Abstract
C29H25BN2O3S, monoclinic, P21/c (no. 14), a = 14.4345(6) Å, b = 9.7073(5) Å, c = 18.3873(8) Å, β = 107.766°, V = 2453.56(19) Å3, Z = 4, Rgt(F) = 0.0587, wRref(F2) = 0.1592, T = 193 K.
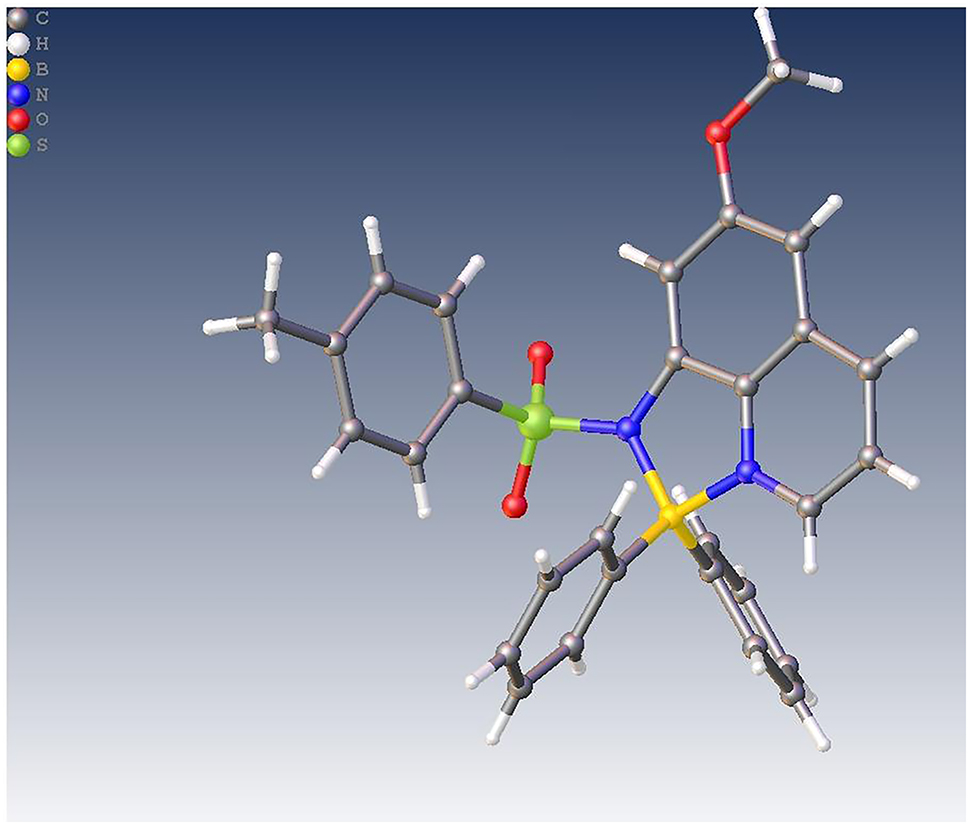
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.20 × 0.15 × 0.14 mm |
| Wavelength: | MoKα radiation (0.71073 Å) |
| μ: | 0.17 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| θmax, completeness: | 29.9°, 99 % |
| N(hkl)measured, N(hkl)unique, Rint: | 26,032, 7065, 0.076 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2σ(Iobs), 5174 |
| N(param)refined: | 327 |
| Programs: | Bruker [1], Shelx [2, 4, 5], Olex2 [3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| S1 | 0.25565 (3) | 0.88064 (5) | 0.56661 (3) | 0.02659 (12) |
| O1 | 0.18385 (10) | 0.85533 (16) | 0.49422 (8) | 0.0343 (3) |
| O2 | 0.11524 (12) | 0.36235 (16) | 0.48890 (8) | 0.0438 (4) |
| O3 | 0.26754 (10) | 1.01731 (14) | 0.59740 (8) | 0.0337 (3) |
| N1 | 0.22866 (10) | 0.67298 (17) | 0.74577 (8) | 0.0257 (3) |
| N2 | 0.23168 (11) | 0.78332 (16) | 0.63043 (8) | 0.0264 (3) |
| C1 | 0.20195 (12) | 0.5838 (2) | 0.68620 (10) | 0.0252 (4) |
| C2 | 0.16825 (15) | 0.9725 (2) | 0.80142 (12) | 0.0360 (4) |
| H2 | 0.219312 | 0.942358 | 0.844619 | 0.043* |
| C3 | 0.54709 (15) | 0.7575 (2) | 0.53841 (12) | 0.0366 (5) |
| C4 | 0.20066 (13) | 0.64640 (19) | 0.61619 (10) | 0.0267 (4) |
| C5 | 0.02071 (14) | 1.0667 (2) | 0.67797 (13) | 0.0377 (5) |
| H5 | −0.029726 | 1.099224 | 0.635027 | 0.045* |
| C6 | 0.36110 (13) | 0.8641 (2) | 0.76375 (10) | 0.0276 (4) |
| C7 | 0.64428 (16) | 0.7189 (3) | 0.52936 (15) | 0.0502 (6) |
| H7A | 0.638489 | 0.630615 | 0.502523 | 0.075* |
| H7B | 0.692673 | 0.710828 | 0.579852 | 0.075* |
| H7C | 0.664762 | 0.790316 | 0.499827 | 0.075* |
| C8 | 0.36856 (13) | 0.8293 (2) | 0.55706 (10) | 0.0281 (4) |
| C9 | 0.14494 (15) | 0.4274 (2) | 0.55769 (11) | 0.0323 (4) |
| C10 | 0.16765 (13) | 0.9288 (2) | 0.72878 (11) | 0.0281 (4) |
| C11 | 0.55795 (15) | 0.9286 (2) | 0.83610 (12) | 0.0374 (5) |
| H11 | 0.624416 | 0.950915 | 0.859876 | 0.045* |
| C12 | 0.17854 (13) | 0.4456 (2) | 0.69296 (11) | 0.0283 (4) |
| C13 | 0.08016 (19) | 0.2237 (2) | 0.48676 (14) | 0.0473 (6) |
| H13A | 0.061148 | 0.189698 | 0.434080 | 0.071* |
| H13B | 0.023690 | 0.221780 | 0.505755 | 0.071* |
| H13C | 0.131659 | 0.164947 | 0.518982 | 0.071* |
| C14 | 0.09128 (13) | 0.9784 (2) | 0.66746 (11) | 0.0319 (4) |
| H14 | 0.087598 | 0.950794 | 0.617082 | 0.038* |
| C15 | 0.21256 (13) | 0.4926 (2) | 0.82749 (11) | 0.0318 (4) |
| H15 | 0.216591 | 0.462716 | 0.877563 | 0.038* |
| C16 | 0.18560 (14) | 0.4007 (2) | 0.76779 (11) | 0.0326 (4) |
| H16 | 0.171794 | 0.307596 | 0.776674 | 0.039* |
| C17 | 0.46201 (15) | 0.6924 (2) | 0.49596 (11) | 0.0348 (4) |
| H17 | 0.464798 | 0.623441 | 0.460040 | 0.042* |
| C18 | 0.43366 (13) | 0.7625 (2) | 0.78185 (11) | 0.0327 (4) |
| H18 | 0.415589 | 0.669227 | 0.769582 | 0.039* |
| C19 | 0.45249 (15) | 0.8952 (2) | 0.60006 (13) | 0.0375 (5) |
| H19 | 0.449588 | 0.964189 | 0.635964 | 0.045* |
| C20 | 0.37326 (14) | 0.7268 (2) | 0.50531 (10) | 0.0308 (4) |
| H20 | 0.315740 | 0.680550 | 0.476530 | 0.037* |
| C21 | 0.02359 (15) | 1.1073 (2) | 0.75067 (13) | 0.0399 (5) |
| H21 | −0.024273 | 1.168366 | 0.757947 | 0.048* |
| C22 | 0.17028 (14) | 0.5672 (2) | 0.55136 (11) | 0.0298 (4) |
| H22 | 0.166271 | 0.605489 | 0.502935 | 0.036* |
| C23 | 0.54064 (15) | 0.8594 (3) | 0.59010 (14) | 0.0423 (5) |
| H23 | 0.598123 | 0.905393 | 0.619162 | 0.051* |
| C24 | 0.39133 (15) | 0.9992 (2) | 0.78451 (11) | 0.0323 (4) |
| H24 | 0.344414 | 1.071054 | 0.773699 | 0.039* |
| C25 | 0.53079 (14) | 0.7935 (2) | 0.81701 (12) | 0.0379 (5) |
| H25 | 0.578339 | 0.722455 | 0.827920 | 0.045* |
| C26 | 0.48829 (15) | 1.0308 (2) | 0.82053 (11) | 0.0364 (5) |
| H26 | 0.506773 | 1.123328 | 0.834543 | 0.044* |
| C27 | 0.09685 (16) | 1.0582 (2) | 0.81259 (13) | 0.0399 (5) |
| H27 | 0.098382 | 1.083188 | 0.862907 | 0.048* |
| C28 | 0.14961 (15) | 0.3649 (2) | 0.62579 (11) | 0.0338 (4) |
| H28 | 0.133868 | 0.270198 | 0.627685 | 0.041* |
| C29 | 0.23401 (13) | 0.6289 (2) | 0.81538 (11) | 0.0299 (4) |
| H29 | 0.252643 | 0.690964 | 0.857264 | 0.036* |
| B1 | 0.24958 (15) | 0.8251 (2) | 0.71844 (12) | 0.0262 (4) |
1 Source of materials
The title complex was synthesized from a mixture of 6-methoxyl-quinolin-8-amine, 4-methylbenzenesulfonyl chloride and potassium trifluoro(phenyl)-λ4-borane suspended in MeCN under air atmosphere at 130 °C for 24 h. Ater the completion of this reaction, the resulting residue was adsorbed onto silica gel by rotary evaporation of a DCM solution, loaded directly onto a silica gel column, and purified by rotary evaporation by eluting first with EtOAc:PE 1:5 to get the target product as yellow solid. Crystals of this target product formed and were obtained in a mixed system of n-hexane and dichloromethane (DCM as the good solvent and n-hexane as the poor solvent).
2 Experimental details
All the H atoms on the benzene rings were placed geometrically and refined without any constraints or restraints.
3 Comment
Four-coordinated organoboron complexes have been widely used as organic fluorescent materials, but the synthesis of N,N-chelated B,B-diaryl tetra-coordinated boron complexes is greatly limited due to the use of organometallic reagents which are extremely sensitive to air and water. A one-pot three-component synthetic strategy of N,N-chelated-B,B-diaryl tetra-coordinated boron complexes is reported. 8-Aminoquinoline is used as the precursor of N,N-chelated ligands, and potassium aryltrifluoroborates (ArBF3K), which are chemically stable and available in the market, are used as the source of diaryl groups on the boron centers. A series of these compounds are obtained in moderate to excellent yields. This reaction route has good substrate applicability and functional group compatibility, which provides a convenient and efficient reaction route for the synthesis of diarylboron complexes. The compound mentioned in this article was synthesized through this route in an excellent yield.
The integration of diffraction data and intensity corrections for the Lorentz and polarization effects were performed by using Saint program. Semi-empirical absorption corrections were applied using Sadabs program. All the hydrogen atoms were introduced at the calculated positions.
The asymmetric unit contains one title molecule. As exhibited in Figure, the central boron atom B1 is connected to two nitrogen atoms (N1, N2) from the quinoline unit and two carbon atoms (C6, C10) from two different benzene units to form a typical tetrahedral geometry. The B–N and B–C bond lengths range from 1.608(3) to 1.618(3) Å and the C/N–B–C/N bond angles are in the range of 95.42(13)°–116.42(16)°, which are comparable to some previously reported boron-containing compounds [5], [6], [7], [8], [9]. Due to the tetrahedral geometry of central B atom, the dihedral angles among the two benzene rings connected to the B atom and the quinoline unit are 64.50°, 68.44° and 75.98°, respectively. Moreover, the presence of the sulfanilamide group causes the methylbenzene unit closely perpendicular to the quinoline unit with the dihedral angles of 78.63° and the C–H⋯π interactions between the methylbenzene unit and one of the benzene rings leads to their dihedral angle of as low as 39.34°. In addition, the molecules are assembled by the intermolecular π⋯π and C–H⋯π interactions among the quinoline units, benzene rings and methylbenzene units and C–H⋯O hydrogen bonds between the sulfanilamide oxygen atom and the methoxy group into two-dimensional (2D) supramolecular layers, which are further joined together by the C–H⋯π interactions between the benzene rings and the methylbenzene units to form the final three-dimensional (3D) supramolecular structure.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This project was funded by Xijing University Special Fund for Talent Research in Special Zone (XJ22T03).
References
1. SAINT. Program for Data Extraction and Reduction; Bruker AXS, Inc: Madison, WI, 2001.Search in Google Scholar
2. Sheldrick, G. M. SADABS, Program for Empirical Adsorption Correction of Area Detector Data; University of Göttingen: Germany, 2003.Search in Google Scholar
3. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
4. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
5. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar PubMed PubMed Central
6. Rodrigues, A. I., Figueira, C. A., Gomes, C. S. B., Suresh, D., Ferreira, B., Di Paolo, R. E., Pereira, D. S., Dias, F. B., Calhorda, M. J., Morgado, J., Maçanita, A. L., Gomes, P. T. Boron complexes of aromatic 5-substituted iminopyrrolyl ligands: synthesis, structure, and luminescence properties. Dalton Trans. 2019, 48, 13337–13352; https://doi.org/10.1039/c9dt02718a.Search in Google Scholar PubMed
7. Nagata, Y., Chujo, Y. Main-chain-type N,N-chelate organoboron aminoquinolate polymers: synthesis, luminescence, and energy transfer behavior. Macromolecules 2008, 41, 3488–3492; https://doi.org/10.1021/ma702873a.Search in Google Scholar
8. Más-Montoya, M., Usea, L., Espinosa Ferao, A., Montenegro, M. F., Arellano, C. R., Tárraga, A., Rodríguez-López, J. N., Curiel, D. Single heteroatom fine-tuning of the emissive properties in organoboron complexes with 7-(azaheteroaryl)indole systems. J. Org. Chem. 2016, 81, 3296–3302; https://doi.org/10.1021/acs.joc.6b00265.Search in Google Scholar PubMed
9. Ding, S. The crystal structure of 2,2-bis(3-methoxyphenyl)-1-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ij]quinoline-dichloromethane (1/1). Z. Kristallogr. N. Cryst. Struct. 2023, 238, 767–769; https://doi.org/10.1515/ncrs-2023-0200.Search in Google Scholar
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of poly[diaqua-(μ4-3,3′-di(1H-1,2,4-triazol-1-yl)-[1,1′-biphenyl]-4,4′-dicarboxylate-N:N′:O:O′)cadmium(II)], C18H14N6O6Cd
- Crystal structure of (8R,8′S,13S,13′R)-8,8′-bis(hydroxymethyl)-9,9′,10,10′-tetramethoxy-5,5′,6,6′,8,8′,13,13′-octahydro-[13,13′-bi[1,3]dioxolo[4,5-g]isoquinolino[3,2-a]isoquinoline]-7,7′-diium chloride-methanol (1/2), C46H58N2O14Cl2
- The crystal structure of 8-methoxy-2,2-diphenyl-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C29H25BN2O3S
- Crystal structure of aqua-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N″,N‴)copper(II) 5-carboxyisophthalate tetrahydrate, C25H50N4CuO11
- The crystal structure of 1-(naphthalen-2-ylsulfonyl)-2,2-diphenyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ij]quinoline, C31H23BN2O2S
- Crystal structure of iodido-(η6-benzene) (1-(pyridin-2-yl)-N-(p-fluoro-methanamine)-κ2N,Nʹ)ruthenium(II) hexaflourophosphate, (C18H15F7IN2RuP)
- The crystal structure of 1-(3-oxo-1-phenyl-3-(p-tolyl) propylidene)-1,3-dihydro-2H-inden-2-one, C25H20O2
- Crystal structure of tricyclohexyl[4-(4H-1,2,4-triazol-4-yl)-benzoato-κO]tin(IV), C27H39N3O2Sn
- Crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ4N,O,O,O)cadmium(II)]monohydrate, C12H15NO9Cd
- Crystal structure of ethyl 2-((4-(3,5-dimethylisoxazol-4-yl)-2,6-difluorophenyl)amino)benzoate, C20H18F2N2O3
- The crystal structure of 2-(hydroxymethyl)-2-(4H-1,2,4-triazol-4-yl)propane-1,3-diol, C6H11N3O3
- The crystal structure of 1,2-bis(2,4-dinitrophenyl) hydrazine, C12H8N6O8
- Crystal structure of 1-(2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)-1,2-dihydro-4H-benzo[d][1,3]oxazin-4-one, C19H14Cl2N2O3
- The crystal structure of 5-amino-5-oxo-4-(1-oxo-4-(2-oxopyrrolidin-1-yl)isoindolin-2-yl)pentanoic acid, C17H19N3O5
- Crystal structure of N2,N6-bis(2-(((Z)-5-bromo-2-hydroxybenzylidene)amino) phenyl)pyridine-2,6-dicarboxamide, C33H23Br2N5O4
- The crystal structure of (E)-2-methoxy-6-(((5-methyl-1,3,4-thiadiazol-2-yl)imino)methyl)phenol, C11H11N3O2S
- The crystal structure of 3-((tert-butyldiphenylsilyl)methyl)-5,5-diphenyl-6-(p-tolyl) tetrahydro-2H-pyran-2-one, C41H42O2Si
- Crystal structure of 9-fluoro-4-(6-methoxypyridin-3-yl)-5,6-dihydrobenzo[h]quinazolin-2-amine, C18H15FN4O
- The crystal structure of 2-bromo-5-(4-cyanophenoxy)benzyl 1-methyl-1,2,5,6-tetrahydropyridine-3-carboxylate, C21H19BrN2O3
- Crystal structure of 3,3′-(1,4-phenylenebis(methylene))bis(1-isopropyl-1H-imidazol-3-ium) bis(hexafluorophosphate(V)), C10H14F6N2P
- The crystal structure of 2,2-di(thiophen-3-yl)-1-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C24H19BN2O2S3
- Crystal structure of 5-bromo-1-(2-iodobenzoyl)-1H-indole-3-carbaldehyde, C16H9BrINO2
- The crystal structure of monocarbonyl-2-carboxypyridinato-κ2N,O-triphenylphosphine-rhodium(I) acetonitrile solvate, C26H20.50N1.50O3PRh
- Crystal structure of dichlorido-tetrakis(1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ1N)manganese(II), C60H68O4N12Cl10Mn
- Crystal structure of 3-(tert-butyldiphenylsilyl)-1-(2,6-dichlorophenyl)-2,2-diphenylpropan-1-ol, C37H36Cl2OSi
- Crystal structure of langite from Mine du Pradet (France)
- The crystal structure of 5′-(furan-2-yl)-3′-((4-methylphenyl)sulfonamido)-3′,4′,5′,6′-tetrahydro-[1,1′:3′,1″-terphenyl]-4′-carboxylic acid, C30H27NO5S
- Synthesis and crystal structure of bis{2-(((4-acetophenone)imino)methyl)-4-fluorophenolato-κ2N,O}zinc(II), C30H22F2N2O4Zn
- The crystal structure of poly[(tripyridine-κ3N,N′,N″) μ3-(pyridine-3,4-dicarboxylate-κ3N:O:O′) manganese(II)], C22H22N4O8Mn
- The crystal structure of (E)-4-chloro-N′-(1-(4-hydroxyphenyl)propylidene)benzohydrazide, C16H15ClN2O2
- Synthesis and crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino) ethyl)phenyl)imino)methyl)phenolato-κ2N,O}cobalt(II), C40H46CoN4O4
- Crystal structure of tetraaqua-[(1-(carboxymethyl)-1H-pyrazole-3-carboxylato-κ2N,O)cobalt(II)], C6H12CoN2O8
- (6R,7S)-2,3,13-trimethoxy-6,7-dimethyl-5,6,7,8-tetrahydrobenzo[3′,4′]cycloocta [1′,2′:4,5]benzo[1,2-d][1,3]dioxol-1-ol, C22H26O6
- Crystal structure of 2-((2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)amino)benzoic acid, C18H14Cl2N2O3
- Crystal structure of (5aS,6aS,8aR,9R,11aS, 11bS,13R,13aS)-1,1,8a,11a-tetramethyl-9-((S)-1-((S)-5-methyl-6-oxo-3,6-dihydro-2H-pyran-2-yl)ethyl)-3-oxo-1,7,8,8a,9,10,11,11a,11b,12,13,13a-dodecahydro-3H,6H-cyclopenta[5,6]cyclopropa[1,8a]naphtho[2,1-c]oxepin-13-yl acetate, C32H44O6
- Crystal structure of catena-poly[triaqua-(μ2-1-(4-carboxylatophenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylato-O,O′:O″)cobalt(II)], C12H12N2O8Co
- Crystal structure of 3-[(furan-2-ylmethyl)-amino]-2-(2,3,4,5-tetrafluoro-benzoyl)-acrylic acid ethyl ester, C17H13F4NO4
- Crystal structure of methyl 4-(2-ethoxy-2-oxoethoxy)-3-methoxybenzoate, C13H16O6
- Crystal structure of 4-bromo-2-(4-chlorophenyl)-1-methyl-5-(trifluoromethyl)-1H-pyrrole-3-carbonitrile, C13H7BrClF3N2
- The crystal structure of triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ3N,O,O)nickel(II) monohydrate, C12H15NO9Ni
- Crystal structure of dihydroxy(2,4,6-triisopro-pylphenyl)telluronium trifluoromethanesulfonate, C16H25F3O5STe
- The crystal structure of 1-(carboxymethyl)-1H-imidazole 3-oxide
- The crystal structure of 1,3,5-tris(dibromomethyl)benzene, C9H6Br6
- Crystal structure of (Z)-3-(4-methoxyphenyl)-4-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-N-phenylthiazol-2(3H)-imine, C25H21N5OS
- Crystal structure of (Z)-3-(3-(4-hydroxyphenyl)-2-(phenylimino)-2,3-dihydrothiazol-4-yl)-2H-chromen-2-one, C24H16N2O3S
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of poly[diaqua-(μ4-3,3′-di(1H-1,2,4-triazol-1-yl)-[1,1′-biphenyl]-4,4′-dicarboxylate-N:N′:O:O′)cadmium(II)], C18H14N6O6Cd
- Crystal structure of (8R,8′S,13S,13′R)-8,8′-bis(hydroxymethyl)-9,9′,10,10′-tetramethoxy-5,5′,6,6′,8,8′,13,13′-octahydro-[13,13′-bi[1,3]dioxolo[4,5-g]isoquinolino[3,2-a]isoquinoline]-7,7′-diium chloride-methanol (1/2), C46H58N2O14Cl2
- The crystal structure of 8-methoxy-2,2-diphenyl-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C29H25BN2O3S
- Crystal structure of aqua-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N″,N‴)copper(II) 5-carboxyisophthalate tetrahydrate, C25H50N4CuO11
- The crystal structure of 1-(naphthalen-2-ylsulfonyl)-2,2-diphenyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ij]quinoline, C31H23BN2O2S
- Crystal structure of iodido-(η6-benzene) (1-(pyridin-2-yl)-N-(p-fluoro-methanamine)-κ2N,Nʹ)ruthenium(II) hexaflourophosphate, (C18H15F7IN2RuP)
- The crystal structure of 1-(3-oxo-1-phenyl-3-(p-tolyl) propylidene)-1,3-dihydro-2H-inden-2-one, C25H20O2
- Crystal structure of tricyclohexyl[4-(4H-1,2,4-triazol-4-yl)-benzoato-κO]tin(IV), C27H39N3O2Sn
- Crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ4N,O,O,O)cadmium(II)]monohydrate, C12H15NO9Cd
- Crystal structure of ethyl 2-((4-(3,5-dimethylisoxazol-4-yl)-2,6-difluorophenyl)amino)benzoate, C20H18F2N2O3
- The crystal structure of 2-(hydroxymethyl)-2-(4H-1,2,4-triazol-4-yl)propane-1,3-diol, C6H11N3O3
- The crystal structure of 1,2-bis(2,4-dinitrophenyl) hydrazine, C12H8N6O8
- Crystal structure of 1-(2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)-1,2-dihydro-4H-benzo[d][1,3]oxazin-4-one, C19H14Cl2N2O3
- The crystal structure of 5-amino-5-oxo-4-(1-oxo-4-(2-oxopyrrolidin-1-yl)isoindolin-2-yl)pentanoic acid, C17H19N3O5
- Crystal structure of N2,N6-bis(2-(((Z)-5-bromo-2-hydroxybenzylidene)amino) phenyl)pyridine-2,6-dicarboxamide, C33H23Br2N5O4
- The crystal structure of (E)-2-methoxy-6-(((5-methyl-1,3,4-thiadiazol-2-yl)imino)methyl)phenol, C11H11N3O2S
- The crystal structure of 3-((tert-butyldiphenylsilyl)methyl)-5,5-diphenyl-6-(p-tolyl) tetrahydro-2H-pyran-2-one, C41H42O2Si
- Crystal structure of 9-fluoro-4-(6-methoxypyridin-3-yl)-5,6-dihydrobenzo[h]quinazolin-2-amine, C18H15FN4O
- The crystal structure of 2-bromo-5-(4-cyanophenoxy)benzyl 1-methyl-1,2,5,6-tetrahydropyridine-3-carboxylate, C21H19BrN2O3
- Crystal structure of 3,3′-(1,4-phenylenebis(methylene))bis(1-isopropyl-1H-imidazol-3-ium) bis(hexafluorophosphate(V)), C10H14F6N2P
- The crystal structure of 2,2-di(thiophen-3-yl)-1-tosyl-1,2-dihydro-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ig]quinoline, C24H19BN2O2S3
- Crystal structure of 5-bromo-1-(2-iodobenzoyl)-1H-indole-3-carbaldehyde, C16H9BrINO2
- The crystal structure of monocarbonyl-2-carboxypyridinato-κ2N,O-triphenylphosphine-rhodium(I) acetonitrile solvate, C26H20.50N1.50O3PRh
- Crystal structure of dichlorido-tetrakis(1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ1N)manganese(II), C60H68O4N12Cl10Mn
- Crystal structure of 3-(tert-butyldiphenylsilyl)-1-(2,6-dichlorophenyl)-2,2-diphenylpropan-1-ol, C37H36Cl2OSi
- Crystal structure of langite from Mine du Pradet (France)
- The crystal structure of 5′-(furan-2-yl)-3′-((4-methylphenyl)sulfonamido)-3′,4′,5′,6′-tetrahydro-[1,1′:3′,1″-terphenyl]-4′-carboxylic acid, C30H27NO5S
- Synthesis and crystal structure of bis{2-(((4-acetophenone)imino)methyl)-4-fluorophenolato-κ2N,O}zinc(II), C30H22F2N2O4Zn
- The crystal structure of poly[(tripyridine-κ3N,N′,N″) μ3-(pyridine-3,4-dicarboxylate-κ3N:O:O′) manganese(II)], C22H22N4O8Mn
- The crystal structure of (E)-4-chloro-N′-(1-(4-hydroxyphenyl)propylidene)benzohydrazide, C16H15ClN2O2
- Synthesis and crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino) ethyl)phenyl)imino)methyl)phenolato-κ2N,O}cobalt(II), C40H46CoN4O4
- Crystal structure of tetraaqua-[(1-(carboxymethyl)-1H-pyrazole-3-carboxylato-κ2N,O)cobalt(II)], C6H12CoN2O8
- (6R,7S)-2,3,13-trimethoxy-6,7-dimethyl-5,6,7,8-tetrahydrobenzo[3′,4′]cycloocta [1′,2′:4,5]benzo[1,2-d][1,3]dioxol-1-ol, C22H26O6
- Crystal structure of 2-((2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)amino)benzoic acid, C18H14Cl2N2O3
- Crystal structure of (5aS,6aS,8aR,9R,11aS, 11bS,13R,13aS)-1,1,8a,11a-tetramethyl-9-((S)-1-((S)-5-methyl-6-oxo-3,6-dihydro-2H-pyran-2-yl)ethyl)-3-oxo-1,7,8,8a,9,10,11,11a,11b,12,13,13a-dodecahydro-3H,6H-cyclopenta[5,6]cyclopropa[1,8a]naphtho[2,1-c]oxepin-13-yl acetate, C32H44O6
- Crystal structure of catena-poly[triaqua-(μ2-1-(4-carboxylatophenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylato-O,O′:O″)cobalt(II)], C12H12N2O8Co
- Crystal structure of 3-[(furan-2-ylmethyl)-amino]-2-(2,3,4,5-tetrafluoro-benzoyl)-acrylic acid ethyl ester, C17H13F4NO4
- Crystal structure of methyl 4-(2-ethoxy-2-oxoethoxy)-3-methoxybenzoate, C13H16O6
- Crystal structure of 4-bromo-2-(4-chlorophenyl)-1-methyl-5-(trifluoromethyl)-1H-pyrrole-3-carbonitrile, C13H7BrClF3N2
- The crystal structure of triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ3N,O,O)nickel(II) monohydrate, C12H15NO9Ni
- Crystal structure of dihydroxy(2,4,6-triisopro-pylphenyl)telluronium trifluoromethanesulfonate, C16H25F3O5STe
- The crystal structure of 1-(carboxymethyl)-1H-imidazole 3-oxide
- The crystal structure of 1,3,5-tris(dibromomethyl)benzene, C9H6Br6
- Crystal structure of (Z)-3-(4-methoxyphenyl)-4-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-N-phenylthiazol-2(3H)-imine, C25H21N5OS
- Crystal structure of (Z)-3-(3-(4-hydroxyphenyl)-2-(phenylimino)-2,3-dihydrothiazol-4-yl)-2H-chromen-2-one, C24H16N2O3S

