chlorophenyl)-1-methyl-5-(trifluoromethyl)-1H-pyrrole-3-carbonitrile, C13H7BrClF3N2
- The crystal structure of triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ3N,O,O)nickel(II) monohydrate, C12H15NO9Ni
- Crystal structure of dihydroxy(2,4,6-triisopro-pylphenyl)telluronium trifluoromethanesulfonate, C16H25F3O5STe
- The crystal structure of 1-(carboxymethyl)-1H-imidazole 3-oxide
- The crystal structure of 1,3,5-tris(dibromomethyl)benzene, C9H6Br6
- Crystal structure of (Z)-3-(4-methoxyphenyl)-4-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-N-phenylthiazol-2(3H)-imine, C25H21N5OS
- Crystal structure of (Z)-3-(3-(4-hydroxyphenyl)-2-(phenylimino)-2,3-dihydrothiazol-4-yl)-2H-chromen-2-one, C24H16N2O3S

