Abstract
C17H12F2O1, monoclinic,
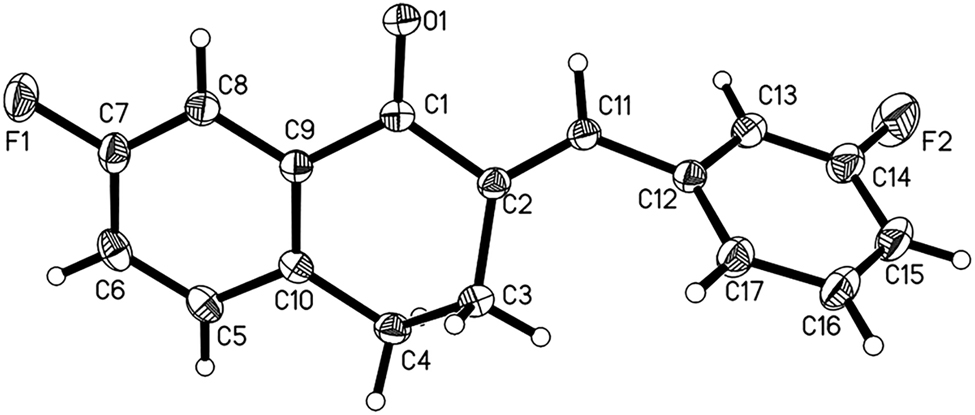
The crystal structure is shown in the figure. Displacement ellipsoids are drawn at the 35% probability level. Tables 1 and 2 contain details on crystal structure and measurement conditions and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Yellow block |
| Size: | 0.14 × 0.11 × 0.10 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.10 mm−1 |
| Diffractometer, scan mode: | Xcalibur, CCD plate scans |
| θmax, completeness: | 25.5°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 12423, 2433, 0.024 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 1891 |
| N(param)refined: | 182 |
| Programs: | CrysAlisPRO [1], Shelx [2, 3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| x | y | z | Uiso*/Ueq | |
|---|---|---|---|---|
| F1 | 0.29139 (17) | 0.57875 (14) | −0.24594 (9) | 0.0833 (4) |
| F2 | −0.1989 (2) | −0.2361 (2) | 0.43629 (12) | 0.1241 (6) |
| O1 | 0.24884 (16) | 0.06387 (13) | −0.05141 (9) | 0.0550 (3) |
| C1 | 0.25997 (19) | 0.14906 (17) | 0.02942 (12) | 0.0418 (3) |
| C2 | 0.2600 (2) | 0.09233 (17) | 0.15540 (12) | 0.0422 (3) |
| C3 | 0.3449 (2) | 0.17173 (19) | 0.24109 (13) | 0.0503 (4) |
| H3A | 0.488109 | 0.130181 | 0.228156 | 0.060* |
| H3B | 0.320711 | 0.140288 | 0.321359 | 0.060* |
| C4 | 0.2471 (2) | 0.36500 (19) | 0.22417 (13) | 0.0512 (4) |
| H4A | 0.108523 | 0.407188 | 0.250485 | 0.061* |
| H4B | 0.313802 | 0.414192 | 0.273047 | 0.061* |
| C5 | 0.2493 (2) | 0.58209 (18) | 0.07057 (15) | 0.0527 (4) |
| H5 | 0.238161 | 0.654546 | 0.131518 | 0.063* |
| C6 | 0.2588 (2) | 0.63650 (19) | −0.04364 (16) | 0.0563 (4) |
| H6 | 0.253793 | 0.744300 | −0.060847 | 0.068* |
| C7 | 0.2760 (2) | 0.5271 (2) | −0.13208 (15) | 0.0544 (4) |
| C8 | 0.2805 (2) | 0.36936 (19) | −0.11116 (13) | 0.0491 (4) |
| H8 | 0.291154 | 0.298893 | −0.173317 | 0.059* |
| C9 | 0.26871 (18) | 0.31584 (17) | 0.00553 (12) | 0.0408 (3) |
| C10 | 0.25574 (19) | 0.42177 (17) | 0.09832 (13) | 0.0433 (3) |
| C11 | 0.1731 (2) | −0.01351 (17) | 0.18075 (12) | 0.0458 (4) |
| H11 | 0.114476 | −0.038397 | 0.117063 | 0.055* |
| C12 | 0.1557 (2) | −0.09667 (17) | 0.29353 (13) | 0.0470 (4) |
| C13 | −0.0152 (2) | −0.1248 (2) | 0.31512 (14) | 0.0579 (4) |
| H13 | −0.118129 | −0.086048 | 0.261061 | 0.070* |
| C14 | −0.0305 (3) | −0.2099 (3) | 0.41639 (16) | 0.0719 (5) |
| C15 | 0.1148 (3) | −0.2720 (3) | 0.49790 (17) | 0.0822 (6) |
| H15 | 0.099512 | −0.329508 | 0.566053 | 0.099* |
| C16 | 0.2853 (3) | −0.2471 (3) | 0.47642 (17) | 0.0782 (6) |
| H16 | 0.387824 | −0.289303 | 0.530666 | 0.094* |
| C17 | 0.3074 (3) | −0.1604 (2) | 0.37551 (15) | 0.0608 (4) |
| H17 | 0.424065 | −0.144812 | 0.362521 | 0.073* |
Source of material
The amounts of 5 ml methanol, 5 ml dichloromethane, 7-fluoro-1-naphthalene ketone and 3-fluorobenzaldehyde were added to a 50 ml flask, stirred in the ice salt bath at −5 °C until the system became soluble and clear. Five millilitre (25%) of sodium hydroxide aqueous solution was added dropwise to the mixture and stirred at room temperature for 3 h. The reaction was monitored by silica gel thin layer chromatography (TLC, 254 nm). When the reaction was finished, the precipitate was filtered from the reaction mixture and dissolved with ethyl acetate. The organic phase was washed successively with water and brine in turn and dried with anhydrous sodium sulfate. After filtration, the filtrate was condensed in vacuum to yield a white solid, which was purified by silica-gel column chromatography (petroleum ether: ethyl acetate = 1:2, v/v). Suitable crystals of the title compound were obtained by recrystallization in the dichloromethane and methanol (1:1, v/v) system and dried in a vacuum at 65 °C for 3 h.
Experimental details
The H atoms were placed in idealized positions and treated as riding on their parent atoms, with d(C–H) = 0.97 Å (methylene), Uiso(H) = 1.2Ueq(C), and d(C–H) = 0.93 Å (aromatic), Uiso(H) = 1.2Ueq(C).
Comment
Central nervous system degenerative disease refers to a group of diseases are caused by chronic progressive degeneration of the central nervous system. Pathologically, degeneration and loss of neurons occurred in the brain and/or spinal cord [4]. Inflammation in the brain is induced by activated microglia and reactive astrocytes [5]. Potential drugs should be able to cross the blood-brain barrier and activate the increase of circulating inflammatory markers of microglia, resulting in the production of cellular inflammation [6]. The cytotoxicity and anti-inflammatory activity of such compounds were evaluated. It is found that they can inhibit tumor growth and has anti-inflammatory activity. Some piperidones may inhibit the expression of NF-/KB and make activated microglia produce an anti-inflammatory effect [7–9]. Combined with the data of cytotoxicity, cytocompatibility and anti-inflammatory activity, some of these compounds may be a new NF KB inhibitor with anti-inflammatory and anti hepatoma activities, which is expected to be further developed into a multifunctional drug for clinical treatment of liver cancer and inflammatory diseases [10, 11]. However, DHN derivatives are rarely developed as anti-neuroinflammatory drugs. Our group also synthesized some of these compounds, and studied their anti-neuroinflammatory activity [12, 13]. In this paper, we report on a benzylidene-substituted DHN derivative which was designed and synthesized through Claisen–Schmidt condensation reactions (see the figure) [14]. Single crystal of the title compound were prepared under ambient conditions, with crystallization obtained via solvent evaporation in the mixed solution of methanol and dichloromethane (1:1, v/v) [15].
There is one molecule in the asymmetric unit of the title structure (see the Figure). Bond lengths and angles are all in the expected ranges [16]. Because of the disto 3,4-dihydronaphthalen-1(2H)-one, the 7-fluorophenyl and 3-fluorophenyl groups are not coplanar with each other, with a dihedral angle of approximately 65°. This twisted configuration may increase likelihood of interactions with bioactive molecules, for the purposes of creating more potent biological activity [17, 18].
Funding source: Science and Technology Innovation Development Plan of Yantai
Award Identifier / Grant number: 2020XDRH105
Funding source: National Natural Science Foundation of China
Award Identifier / Grant number: 81473104
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work was supported by Science and Technology Innovation Development Plan of Yantai (No. 2020XDRH105) and the National Natural Science Foundation of China (No. 81473104).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Rigaku OD. CrysAlisPRO; Rigaku Oxford Diffraction Ltd: Yarnton, Oxfordshire, England, 2017.Search in Google Scholar
2. Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Search in Google Scholar
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Stephenson, J., Nutma, E., van der Valk, P., Amor, S. Inflammation in CNS neurodegenerative diseases. Immunology 2018, 154, 204–219; https://doi.org/10.1111/imm.12922.Search in Google Scholar
5. Goldmann, T., Prinz, M. Role of microglia in CNS autoimmunity. Clin. Dev. Immunol. 2013, 2013, 208093; https://doi.org/10.1155/2013/208093.Search in Google Scholar
6. Gao, C. L., Hou, G. G., Liu, J., Ru, T., Xu, Y. Z., Zhao, S. Y., Ye, H., Zhang, L. Y., Chen, K. X., Guo, Y. W., Pang, T., Li, X. W. Synthesis and target identification of benzoxepane derivatives as potential anti-neuroinflammatory agents for ischemic stroke. Angew. Chem. Int. Ed. 2020, 59, 2429–2439; https://doi.org/10.1002/anie.201912489.Search in Google Scholar
7. Troubat, R., Barone, P., Leman, S., Desmidt, T., Cressant, A., Atanasova, B., Brizard, B., El Hage, W., Surget, A., Belzung, C., Camus, V. Neuroinflammation and depression: a review. Eur. J. Neurosci. 2021, 154, 151–171; https://doi.org/10.1111/ejn.14720.Search in Google Scholar
8. Li, N., Xin, W. Y., Yao, B. R., Cong, W., Wang, C. H., Hou, G. G. N-phenylsulfonyl-3,5-bis(arylidene)-4-piperidone derivatives as activation NF-kappaB inhibitors in hepatic carcinoma cell lines. Eur. J. Med. Chem. 2018, 155, 531–544; https://doi.org/10.1016/j.ejmech.2018.06.027.Search in Google Scholar
9. Yao, B. R., Sun, Y., Chen, S. L., Suo, H. D., Zhang, Y. L., Wei, H., Wang, C. H., Zhao, F., Cong, W., Xin, W. Y., Hou, G. G. Dissymmetric pyridyl-substituted 3,5-bis(arylidene)-4-piperidones as anti-hepatoma agents by inhibiting NF-κB pathway activation. Eur. J. Med. Chem. 2019, 167, 187–199; https://doi.org/10.1016/j.ejmech.2019.02.020.Search in Google Scholar
10. Sun, Y., Gao, Z. F., Yan, W. B., Yao, B. R., Xin, W. Y., Wang, C. H., Meng, Q. G., Hou, G. G. Discovery of novel NF-κB inhibitor based on scaffold hopping: 1,4,5,6,7,8-hexahydropyrido[4,3-d]pyrimidine. Eur. J. Med. Chem. 2020, 198, 112366; https://doi.org/10.1016/j.ejmech.2020.112366.Search in Google Scholar
11. Sun, Y., Gao, Z. F., Wang, C. H., Hou, G. G. Synthesis, crystal structures and anti-inflammatory activity of fluorine-substituted 1,4,5,6-tetrahydrobenzo[h]quinazolin-2-amine derivatives. Acta Crystallogr. 2019, 75, 1157–1165; https://doi.org/10.1107/s2053229619010118.Search in Google Scholar
12. Sun, Y., Zhou, Y. Q., Liu, Y. K., Zhang, H. Q., Hou, G. G., Meng, Q. G., Hou, Y. Potential anti-neuroinflammatory NF-κB inhibitors based on 3,4-dihydronaphthalen-1(2H)-one derivatives. J. Enzym. Inhib. Med. Chem. 2020, 35, 1631–1640; https://doi.org/10.1080/14756366.2020.1804899.Search in Google Scholar
13. Li, N., Xin, W. Y., Yao, B. R., Wang, C. H., Cong, W., Zhao, F., Li, H. J., Hou, Y., Meng, Q. G., Hou, G. G. Novel dissymmetric 3,5-bis(arylidene)-4-piperidones as potential antitumor agents with biological evaluation in vitro and in vivo. Eur. J. Med. Chem. 2018, 147, 21–33; https://doi.org/10.1016/j.ejmech.2018.01.088.Search in Google Scholar
14. Li, N., Yao, B. Y., Wang, C. H., Meng, Q. G., Hou, G. G. Synthesis, crystal structure and activity evaluation of novel 3,4-dihydro-1-benzoxepin-5(2H)-one derivatives as protein-tyrosine kinase (PTK) inhibitors. Acta Crystallogr. 2017, C73, 1003–1009; https://doi.org/10.1107/s2053229617015145.Search in Google Scholar
15. Xiong, C. L., Lan, Y. D., Song, X. Y ., Xiong, W. M., Nie, X. L. Crystal structure of methyl 4-acetoxy3,5-dimethoxybenzoate, C12H14O6. Z. Kristallogr. N. Cryst. Struct. 2021, 236, 573–575; https://doi.org/10.1515/ncrs-2020-0632.Search in Google Scholar
16. Luan, M.-Z., Meng, Q.-G. Crystal structure of (E)-7-methoxy-2-((5-methoxypyridin-3-yl)methylene)-3,4- dihydronaphthalen-1(2H)-one, C18H17NO3. Z. Kristallogr. N. Cryst. Struct. 2021, 236, 387–389.10.1515/ncrs-2020-0602Search in Google Scholar
17. Sun, Y., Liu, Y. K., Li, J. D., Meng, Q. G., Hou, G. G. Crystal structure and anti-inflammatory activity of (3E,5E)-3-(2-fluorobenzylidene)-1-((4-fluorophenyl)sulfonyl)-5-(pyridin-3-ylmethylene)piperidin-4-one, C24H18F2N2O3S. Z. Kristallogr. N. Cryst. Struct. 2020, 235, 377–379; https://doi.org/10.1515/ncrs-2019-0683.Search in Google Scholar
18. Zhou, Y. Q., Hou, G. G., Meng, Q. G., Hou, Y. Crystal structure and anti-inflammatory activity of (3E,5E)-1-((4-chlorophenyl)sulfonyl)-3-(pyridin-4-ylmethylene)-5-(2-(trifluoromethyl) benzylidene)piperidin-4-one, C25H18ClF3N2O3S. Z. Kristallogr. N. Cryst. Struct. 2020, 235, 411–413; https://doi.org/10.1515/ncrs-2019-0716.Search in Google Scholar
© 2021 Jia Song et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of (E)-7-hydroxy-2-((6-methoxypyridin-3-yl)methylene)-3, 4-dihydronaphthalen-1(2H)-one, C17H15NO3
- Crystal structure of (E)-7-methoxy-2-((2-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1 (2H)-one, C18H17NO3
- The crystal structure of N 6,N 6′-di(pyridin-2-yl)-[2,2′-bipyridine]-6,6′-diamine, C20H16N6
- The crystal structure of {N 1,N 2-bis[2,4-dimethyl-6-(4-(tert-butyl)phenyl)(phenyl)methyl]acenaphthylene-1,2-diimino-κ2 N, N′}-dibromido-nickel(II) – dichloromethane(1/2), C64H64Br2Cl4N2Ni
- Synthesis and crystal structure of nonacarbonyltris[(2-thia-1,3,5-triaza-7-phosphatricylco[3.3.1.1]decane-κ1 P)-2,2-dioxide]triruthenium(0) – acetonitrile (7/6), C25.71H32.57N9.86O15P3S3Ru3
- A new polymorph of 1-(4-nitrophenyl)-1H-benzimidazole (C13H9N3O2)
- The crystal structure of 2,2′-((1E,1′E)-(naphthalene-2,3 diylbis(azanylylidene)) bis(methanylylidene))bis(4-methylphenol), C26H22N2O2
- The crystal structure of bis(μ2-iodido)-bis(η6-benzene)-bis(iodido)-diosmium(II), C12H12I4Os2
- Redetermination of the crystal structure of bis{hydridotris(3,5-dimethylpyrazol-1-yl-κN 3)borato}copper(II), C30H44B2CuN12
- Crystal structure of (E)-3-((4-(tert-butyl)phenyl)thio)-4-hydroxypent-3-en-2-one, C15H20O2S
- Crystal structure of 2,2′-(p-tolylazanediyl)bis(1-phenylethan-1-one), C23H21NO2
- Redetermination of the crystal structure of the crystal sponge the poly[tetrakis(μ3-2,4,6-tris(pyridin-4-yl)-1,3,5-triazine)-dodecaiodidohexazinc(II) nitrobenzene solvate], C72H48I12N24Zn6⋅10(C6H5NO2)
- Crystal structure of (4′E)-6′-(diethylamino)-2-[(E)-[(6-methylpyridin-2-yl)methylidene]amino]-4′-{2-[(2E)-1,3,3-trimethyl-2,3-dihydro-1H-indol-2-ylidene]ethylidene}-1′,2,2′,3,3′,4′-hexahydrospiro[isoindole-1,9′-xanthene]-3-one, C44H45N5O2
- Crystal structure of (E)-7-fluoro-2-(3-fluorobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C17H12F2O1
- Crystal structure of tetrabutylammonium sulfanilate – 1-(diaminomethylene)thiourea (1/2)
- Crystal structure of [2,2′-{azanediyl)bis[(propane-3,1-diyl)(azanylylidene)methylylidene]} bis(3,5-dichlorophenolato)-κ2O,O′]-isothiocyanato-κN-iron(III), C21H19Cl4FeN4O2S
- Crystal structure of (4-chlorophenyl)(4-hydroxyphenyl)methanone, C13H9ClO2
- Crystal structure of 6,6′-((pentane-1,3-diylbis(azaneylylidene))bis(methaneylylidene))bis(2,4-dibromolphenolato-κ4 N,N′,O,O′)copper(II),) C19H16Br4CuN2O2
- Chlorido-(2,2′-(ethane-bis(5-methoxyphenolato))-κ4 N,N′,O,O′)manganese(III) monohydrate, C19H18Cl2CuN2O2
- Crystal structure of 2,6-di-tert-butyl-4-(4-methoxybenzylidene)cyclohexa-2,5-dien-1-one, C22H28O2
- Crystal structure of [6,6′-(((2,2-dimethylpropane-1,3-diyl)bis(azanylylidene))bis(methanylylidene))bis(2-chlorophenolato)-κ4N,N′,O,O′]copper(II)
- Crystal structure of 2-chloro-3-((thiophen-2-ylmethyl)amino)naphthalene-1,4-dione, C30H20O4N2Cl2S2
- Crystal structure of bis{hydridotris(3-trifluoromethyl-5-methylpyrazolyl-1-yl)borato-κN 3}manganese(II), C30H26B2F18MnN12
- Crystal structure of 1-(2-methylphenyl)-2-(2-methylbenzo[b]thienyl)-3,3,4,4,5,5-hexafluorocyclopent-ene, C21H14F6S
- Crystal structure of 2-(3-((carbamimidoylthio)methyl)benzyl)isothiouronium hexafluorophosphate monohydrate, C10H17F6N4OPS2
- Crystal structure of 4,5-diiodo-1,3-dimesityl-1H-1,2,3-triazol-3-ium chloride – chloroform (1/1), C21H23Cl4I2N3
- Crystal structure of azido-k1 N-{6,6′-((((methylazanediyl)bis(propane-3,1-diyl))bis(azanylylidene))bis(methanylylidene))bis(2,4-dibromophenolato)k5 N,N′,N″,O,O′}cobalt(III)-methanol (1/1)), C21H23Br4CoN6O3
- The crystal structure of 2-(4-((carbamimidoylthio)methyl)benzyl)isothiouronium hexafluorophosphate monohydrate, C10H17F6N4OPS2
- Crystal structure of 1,1′-(methane-1,1-diyl)bis(3-methyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C9H14F12N4P2
- Crystal structure of (4′E)-6′-(diethylamino)-2-[(E)-[(pyren-1-yl)methylidene]amino]-4′-{2-[(2E)-1,3,3-trimethyl-2,3-dihydro-1H-indol-2-ylidene]ethylidene}-1′,2,2′,3,3′,4′-hexahydrospiro[isoindole-1,9′-xanthene]-3-one, C54H48N4O2
- Crystal structure of poly[bis(μ2-2,6-bis(1-imidazoly)pyridine-κ2 N,N′)-bis(thiocyanato-κ1 N)copper(II)] dithiocyanate, C24H18CuN12S2
- Cones with a three-fold symmetry constructed from three hydrogen bonded theophyllinium cations that coat [FeCl4]− anions in the crystal structure of tris(theophyllinium) bis(tetrachloridoferrate(III)) chloride trihydrate, C21H33Cl9Fe2N12O9
- Crystal structure of 14-O-[(4-(4-hydroxypiperidine-1-yl)-6-methylpyrimidine-2-yl)thioacetyl]-mutilin monohydrate, C32H49N3O6S
- The crystal structure of (E)-3-chloro-2-(2-(4-methylbenzylidene)hydrazinyl)pyridine, C13H12ClN3
- The crystal structure of 4-phenyl-4-[2-(pyridine-4-carbonyl)hydrazinylidene]butanoic acid, C16H15N3O3
- The crystal structure of 6-amino-5-carboxypyridin-1-ium pentaiodide monohydrate C6H9I5N2O3
- Crystal structure of bis(μ3-oxido)-bis(μ2-2-formylbenzoato-k2O:O′)-bis(2-(dimethoxymethyl)-benzoato-κO)-oktakismethyl-tetratin(IV)
- Crystal structure of 2-((E)-(((E)-2-hydroxy-4-methylbenzylidene) hydrazineylidene)methyl)-4-methylphenol, C16H16N2O2
- Crystal structure of (E)-amino(2-((5-methylfuran-2-yl)methylene)hydrazinyl) methaniminium nitrate monohydrate, C14H26N10O10
- The crystal structure of N′-(2-chloro-6-hydroxybenzylidene)thiophene-2-carbohydrazide monohydrate, C12H11ClN2O3S
- Crystal structure of catena-poly[(μ2-1,1′-(biphenyl-4,4′-diyl)bis(1H-imidazol)-κ2N:N′)-bis(4-bromobenzoate-κ1O)zinc(II)], C64H44Br4N8O8Zn2
- The crystal structure of catena-poly[(1-(4-carboxybenzyl)pyridin-1-ium-4-carboxylato-κ1O)-(μ2-oxalato-κ4 O:O′:O″:O‴)dioxidouranium(VI)], C16H11NO10U
- Crystal structure of 3-allyl-4-(2-bromoethyl)-5-(4-methoxyphenyl)-2-phenylfuran, C22H21BrO2
- Halogen bonds in the crystal structure of 4,3′:5′,4″-terpyridine — 1,3-diiodotetrafluorobenzene (1/1), C21H11F4I2N3
- Crystal structure of 2-(1H-indol-3-yl)ethan-1-aminium 2-(4-acetylphenoxy)acetate, C20H22N2O4
- Chalcogen bonds in the crystal structure of 4,7-dibromo-2,1,3-benzoselenadiazole, C6H2Br2N2Se
- The crystal structure of 1,4-bis((1H-benzimidazol-2-yl)methyl)-piperazine-2,5-dione dihydrate, C20H22N6O4
- The crystal structure of C19H20O8
- The crystal structure of KNa3Te8O18·5H2O exhibiting a ∞2[Te4O9]2− layer
- Erratum
- Erratum to: Crystal structure of (Z)-3-(6-bromo-1H-indol-3-yl)-1,3-diphenylprop-2-en-1-one, C23H16BrNO
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of (E)-7-hydroxy-2-((6-methoxypyridin-3-yl)methylene)-3, 4-dihydronaphthalen-1(2H)-one, C17H15NO3
- Crystal structure of (E)-7-methoxy-2-((2-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1 (2H)-one, C18H17NO3
- The crystal structure of N 6,N 6′-di(pyridin-2-yl)-[2,2′-bipyridine]-6,6′-diamine, C20H16N6
- The crystal structure of {N 1,N 2-bis[2,4-dimethyl-6-(4-(tert-butyl)phenyl)(phenyl)methyl]acenaphthylene-1,2-diimino-κ2 N, N′}-dibromido-nickel(II) – dichloromethane(1/2), C64H64Br2Cl4N2Ni
- Synthesis and crystal structure of nonacarbonyltris[(2-thia-1,3,5-triaza-7-phosphatricylco[3.3.1.1]decane-κ1 P)-2,2-dioxide]triruthenium(0) – acetonitrile (7/6), C25.71H32.57N9.86O15P3S3Ru3
- A new polymorph of 1-(4-nitrophenyl)-1H-benzimidazole (C13H9N3O2)
- The crystal structure of 2,2′-((1E,1′E)-(naphthalene-2,3 diylbis(azanylylidene)) bis(methanylylidene))bis(4-methylphenol), C26H22N2O2
- The crystal structure of bis(μ2-iodido)-bis(η6-benzene)-bis(iodido)-diosmium(II), C12H12I4Os2
- Redetermination of the crystal structure of bis{hydridotris(3,5-dimethylpyrazol-1-yl-κN 3)borato}copper(II), C30H44B2CuN12
- Crystal structure of (E)-3-((4-(tert-butyl)phenyl)thio)-4-hydroxypent-3-en-2-one, C15H20O2S
- Crystal structure of 2,2′-(p-tolylazanediyl)bis(1-phenylethan-1-one), C23H21NO2
- Redetermination of the crystal structure of the crystal sponge the poly[tetrakis(μ3-2,4,6-tris(pyridin-4-yl)-1,3,5-triazine)-dodecaiodidohexazinc(II) nitrobenzene solvate], C72H48I12N24Zn6⋅10(C6H5NO2)
- Crystal structure of (4′E)-6′-(diethylamino)-2-[(E)-[(6-methylpyridin-2-yl)methylidene]amino]-4′-{2-[(2E)-1,3,3-trimethyl-2,3-dihydro-1H-indol-2-ylidene]ethylidene}-1′,2,2′,3,3′,4′-hexahydrospiro[isoindole-1,9′-xanthene]-3-one, C44H45N5O2
- Crystal structure of (E)-7-fluoro-2-(3-fluorobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C17H12F2O1
- Crystal structure of tetrabutylammonium sulfanilate – 1-(diaminomethylene)thiourea (1/2)
- Crystal structure of [2,2′-{azanediyl)bis[(propane-3,1-diyl)(azanylylidene)methylylidene]} bis(3,5-dichlorophenolato)-κ2O,O′]-isothiocyanato-κN-iron(III), C21H19Cl4FeN4O2S
- Crystal structure of (4-chlorophenyl)(4-hydroxyphenyl)methanone, C13H9ClO2
- Crystal structure of 6,6′-((pentane-1,3-diylbis(azaneylylidene))bis(methaneylylidene))bis(2,4-dibromolphenolato-κ4 N,N′,O,O′)copper(II),) C19H16Br4CuN2O2
- Chlorido-(2,2′-(ethane-bis(5-methoxyphenolato))-κ4 N,N′,O,O′)manganese(III) monohydrate, C19H18Cl2CuN2O2
- Crystal structure of 2,6-di-tert-butyl-4-(4-methoxybenzylidene)cyclohexa-2,5-dien-1-one, C22H28O2
- Crystal structure of [6,6′-(((2,2-dimethylpropane-1,3-diyl)bis(azanylylidene))bis(methanylylidene))bis(2-chlorophenolato)-κ4N,N′,O,O′]copper(II)
- Crystal structure of 2-chloro-3-((thiophen-2-ylmethyl)amino)naphthalene-1,4-dione, C30H20O4N2Cl2S2
- Crystal structure of bis{hydridotris(3-trifluoromethyl-5-methylpyrazolyl-1-yl)borato-κN 3}manganese(II), C30H26B2F18MnN12
- Crystal structure of 1-(2-methylphenyl)-2-(2-methylbenzo[b]thienyl)-3,3,4,4,5,5-hexafluorocyclopent-ene, C21H14F6S
- Crystal structure of 2-(3-((carbamimidoylthio)methyl)benzyl)isothiouronium hexafluorophosphate monohydrate, C10H17F6N4OPS2
- Crystal structure of 4,5-diiodo-1,3-dimesityl-1H-1,2,3-triazol-3-ium chloride – chloroform (1/1), C21H23Cl4I2N3
- Crystal structure of azido-k1 N-{6,6′-((((methylazanediyl)bis(propane-3,1-diyl))bis(azanylylidene))bis(methanylylidene))bis(2,4-dibromophenolato)k5 N,N′,N″,O,O′}cobalt(III)-methanol (1/1)), C21H23Br4CoN6O3
- The crystal structure of 2-(4-((carbamimidoylthio)methyl)benzyl)isothiouronium hexafluorophosphate monohydrate, C10H17F6N4OPS2
- Crystal structure of 1,1′-(methane-1,1-diyl)bis(3-methyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C9H14F12N4P2
- Crystal structure of (4′E)-6′-(diethylamino)-2-[(E)-[(pyren-1-yl)methylidene]amino]-4′-{2-[(2E)-1,3,3-trimethyl-2,3-dihydro-1H-indol-2-ylidene]ethylidene}-1′,2,2′,3,3′,4′-hexahydrospiro[isoindole-1,9′-xanthene]-3-one, C54H48N4O2
- Crystal structure of poly[bis(μ2-2,6-bis(1-imidazoly)pyridine-κ2 N,N′)-bis(thiocyanato-κ1 N)copper(II)] dithiocyanate, C24H18CuN12S2
- Cones with a three-fold symmetry constructed from three hydrogen bonded theophyllinium cations that coat [FeCl4]− anions in the crystal structure of tris(theophyllinium) bis(tetrachloridoferrate(III)) chloride trihydrate, C21H33Cl9Fe2N12O9
- Crystal structure of 14-O-[(4-(4-hydroxypiperidine-1-yl)-6-methylpyrimidine-2-yl)thioacetyl]-mutilin monohydrate, C32H49N3O6S
- The crystal structure of (E)-3-chloro-2-(2-(4-methylbenzylidene)hydrazinyl)pyridine, C13H12ClN3
- The crystal structure of 4-phenyl-4-[2-(pyridine-4-carbonyl)hydrazinylidene]butanoic acid, C16H15N3O3
- The crystal structure of 6-amino-5-carboxypyridin-1-ium pentaiodide monohydrate C6H9I5N2O3
- Crystal structure of bis(μ3-oxido)-bis(μ2-2-formylbenzoato-k2O:O′)-bis(2-(dimethoxymethyl)-benzoato-κO)-oktakismethyl-tetratin(IV)
- Crystal structure of 2-((E)-(((E)-2-hydroxy-4-methylbenzylidene) hydrazineylidene)methyl)-4-methylphenol, C16H16N2O2
- Crystal structure of (E)-amino(2-((5-methylfuran-2-yl)methylene)hydrazinyl) methaniminium nitrate monohydrate, C14H26N10O10
- The crystal structure of N′-(2-chloro-6-hydroxybenzylidene)thiophene-2-carbohydrazide monohydrate, C12H11ClN2O3S
- Crystal structure of catena-poly[(μ2-1,1′-(biphenyl-4,4′-diyl)bis(1H-imidazol)-κ2N:N′)-bis(4-bromobenzoate-κ1O)zinc(II)], C64H44Br4N8O8Zn2
- The crystal structure of catena-poly[(1-(4-carboxybenzyl)pyridin-1-ium-4-carboxylato-κ1O)-(μ2-oxalato-κ4 O:O′:O″:O‴)dioxidouranium(VI)], C16H11NO10U
- Crystal structure of 3-allyl-4-(2-bromoethyl)-5-(4-methoxyphenyl)-2-phenylfuran, C22H21BrO2
- Halogen bonds in the crystal structure of 4,3′:5′,4″-terpyridine — 1,3-diiodotetrafluorobenzene (1/1), C21H11F4I2N3
- Crystal structure of 2-(1H-indol-3-yl)ethan-1-aminium 2-(4-acetylphenoxy)acetate, C20H22N2O4
- Chalcogen bonds in the crystal structure of 4,7-dibromo-2,1,3-benzoselenadiazole, C6H2Br2N2Se
- The crystal structure of 1,4-bis((1H-benzimidazol-2-yl)methyl)-piperazine-2,5-dione dihydrate, C20H22N6O4
- The crystal structure of C19H20O8
- The crystal structure of KNa3Te8O18·5H2O exhibiting a ∞2[Te4O9]2− layer
- Erratum
- Erratum to: Crystal structure of (Z)-3-(6-bromo-1H-indol-3-yl)-1,3-diphenylprop-2-en-1-one, C23H16BrNO

