Abstract
C25H20O2, triclininc, P
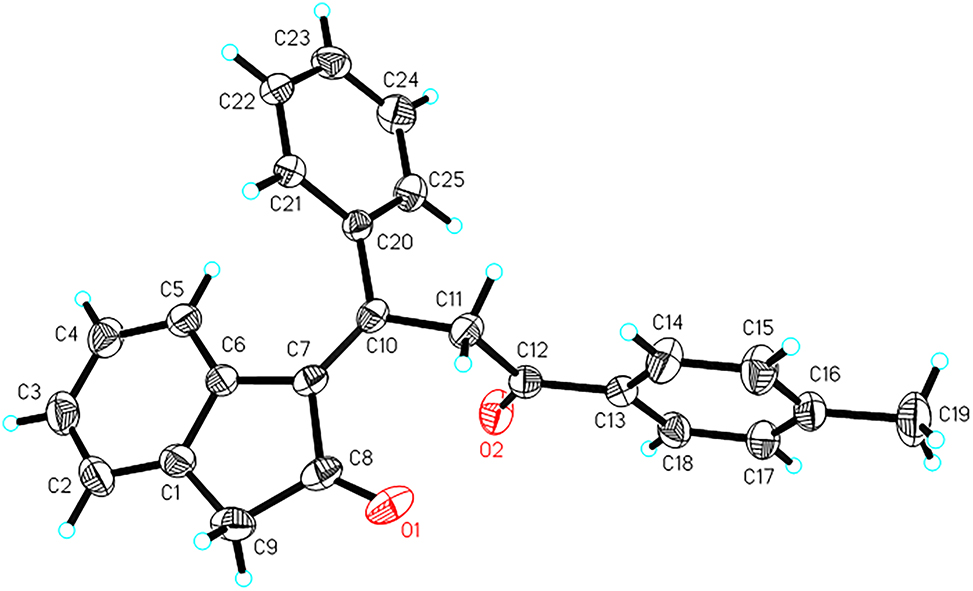
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless prismatic |
| Size: | 0.20 × 0.16 × 0.13 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.08 mm−1 |
| Diffractometer, scan mode: | φ and ω |
| θmax, completeness: | 25.0°, 94 % |
| N(hkl)measured, N(hkl)unique, Rint: | 4808, 3160, 0.032 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2σ(Iobs), 2395 |
| N(param)refined: | 245 |
| Programs: | Bruker [1], Olex2 [2], SHELX [3, 4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| O1 | 1.16664 (16) | 0.4745 (2) | 0.16075 (18) | 0.0716 (5) |
| O2 | 1.09645 (17) | 0.13049 (17) | 0.37400 (15) | 0.0644 (5) |
| C1 | 0.9374 (2) | 0.6383 (2) | 0.3278 (2) | 0.0505 (5) |
| C2 | 0.8906 (3) | 0.7222 (2) | 0.3955 (3) | 0.0652 (7) |
| H2 | 0.9443 | 0.7926 | 0.3956 | 0.078* |
| C3 | 0.7640 (3) | 0.7006 (3) | 0.4624 (3) | 0.0696 (7) |
| H3 | 0.7316 | 0.7568 | 0.5077 | 0.083* |
| C4 | 0.6852 (3) | 0.5963 (3) | 0.4626 (2) | 0.0656 (7) |
| H4 | 0.5998 | 0.5824 | 0.5085 | 0.079* |
| C5 | 0.7306 (2) | 0.5116 (2) | 0.3959 (2) | 0.0533 (6) |
| H5 | 0.6764 | 0.4405 | 0.3979 | 0.064* |
| C6 | 0.8572 (2) | 0.5328 (2) | 0.3257 (2) | 0.0436 (5) |
| C7 | 0.93268 (19) | 0.4553 (2) | 0.25143 (19) | 0.0427 (5) |
| C8 | 1.0715 (2) | 0.5146 (2) | 0.2154 (2) | 0.0520 (6) |
| C9 | 1.0723 (2) | 0.6375 (2) | 0.2562 (2) | 0.0616 (6) |
| H9A | 1.1462 | 0.6168 | 0.3203 | 0.074* |
| H9B | 1.0833 | 0.7336 | 0.1726 | 0.074* |
| C10 | 0.89310 (19) | 0.3540 (2) | 0.21590 (19) | 0.0415 (5) |
| C11 | 0.9896 (2) | 0.2779 (2) | 0.1501 (2) | 0.0479 (5) |
| H11A | 1.0419 | 0.3535 | 0.0741 | 0.057* |
| H11B | 0.9360 | 0.2349 | 0.1076 | 0.057* |
| C12 | 1.0890 (2) | 0.1548 (2) | 0.2521 (2) | 0.0446 (5) |
| C13 | 1.17693 (19) | 0.0649 (2) | 0.19648 (19) | 0.0418 (5) |
| C14 | 1.1787 (2) | 0.0933 (3) | 0.0564 (2) | 0.0610 (6) |
| H14 | 1.1235 | 0.1736 | −0.0084 | 0.073* |
| C15 | 1.2603 (3) | 0.0055 (3) | 0.0109 (3) | 0.0690 (7) |
| H15 | 1.2584 | 0.0264 | −0.0837 | 0.083* |
| C16 | 1.3451 (2) | −0.1134 (2) | 0.1033 (3) | 0.0579 (6) |
| C17 | 1.3437 (2) | −0.1408 (2) | 0.2425 (3) | 0.0581 (6) |
| H17 | 1.4001 | −0.2201 | 0.3069 | 0.070* |
| C18 | 1.2617 (2) | −0.0547 (2) | 0.2888 (2) | 0.0516 (5) |
| H18 | 1.2628 | −0.0769 | 0.3838 | 0.062* |
| C19 | 1.4312 (3) | −0.2121 (3) | 0.0548 (3) | 0.0881 (9) |
| H19A | 1.3746 | −0.2793 | 0.0466 | 0.132* |
| H19B | 1.5009 | −0.2692 | 0.1228 | 0.132* |
| H19C | 1.4733 | −0.1510 | −0.0360 | 0.132* |
| C20 | 0.75196 (19) | 0.3076 (2) | 0.23806 (18) | 0.0390 (5) |
| C21 | 0.6419 (2) | 0.4113 (2) | 0.1751 (2) | 0.0459 (5) |
| H21 | 0.6573 | 0.5120 | 0.1181 | 0.055* |
| C22 | 0.5110 (2) | 0.3679 (2) | 0.1955 (2) | 0.0532 (6) |
| H22 | 0.4384 | 0.4392 | 0.1534 | 0.064* |
| C23 | 0.4868 (2) | 0.2191 (3) | 0.2783 (2) | 0.0602 (6) |
| H23 | 0.3979 | 0.1895 | 0.2937 | 0.072* |
| C24 | 0.5950 (3) | 0.1146 (3) | 0.3378 (3) | 0.0632 (6) |
| H24 | 0.5792 | 0.0136 | 0.3922 | 0.076* |
| C25 | 0.7258 (2) | 0.1575 (2) | 0.3180 (2) | 0.0523 (5) |
| H25 | 0.7980 | 0.0853 | 0.3587 | 0.063* |
1 Source of material
In a Schlenk tube 3-phenyl-1-(p-tolyl)prop-2-yn-1-one (66.1 mg, 0.30 mmol), 1,3-dihydro-2H-inden-2-one (79.3 mg, 0.60 mmol), Cs2CO3 (195.5 mg, 0.60 mmol) and toluene (3.0 mL) were stirred at 100 °C under N2 for 3 h. The reaction mixture was then quenched by water, and the water layers were extracted with ethyl acetate (10 mL × 3). The combined organic layers were washed with brine, dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure. Purification by chromatography on silica gel (petroleum ether/ethyl acetate = 5:1) afforded desired compound (yellow solid, 91.8 mg, 87 %). Single crystals were obtained by crystallization of the title compound from a mixture of dichloromethane (10 mL) and petroleum ether (2 mL). Melting point: 177–178 °C. 1H NMR (400 MHz, CDCl3) δ 2.39 (s, 3H), 3.52 (s, 2H), 4.80 (s, 2H), 6.38–6.35 (m, 1H), 6.92–6.87 (m, 1H), 7.46–7.13 (m, 9H), 7.91–7.88 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 206.0, 196.3, 145.3, 144.0, 142.5, 139.5, 137.7, 135.0, 133.7, 129.5, 129.4, 128.5, 128.5, 128.4, 127.1, 126.9, 125.1, 124.4, 46.0, 42.3, 21.3.
2 Experimental details
Using Olex2 [2], the structure was solved with the SHELXT [3] structure solution program and refined with the SHELXL [4] refinement package.
3 Comment
α,β-Unsaturated carbonyls that contain an electron-deficient carbon–carbon double bond are not only versatile synthetic intermediates but also a structural motif in biologically active molecules [5], [6], [7], [8]. In this paper we report the synthesis and crystal structure of a novel α,β-unsaturated carbonyl compound. In the crystal structure, there are two carbonyl groups and a carbon–carbon double bond. The two carbonyl groups are linked by carbon–carbon double bond. The carbon–carbon double bond length of C(7)–C(10) is 1.350(3) Å and this is typical for carbon–carbon double bond [9]. The bond lengths of the two carbonyl groups are almost the same (1.205 Å and 1.211 Å), which is similar to that reported [10].
The complete set of X-ray diffraction data for the title compound was deposited to the Cambridge Crystallographic Data Centre (CCDC entry no. 2295930).
-
Research funding: This work was supported by the Doctor Start-up Funds of Henan University of Science and Technology (no. 13480078).
-
Competing interests: The authors declare no conflicts of interest regarding this article.
References
1. BRUKER. SAINT, APEX2 and SADABS; Bruker AXS Inc.: Madison, Wisconsin, USA, 2009.Search in Google Scholar
2. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
3. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar PubMed PubMed Central
4. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
5. Muzart, J. One-pot syntheses of α,β-unsaturated carbonyl compounds through palladium-mediated dehydrogenation of ketones, aldehydes, esters, lactones and amides. Eur. J. Org Chem. 2010, 2010, 3779–3790; https://doi.org/10.1002/ejoc.201000278.Search in Google Scholar
6. Hayashi, T., Yamasaki, K. Rhodium-catalyzed asymmetric 1,4-addition and its related asymmetric reactions. Chem. Rev. 2003, 103, 2829–2844; https://doi.org/10.1021/cr020022z.Search in Google Scholar PubMed
7. Fang, Z., Ning, Y., Mi, P., Liao, P., Bi, X. Catalytic C–H α-trifluoromethylation of α,β-unsaturated carbonyl compounds. Org. Lett. 2014, 16, 1522–1525; https://doi.org/10.1021/ol5004498.Search in Google Scholar PubMed
8. Xiao, Q., Li, J., Wang, J. 1,4-Diazabicyclo[2.2.2]octane-promoted aminotrifluoromethylthiolation of α,β-unsaturated carbonyl compounds: N-trifluoromethylthio-4-nitrophthalimide acts as both the nitrogen and SCF3 sources. Org. Lett. 2015, 17, 6090–6093; https://doi.org/10.1021/acs.orglett.5b03116.Search in Google Scholar PubMed
9. Ghorab, M., Alsaid, M., Al-Dosari, M., Ghabbour, H. Crystal structure of the co-crystalline adduct 4-((4,4-dimethyl-2,6-dioxocyclohexylidene) methylamino)-N-(4,6-dimethylpyrimidin-2-yl)benzenesulfonamide – acetic acid (1/1), C21H24N4O4S·C2H4O2. Z. Kristallogr. N. Cryst. Struct. 2017, 232, 417–419; https://doi.org/10.1515/ncrs-2016-0295.Search in Google Scholar
10. Chen, Q.-W., Shen, D.-L. Crystal structure of 2-[(2-oxo-thiazolidine-3-carbonyl)sulfamoyl]-methy-benzoic acid methyl ester, C13H14N2O6S2. Z. Kristallogr. N. Cryst. Struct. 2017, 232, 67–68; https://doi.org/10.1515/ncrs-2016-0150.Search in Google Scholar
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of poly[diaqua-(μ4-3,3′-di(1H-1,2,4-triazol-1-yl)-[1,1′-biphenyl]-4,4′-dicarboxylate-N:N′:O:O′)cadmium(II)], C18H14N6O6Cd
- Crystal structure of (8R,8′S,13S,13′R)-8,8′-bis(hydroxymethyl)-9,9′,10,10′-tetramethoxy-5,5′,6,6′,8,8′,13,13′-octahydro-[13,13′-bi[1,3]dioxolo[4,5-g]isoquinolino[3,2-a]isoquinoline]-7,7′-diium chloride-methanol (1/2), C46H58N2O14Cl2
- The crystal structure of 8-methoxy-2,2-diphenyl-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C29H25BN2O3S
- Crystal structure of aqua-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N″,N‴)copper(II) 5-carboxyisophthalate tetrahydrate, C25H50N4CuO11
- The crystal structure of 1-(naphthalen-2-ylsulfonyl)-2,2-diphenyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ij]quinoline, C31H23BN2O2S
- Crystal structure of iodido-(η6-benzene) (1-(pyridin-2-yl)-N-(p-fluoro-methanamine)-κ2N,Nʹ)ruthenium(II) hexaflourophosphate, (C18H15F7IN2RuP)
- The crystal structure of 1-(3-oxo-1-phenyl-3-(p-tolyl) propylidene)-1,3-dihydro-2H-inden-2-one, C25H20O2
- Crystal structure of tricyclohexyl[4-(4H-1,2,4-triazol-4-yl)-benzoato-κO]tin(IV), C27H39N3O2Sn
- Crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ4N,O,O,O)cadmium(II)]monohydrate, C12H15NO9Cd
- Crystal structure of ethyl 2-((4-(3,5-dimethylisoxazol-4-yl)-2,6-difluorophenyl)amino)benzoate, C20H18F2N2O3
- The crystal structure of 2-(hydroxymethyl)-2-(4H-1,2,4-triazol-4-yl)propane-1,3-diol, C6H11N3O3
- The crystal structure of 1,2-bis(2,4-dinitrophenyl) hydrazine, C12H8N6O8
- Crystal structure of 1-(2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)-1,2-dihydro-4H-benzo[d][1,3]oxazin-4-one, C19H14Cl2N2O3
- The crystal structure of 5-amino-5-oxo-4-(1-oxo-4-(2-oxopyrrolidin-1-yl)isoindolin-2-yl)pentanoic acid, C17H19N3O5
- Crystal structure of N2,N6-bis(2-(((Z)-5-bromo-2-hydroxybenzylidene)amino) phenyl)pyridine-2,6-dicarboxamide, C33H23Br2N5O4
- The crystal structure of (E)-2-methoxy-6-(((5-methyl-1,3,4-thiadiazol-2-yl)imino)methyl)phenol, C11H11N3O2S
- The crystal structure of 3-((tert-butyldiphenylsilyl)methyl)-5,5-diphenyl-6-(p-tolyl) tetrahydro-2H-pyran-2-one, C41H42O2Si
- Crystal structure of 9-fluoro-4-(6-methoxypyridin-3-yl)-5,6-dihydrobenzo[h]quinazolin-2-amine, C18H15FN4O
- The crystal structure of 2-bromo-5-(4-cyanophenoxy)benzyl 1-methyl-1,2,5,6-tetrahydropyridine-3-carboxylate, C21H19BrN2O3
- Crystal structure of 3,3′-(1,4-phenylenebis(methylene))bis(1-isopropyl-1H-imidazol-3-ium) bis(hexafluorophosphate(V)), C10H14F6N2P
- The crystal structure of 2,2-di(thiophen-3-yl)-1-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C24H19BN2O2S3
- Crystal structure of 5-bromo-1-(2-iodobenzoyl)-1H-indole-3-carbaldehyde, C16H9BrINO2
- The crystal structure of monocarbonyl-2-carboxypyridinato-κ2N,O-triphenylphosphine-rhodium(I) acetonitrile solvate, C26H20.50N1.50O3PRh
- Crystal structure of dichlorido-tetrakis(1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ1N)manganese(II), C60H68O4N12Cl10Mn
- Crystal structure of 3-(tert-butyldiphenylsilyl)-1-(2,6-dichlorophenyl)-2,2-diphenylpropan-1-ol, C37H36Cl2OSi
- Crystal structure of langite from Mine du Pradet (France)
- The crystal structure of 5′-(furan-2-yl)-3′-((4-methylphenyl)sulfonamido)-3′,4′,5′,6′-tetrahydro-[1,1′:3′,1″-terphenyl]-4′-carboxylic acid, C30H27NO5S
- Synthesis and crystal structure of bis{2-(((4-acetophenone)imino)methyl)-4-fluorophenolato-κ2N,O}zinc(II), C30H22F2N2O4Zn
- The crystal structure of poly[(tripyridine-κ3N,N′,N″) μ3-(pyridine-3,4-dicarboxylate-κ3N:O:O′) manganese(II)], C22H22N4O8Mn
- The crystal structure of (E)-4-chloro-N′-(1-(4-hydroxyphenyl)propylidene)benzohydrazide, C16H15ClN2O2
- Synthesis and crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino) ethyl)phenyl)imino)methyl)phenolato-κ2N,O}cobalt(II), C40H46CoN4O4
- Crystal structure of tetraaqua-[(1-(carboxymethyl)-1H-pyrazole-3-carboxylato-κ2N,O)cobalt(II)], C6H12CoN2O8
- (6R,7S)-2,3,13-trimethoxy-6,7-dimethyl-5,6,7,8-tetrahydrobenzo[3′,4′]cycloocta [1′,2′:4,5]benzo[1,2-d][1,3]dioxol-1-ol, C22H26O6
- Crystal structure of 2-((2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)amino)benzoic acid, C18H14Cl2N2O3
- Crystal structure of (5aS,6aS,8aR,9R,11aS, 11bS,13R,13aS)-1,1,8a,11a-tetramethyl-9-((S)-1-((S)-5-methyl-6-oxo-3,6-dihydro-2H-pyran-2-yl)ethyl)-3-oxo-1,7,8,8a,9,10,11,11a,11b,12,13,13a-dodecahydro-3H,6H-cyclopenta[5,6]cyclopropa[1,8a]naphtho[2,1-c]oxepin-13-yl acetate, C32H44O6
- Crystal structure of catena-poly[triaqua-(μ2-1-(4-carboxylatophenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylato-O,O′:O″)cobalt(II)], C12H12N2O8Co
- Crystal structure of 3-[(furan-2-ylmethyl)-amino]-2-(2,3,4,5-tetrafluoro-benzoyl)-acrylic acid ethyl ester, C17H13F4NO4
- Crystal structure of methyl 4-(2-ethoxy-2-oxoethoxy)-3-methoxybenzoate, C13H16O6
- Crystal structure of 4-bromo-2-(4-chlorophenyl)-1-methyl-5-(trifluoromethyl)-1H-pyrrole-3-carbonitrile, C13H7BrClF3N2
- The crystal structure of triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ3N,O,O)nickel(II) monohydrate, C12H15NO9Ni
- Crystal structure of dihydroxy(2,4,6-triisopro-pylphenyl)telluronium trifluoromethanesulfonate, C16H25F3O5STe
- The crystal structure of 1-(carboxymethyl)-1H-imidazole 3-oxide
- The crystal structure of 1,3,5-tris(dibromomethyl)benzene, C9H6Br6
- Crystal structure of (Z)-3-(4-methoxyphenyl)-4-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-N-phenylthiazol-2(3H)-imine, C25H21N5OS
- Crystal structure of (Z)-3-(3-(4-hydroxyphenyl)-2-(phenylimino)-2,3-dihydrothiazol-4-yl)-2H-chromen-2-one, C24H16N2O3S
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of poly[diaqua-(μ4-3,3′-di(1H-1,2,4-triazol-1-yl)-[1,1′-biphenyl]-4,4′-dicarboxylate-N:N′:O:O′)cadmium(II)], C18H14N6O6Cd
- Crystal structure of (8R,8′S,13S,13′R)-8,8′-bis(hydroxymethyl)-9,9′,10,10′-tetramethoxy-5,5′,6,6′,8,8′,13,13′-octahydro-[13,13′-bi[1,3]dioxolo[4,5-g]isoquinolino[3,2-a]isoquinoline]-7,7′-diium chloride-methanol (1/2), C46H58N2O14Cl2
- The crystal structure of 8-methoxy-2,2-diphenyl-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C29H25BN2O3S
- Crystal structure of aqua-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N″,N‴)copper(II) 5-carboxyisophthalate tetrahydrate, C25H50N4CuO11
- The crystal structure of 1-(naphthalen-2-ylsulfonyl)-2,2-diphenyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ij]quinoline, C31H23BN2O2S
- Crystal structure of iodido-(η6-benzene) (1-(pyridin-2-yl)-N-(p-fluoro-methanamine)-κ2N,Nʹ)ruthenium(II) hexaflourophosphate, (C18H15F7IN2RuP)
- The crystal structure of 1-(3-oxo-1-phenyl-3-(p-tolyl) propylidene)-1,3-dihydro-2H-inden-2-one, C25H20O2
- Crystal structure of tricyclohexyl[4-(4H-1,2,4-triazol-4-yl)-benzoato-κO]tin(IV), C27H39N3O2Sn
- Crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ4N,O,O,O)cadmium(II)]monohydrate, C12H15NO9Cd
- Crystal structure of ethyl 2-((4-(3,5-dimethylisoxazol-4-yl)-2,6-difluorophenyl)amino)benzoate, C20H18F2N2O3
- The crystal structure of 2-(hydroxymethyl)-2-(4H-1,2,4-triazol-4-yl)propane-1,3-diol, C6H11N3O3
- The crystal structure of 1,2-bis(2,4-dinitrophenyl) hydrazine, C12H8N6O8
- Crystal structure of 1-(2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)-1,2-dihydro-4H-benzo[d][1,3]oxazin-4-one, C19H14Cl2N2O3
- The crystal structure of 5-amino-5-oxo-4-(1-oxo-4-(2-oxopyrrolidin-1-yl)isoindolin-2-yl)pentanoic acid, C17H19N3O5
- Crystal structure of N2,N6-bis(2-(((Z)-5-bromo-2-hydroxybenzylidene)amino) phenyl)pyridine-2,6-dicarboxamide, C33H23Br2N5O4
- The crystal structure of (E)-2-methoxy-6-(((5-methyl-1,3,4-thiadiazol-2-yl)imino)methyl)phenol, C11H11N3O2S
- The crystal structure of 3-((tert-butyldiphenylsilyl)methyl)-5,5-diphenyl-6-(p-tolyl) tetrahydro-2H-pyran-2-one, C41H42O2Si
- Crystal structure of 9-fluoro-4-(6-methoxypyridin-3-yl)-5,6-dihydrobenzo[h]quinazolin-2-amine, C18H15FN4O
- The crystal structure of 2-bromo-5-(4-cyanophenoxy)benzyl 1-methyl-1,2,5,6-tetrahydropyridine-3-carboxylate, C21H19BrN2O3
- Crystal structure of 3,3′-(1,4-phenylenebis(methylene))bis(1-isopropyl-1H-imidazol-3-ium) bis(hexafluorophosphate(V)), C10H14F6N2P
- The crystal structure of 2,2-di(thiophen-3-yl)-1-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C24H19BN2O2S3
- Crystal structure of 5-bromo-1-(2-iodobenzoyl)-1H-indole-3-carbaldehyde, C16H9BrINO2
- The crystal structure of monocarbonyl-2-carboxypyridinato-κ2N,O-triphenylphosphine-rhodium(I) acetonitrile solvate, C26H20.50N1.50O3PRh
- Crystal structure of dichlorido-tetrakis(1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ1N)manganese(II), C60H68O4N12Cl10Mn
- Crystal structure of 3-(tert-butyldiphenylsilyl)-1-(2,6-dichlorophenyl)-2,2-diphenylpropan-1-ol, C37H36Cl2OSi
- Crystal structure of langite from Mine du Pradet (France)
- The crystal structure of 5′-(furan-2-yl)-3′-((4-methylphenyl)sulfonamido)-3′,4′,5′,6′-tetrahydro-[1,1′:3′,1″-terphenyl]-4′-carboxylic acid, C30H27NO5S
- Synthesis and crystal structure of bis{2-(((4-acetophenone)imino)methyl)-4-fluorophenolato-κ2N,O}zinc(II), C30H22F2N2O4Zn
- The crystal structure of poly[(tripyridine-κ3N,N′,N″) μ3-(pyridine-3,4-dicarboxylate-κ3N:O:O′) manganese(II)], C22H22N4O8Mn
- The crystal structure of (E)-4-chloro-N′-(1-(4-hydroxyphenyl)propylidene)benzohydrazide, C16H15ClN2O2
- Synthesis and crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino) ethyl)phenyl)imino)methyl)phenolato-κ2N,O}cobalt(II), C40H46CoN4O4
- Crystal structure of tetraaqua-[(1-(carboxymethyl)-1H-pyrazole-3-carboxylato-κ2N,O)cobalt(II)], C6H12CoN2O8
- (6R,7S)-2,3,13-trimethoxy-6,7-dimethyl-5,6,7,8-tetrahydrobenzo[3′,4′]cycloocta [1′,2′:4,5]benzo[1,2-d][1,3]dioxol-1-ol, C22H26O6
- Crystal structure of 2-((2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)amino)benzoic acid, C18H14Cl2N2O3
- Crystal structure of (5aS,6aS,8aR,9R,11aS, 11bS,13R,13aS)-1,1,8a,11a-tetramethyl-9-((S)-1-((S)-5-methyl-6-oxo-3,6-dihydro-2H-pyran-2-yl)ethyl)-3-oxo-1,7,8,8a,9,10,11,11a,11b,12,13,13a-dodecahydro-3H,6H-cyclopenta[5,6]cyclopropa[1,8a]naphtho[2,1-c]oxepin-13-yl acetate, C32H44O6
- Crystal structure of catena-poly[triaqua-(μ2-1-(4-carboxylatophenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylato-O,O′:O″)cobalt(II)], C12H12N2O8Co
- Crystal structure of 3-[(furan-2-ylmethyl)-amino]-2-(2,3,4,5-tetrafluoro-benzoyl)-acrylic acid ethyl ester, C17H13F4NO4
- Crystal structure of methyl 4-(2-ethoxy-2-oxoethoxy)-3-methoxybenzoate, C13H16O6
- Crystal structure of 4-bromo-2-(4-chlorophenyl)-1-methyl-5-(trifluoromethyl)-1H-pyrrole-3-carbonitrile, C13H7BrClF3N2
- The crystal structure of triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ3N,O,O)nickel(II) monohydrate, C12H15NO9Ni
- Crystal structure of dihydroxy(2,4,6-triisopro-pylphenyl)telluronium trifluoromethanesulfonate, C16H25F3O5STe
- The crystal structure of 1-(carboxymethyl)-1H-imidazole 3-oxide
- The crystal structure of 1,3,5-tris(dibromomethyl)benzene, C9H6Br6
- Crystal structure of (Z)-3-(4-methoxyphenyl)-4-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-N-phenylthiazol-2(3H)-imine, C25H21N5OS
- Crystal structure of (Z)-3-(3-(4-hydroxyphenyl)-2-(phenylimino)-2,3-dihydrothiazol-4-yl)-2H-chromen-2-one, C24H16N2O3S

