Abstract
C26H26N6O9Ni, triclinic, P
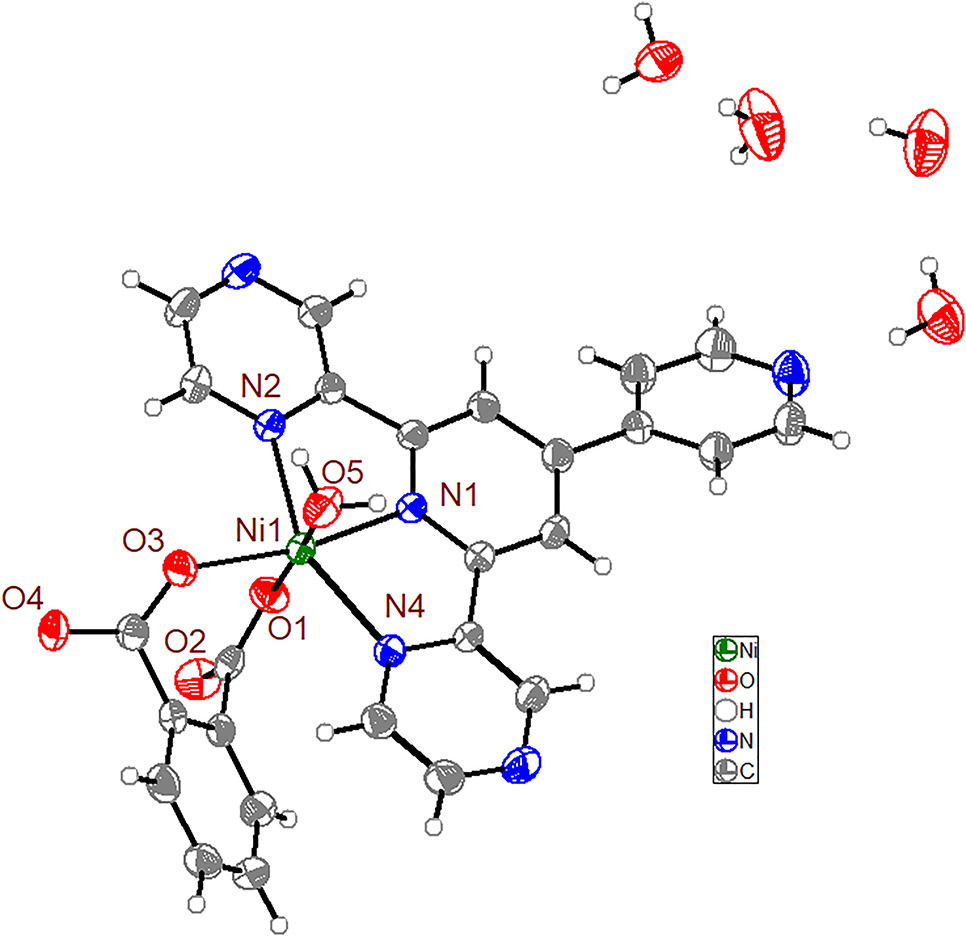
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Green block |
| Size: | 0.18 × 0.18 × 0.18 mm |
| Wavelength: | MoKα radiation (0.71073 Å) |
| μ: | 0.79 mm−1 |
| Diffractometer, scan mode: | SuperNova, ω |
| θ max, completeness: | 25.5°, >99% |
| N(hkl)measured, N(hkl)unique, R int: | 25097, 4994, 0.033 |
| Criterion for I obs, N(hkl)gt: | I obs > 2 σ(I obs), 4479 |
| N(param) refined: | 383 |
| Programs: | CrysAlisPRO [1], Olex2 [2], SHELX [3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| Ni1 | 0.35072 (2) | 0.12411 (2) | 0.20228 (2) | 0.02452 (8) |
| O1 | 0.29144 (14) | 0.00449 (14) | 0.34780 (12) | 0.0329 (3) |
| O2 | 0.19155 (15) | −0.13132 (15) | 0.43954 (13) | 0.0397 (4) |
| O3 | 0.37212 (14) | 0.00281 (15) | 0.10788 (14) | 0.0390 (4) |
| O4 | 0.33128 (15) | −0.13947 (14) | 0.05772 (13) | 0.0374 (4) |
| O5 | 0.40718 (15) | 0.23714 (15) | 0.04638 (13) | 0.0414 (4) |
| H5A | 0.4926 | 0.1996 | 0.0246 | 0.062* |
| H5B | 0.3903 | 0.3138 | 0.0548 | 0.062* |
| N1 | 0.36508 (15) | 0.21314 (15) | 0.31269 (14) | 0.0246 (4) |
| N2 | 0.55193 (16) | 0.01222 (15) | 0.24189 (15) | 0.0272 (4) |
| N3 | 0.79606 (17) | −0.13547 (17) | 0.33220 (17) | 0.0379 (4) |
| N4 | 0.15872 (16) | 0.29271 (15) | 0.20248 (14) | 0.0255 (4) |
| N5 | −0.06954 (18) | 0.52281 (19) | 0.21721 (19) | 0.0448 (5) |
| N6 | 0.4074 (2) | 0.51259 (19) | 0.70930 (17) | 0.0420 (5) |
| C25 | 0.10765 (19) | 0.02286 (18) | 0.26608 (16) | 0.0238 (4) |
| C1 | 0.26134 (19) | 0.31988 (18) | 0.33826 (17) | 0.0253 (4) |
| C2 | 0.2670 (2) | 0.3784 (2) | 0.41770 (18) | 0.0295 (5) |
| H2 | 0.1944 | 0.4531 | 0.4342 | 0.035* |
| C3 | 0.38219 (19) | 0.32516 (19) | 0.47303 (17) | 0.0268 (4) |
| C4 | 0.48814 (19) | 0.21200 (19) | 0.44689 (17) | 0.0270 (4) |
| H4 | 0.5657 | 0.1727 | 0.4836 | 0.032* |
| C5 | 0.47601 (18) | 0.15946 (18) | 0.36584 (17) | 0.0247 (4) |
| C6 | 0.58125 (18) | 0.04232 (18) | 0.32681 (17) | 0.0248 (4) |
| C7 | 0.7028 (2) | −0.0332 (2) | 0.37236 (19) | 0.0318 (5) |
| H7 | 0.7197 | −0.0119 | 0.4327 | 0.038* |
| C8 | 0.7670 (2) | −0.1607 (2) | 0.2453 (2) | 0.0393 (5) |
| H8 | 0.8311 | −0.2299 | 0.2137 | 0.047* |
| C9 | 0.6455 (2) | −0.0885 (2) | 0.19992 (19) | 0.0335 (5) |
| H9 | 0.6289 | −0.1104 | 0.1397 | 0.040* |
| C11 | 0.14592 (19) | 0.36643 (19) | 0.27284 (17) | 0.0265 (4) |
| C10 | 0.0318 (2) | 0.4803 (2) | 0.2789 (2) | 0.0401 (5) |
| H10 | 0.0256 | 0.5293 | 0.3284 | 0.048* |
| C13 | −0.0555 (2) | 0.4492 (2) | 0.1484 (2) | 0.0381 (5) |
| H13 | −0.1242 | 0.4750 | 0.1040 | 0.046* |
| C12 | 0.0575 (2) | 0.3355 (2) | 0.14030 (18) | 0.0315 (5) |
| H12 | 0.0632 | 0.2875 | 0.0901 | 0.038* |
| C14 | 0.39157 (19) | 0.38853 (19) | 0.55568 (17) | 0.0268 (4) |
| C18 | 0.3224 (2) | 0.5229 (2) | 0.54338 (19) | 0.0340 (5) |
| H18 | 0.2690 | 0.5743 | 0.4828 | 0.041* |
| C17 | 0.3332 (2) | 0.5794 (2) | 0.6209 (2) | 0.0389 (5) |
| H17 | 0.2859 | 0.6697 | 0.6110 | 0.047* |
| C16 | 0.4742 (2) | 0.3831 (2) | 0.7211 (2) | 0.0417 (6) |
| H16 | 0.5266 | 0.3344 | 0.7827 | 0.050* |
| C15 | 0.4701 (2) | 0.3177 (2) | 0.64765 (18) | 0.0341 (5) |
| H15 | 0.5190 | 0.2273 | 0.6593 | 0.041* |
| C20 | 0.14942 (19) | 0.01603 (18) | 0.14890 (17) | 0.0253 (4) |
| C21 | 0.0533 (2) | 0.0687 (2) | 0.07194 (19) | 0.0338 (5) |
| H21 | 0.0800 | 0.0608 | −0.0052 | 0.041* |
| C22 | −0.0813 (2) | 0.1327 (2) | 0.1085 (2) | 0.0384 (5) |
| H22 | −0.1444 | 0.1696 | 0.0557 | 0.046* |
| C23 | −0.1216 (2) | 0.1414 (2) | 0.2235 (2) | 0.0365 (5) |
| H23 | −0.2118 | 0.1859 | 0.2480 | 0.044* |
| C24 | −0.0285 (2) | 0.0843 (2) | 0.30248 (19) | 0.0322 (5) |
| H24 | −0.0571 | 0.0870 | 0.3809 | 0.039* |
| C26 | 0.20419 (19) | −0.03962 (19) | 0.35746 (16) | 0.0259 (4) |
| C19 | 0.2951 (2) | −0.04567 (19) | 0.10260 (16) | 0.0270 (4) |
| O7 | 0.3033 (2) | 0.50275 (19) | 1.04469 (19) | 0.0786 (7) |
| H7A | 0.2585 | 0.5554 | 1.0887 | 0.118* |
| H7B | 0.3462 | 0.5404 | 0.9898 | 0.118* |
| O8 | 0.2045 (3) | 0.6817 (2) | 1.1769 (2) | 0.0865 (8) |
| H8A | 0.1639 | 0.6998 | 1.2429 | 0.097 (13)* |
| H8B | 0.2437 | 0.7274 | 1.1407 | 0.105 (15)* |
| O6 | 0.4292 (2) | 0.63299 (19) | 0.85914 (16) | 0.0630 (5) |
| H6A | 0.4164 | 0.6019 | 0.8109 | 0.095* |
| H6B | 0.5095 | 0.5898 | 0.8756 | 0.095* |
| O9 | 0.06329 (17) | 0.72532 (18) | 1.39954 (17) | 0.0593 (5) |
| H9A | −0.0184 | 0.7705 | 1.3875 | 0.086 (11)* |
| H9B | 0.0806 | 0.7784 | 1.4169 | 0.088 (12)* |
Source of material
A mixture of 2,6-di(pyrazin-2-yl)-4,4′-bipyridine (py-pzpypz; 0.1 mmol), phthalic acid (0.1 mmol), Ni(OAc)2·4H2O (0.1 mmol) and H2O (10 mL) was stirred for 30 min, and the pH value of the solution was adjusted to about 4 with 1 M KOH. Then the mixture was transferred to a 25 mL Teflon-lined stainless steel vessel and heated at 140 °C for three days, and then cooled to room temperature over 40 h. Green block crystals of the title compound were obtained. Anal. Calcd. for C26H26NiN6O9 (%): C, 49.95, H 4.19, N 13.44. Found: C 49.76, H 4.21, N 13.40.
Experimental details
Using Olex2, the structure was solved with the SIR2004 structure solution program using Direct Methods and refined with the ShelXL refinement package using least squares minimisation.
Comment
Coordination compounds or frameworks have been receiving extensive attention not only for their aesthetics of structures but also for their potential applications, such as photoluminescence, gas storage, catalysis, etc. [4], [5], [6]. The construction of the compounds involve a variety of building blocks (metal ions, organic ligands, counter anions, solvents, etc.) [7], [8]. The carboxylate ligands have been extensively employed due to their versatile coordination conformations and strong coordination ability. Moreover, carboxylate ligands are usually utilized as hydrophilic groups to form hydrogen bonds. Hydrogen bonds often play a dominant role in crystal engineering because of their selectivity and directionality to control the design in the synthesis of assembiles that contain N-based ligands and carboxylates [9]. The pyrazine analogues ligands have received some attention on account of the weaker σ-donor and stronger π-acceptor nature of pyrazine [10], [11]. This contribution is part of a study on 4-(4-pyridyl)-2,5-dipyrazylpyridine [12], [13], [14]. The interesting feature of the ligand is that it is able to a chelating coordination and a monodentate one.
The ORPEP-type drawing of the title compound is shown in the Figure. The structure consists of the title complex and four isolated water molecules. The center Ni(II) ion resides in a distorted octahedron center and is coordinated by three nitrogen atoms from a py-pzpypz ligand and two oxygen atoms from the phthalate ligand and one coordinated water molecular. The equatorial plane is composed of three nitrogen atoms and one oxygen atom (N1, N2, N4, O3), while atoms O1 and O5 occupy the axial positions. The average Ni–N bond length is 2.1071(16) Å, and the Ni–O bond lengths are in a range of 2.0045(14)–2.0638(15) Å, which are comparable to those found in other reported Ni(II) compounds [15], [16].
The intermolecular hydrogen bonds occur between two oxygen atoms from coordinated water molecules and non-coordinated water molecules and between uncoordinated water molecule. The intermolecular hydrogen bonds also occur between two oxygen atoms from coordinated water molecule and one carboxylate group and between two oxygen atoms from non-coordinated water molecule and a carboxylate group. Thereby, the title compound can be viewed as a three-dimensional architecture extended via these hydrogen bonds.
Funding source: Program for Innovative Research Team (in Science and Technology) in Universities of Henan Province
Award Identifier / Grant number: 21IRTSTHN004
Funding source: Science and Technology Project of Henan Province
Award Identifier / Grant number: 202102110210
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: Program for Innovative Research Team (in Science and Technology) in Universities of Henan Province (No. 21IRTSTHN004) and the Science and Technology Project of Henan Province (No. 202102110210).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Agilent Technologies. CrysAlisPRO Software System, version 1.171.38.41r; Agilent Technologies UK Ltd: Oxford, UK, 2011.Search in Google Scholar
2. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
3. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8. https://doi.org/10.1107/s2053273314026370.Search in Google Scholar PubMed PubMed Central
4. Yang, X. G., Ma, L. F., Yan, D. P. Facile synthesis of 1D organic–inorganic perovskite micro-belts with high water stability for sensing and photonic applications. Chem. Sci. 2019, 10, 4567–4572. https://doi.org/10.1039/c9sc00162j.Search in Google Scholar PubMed PubMed Central
5. Zhang, L., Zou, C., Zhao, M., Jiang, K., Lin, R. B., He, Y. B., Wu, C. D., Cui, Y. J., Chen, B. L., Qian, G. D. Doubly interpenetrated metal-organic framework for highly selective C2H2/CH4 and C2H2/CO2 separation at room temperature. Cryst. Growth Des. 2016, 16, 7194–7197. https://doi.org/10.1021/acs.cgd.6b01382.Search in Google Scholar
6. Zhao, Y., Yang, X. G., Lu, X. M., Yang, C. D., Fan, N. N., Yang, Z. T., Wang, L. Y., Ma, L. F. {Zn6} cluster based metal-organic framework with enhanced room-temperature phosphorescence and optoelectronic performances. Inorg. Chem. 2019, 58, 6215–6221. https://doi.org/10.1021/acs.inorgchem.9b00450.Search in Google Scholar PubMed
7. Mukherjee, S., Desai, A. V., Ghosh, S. K. Potential of metal-organic frameworks for adsorptive separation of industrially and environmentally relevant liquid mixtures. Coord. Chem. Rev. 2018, 367, 82–126. https://doi.org/10.1016/j.ccr.2018.04.001.Search in Google Scholar
8. He, C. J., Chen, J. Q. Synthesis, characterization, and crystal structure of one zinc(II) complex with 4-hydroxypyridine-2,6-dicarboxylic acid and 4,4′bipyridine. Russ. J. Coord. Chem. 2014, 40, 659–663. https://doi.org/10.1134/s1070328414090048.Search in Google Scholar
9. Du, M., Zhang, Z. H., Zhao, X. J. Cocrystallization of trimesic acid and pyromellitic acid with bent dipyridines. Cryst. Growth Des. 2005, 5, 1247–1254. https://doi.org/10.1021/cg0495680.Search in Google Scholar
10. Steel, P. J. Aromatic nitrogen heterocycles as bridging ligands; a survey. Coord. Chem. Rev. 1990, 106, 227–265. https://doi.org/10.1016/0010-8545(60)80005-7.Search in Google Scholar
11. Miller, R. G., Brooker, S. Spin crossover, reversible redox, and supramolecular interactions in 3d complexes of 4-(4-pyridyl)-2,5-dipyrazyl-pyridine. Inorg. Chem. 2015, 54, 5398–5410. https://doi.org/10.1021/acs.inorgchem.5b00428.Search in Google Scholar PubMed
12. Miller, R. G., Warren, M. R., Allan, D. R., Brooke, S. Direct crystallographic observation of CO2 captured in zigzag channels of a copper(I) metal-organic framework. Inorg. Chem. 2020, 59, 6376–6381. https://doi.org/10.1021/acs.inorgchem.0c00471.Search in Google Scholar PubMed
13. Miller, R. G., Southon, P. D., Kepert, C. J., Brooker, S. Commensurate CO2 capture, and shape selectivity for HCCH over H2CCH2, in zigzag channels of a robust Cu1(CN)(L) metal-organic framework. Inorg. Chem. 2016, 55, 6195–6200. https://doi.org/10.1021/acs.inorgchem.6b00813.Search in Google Scholar PubMed
14. Wang, Y. F., Zhang, Y. H. Crystal structure of catena-poly [(μ2-isophthalato-κ2O:O′)-(2,5-di(pyrazin-2-yl)-4,4′-bipyridine-κ2N,N′,N″)zinc(II)] — water (2/5), C26H21N6O6.5Zn. Z. Kristallogr. N. Cryst. Struct. 2019, 234, 1043–1045. https://doi.org/10.1515/ncrs-2019-0250.Search in Google Scholar
15. Li, J. X., Du, Z. X., Xiong, L. Y., Fu, L. L., Bo, W. B. Supramolecular isomerism in two nickel(II) coordination polymers constructed with the flexible 2-carboxyphenoxyacetate linker: syntheses, structure analyses and magnetic properties. J. Solid State Chem. 2021, 293, 121799–121808. https://doi.org/10.1016/j.jssc.2020.121799.Search in Google Scholar
16. Wang, Y. F., Li, S., Wang, L. Y. Crystal structures and magnetic properties of two isomorphic nickel(II) and cobalt(II) coordination polymers-based nitrogen-heterocyclic tricarbolylate ligands. Trans. Met. Chem. 2020, 45, 561–568. https://doi.org/10.1007/s11243-020-00408-6.Search in Google Scholar
© 2021 Wang Yu-Fang and Shu-Qi Zhang, published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of [aqua-(4-iodopyridine-2,6-dicarboxylato-κ3 O,N,O′)-(1,10-phenanothroline-κ2 N,N′)copper(II)] dihydrate, C19H16O7N3CuI
- The crystal structure of tetrakis(1-isopropyl-1H-imidazolium) octamolybdate, C24H44Mo8N8O26
- Crystal structure of catena-poly[bis(µ2-3,5-bis(1-imidazolyl)pyridine-κ2 N:N′)-(µ2-3-nitrophthalato-k3 O,O′:O″)cadmium(II)] dihydrate, C30H25N11O8Cd
- The crystal structure of diaqua-bis(2-(3-(1H-pyrazol-4-yl)-1H-1,2,4-triazol-5-yl)pyridine-κ2 N:N′)-bis(3,5-dicarboxybenzoato-κ1 O)cobalt(II), C38H30CoN12O14
- Crystal structure of the nickel(II) complex aqua-(2,6-di(pyrazin-2-yl)-4,4′-bipyridine-κ3 N,N′,N′′)-(phthalato-κ2 O,O′)nickel(II) tetrahydrate, C26H26N6O9Ni
- The crystal structure of 1-[5-(2-fluorophenyl)-1-(pyridine-3-sulfonyl)-1H-pyrrol-3-yl]-N-methylmethanaminium 3-carboxyprop-2-enoate, C21H20FN3O6S
- The crystal structure of 1,2-bis(4-pyridyl)ethane - 4,4-dihydroxydiphenylmethane (1/1), C25H21N2O2
- Crystal structure of bis(2-((E)-5-chloro-2-hydroxybenzylidene)hydrazineyl)methaniminium trifluoroacetate dihydrate, C34H36Cl4N10O12
- Crystal structure of 1-cyclopropyl-7-ethoxy-6,8-difluoro-4-oxo-1,4-dihydro-quinoline-3-carboxylic acid, C15H13F2NO4
- Crystal structure of methyl 3-(1H-naphtho[1,8-de][1,3,2]diazaborinin-2(3H)-yl)benzoate, C18H15BN2O2
- Crystal structure of (E)-N′-(2-chloro-6-hydroxybenzylidene)-2-hydroxybenzohydrazide, C14H11ClN2O3
- Crystal structure of Al-rich fluorophlogopite, K1.0(Mg2.8Al0.2)(Si2.8Al1.2)O10F2
- The crystal structure of 4,5-diiodo-1,3-dimesityl-1H-1,2,3-triazol-3-ium hexafluoridoantimonate(V), C20H22F6I2N3Sb
- Crystal structure of tris(3-iodopyridin-1-ium) catena-poly[(hexachlorido-κ1 Cl)-(μ2-trichlorido-κ2 Cl:Cl)diantimony(III)], C15H15Cl9I3N3Sb2
- Crystal structure of methyl 2-(1H-naphtho[1,8-de][1.3.2]diazaborinin-2(3H-yl)benzoate C18H15BN2O2
- The crystal structure of 1,8-bis(4-methoxybenzoyl)naphthalene-2,7-diyl dibenzoate, C40H28O8
- Crystal structure of 2-bromo-1,3,6,8-tetramethylBOPHY (BOPHY = bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl)methylene)hydrazine), C14H15B2BrF4N4
- The crystal structure of (E)-3-chloro-2-(2-(2-fluorobenzylidene)hydrazinyl)pyridine, C12H9ClFN3
- Crystal structure of bis(µ2- 4-iodopyridine-2,6-dicarboxylato-κ3O:N:O′)-bis(4-iodopyridine-2,6-dicarboxylato-κ3O:N:O′)-bis(µ2-1-(4-pyridyl)piperazine-κ2N:N′)-hexa-aqua-tetra-copper(II), C46H46Cu4I4N10O22
- Crystal structure of poly[diaqua-(μ2-2,5-dihydroxyterephthalato-κ2O:O′)(μ2-bis(4-pyridylformyl)piperazine-κ2N:N′)cadmium(II)] dihydrate, C24H28CdN4O12
- Crystal structure of poly[aqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)-(μ3-2,3,5,6-tetrafluoroterephthalato-κ3O:O′:O′′)cadmium(II)], C17H14N4O5F4Cd
- Crystal structure of 6-(quinolin-8-yl)benzo[a]phenanthridin-5(6H)-one, C26H16N2O
- The crystal structure of aqua-bis(6-chloropicolinato-κ2N,O)copper(II), C12H8Cl2N2O5Cu
- Crystal structure of catena-poly[diaqua-bis(μ2-4,4′-bipyridyl-κ2N:N′) disilver(I)] 4-oxidopyridine-3-sulfonate trihydrate, C25H29Ag2N5O9S
- The crystal structure of 4-(3-bromophenyl)pyrimidin-2-amine, C10H8BrN3
- Crystal structure of 6-oxo-4-phenyl-1-propyl-1,6-dihydropyridine-3-carbonitrile, C15H14N2O
- Crystal structure of 4-(2,2-difluoroethyl)-2,4-dimethyl-6-(trifluoromethyl)isoquinoline-1,3(2H,4H)-dione, C14H12F5NO2
- Crystal structure of dibromido-(1-methyl-1H-imidazole-κ1N)-(3-(3-methyl-1H-imidazol-3-ium-1-yl)propanoato-κ1O)zinc(II), C11H16Br2N4O2Zn
- The crystal structure of 1,1′-(((2 (dimethylamino)ethyl)azanediyl)bis(methylene)) bis(naphthalen-2-olato-κ4 N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)- titanium(IV) ─ dichloromethane (2/1), C33H29N3O6Ti
- The layered crystal structure of bis(theophyllinium) hexachloridostannate (IV), C14H18N8O8SnCl6
- The crystal structre of 3-(1-ethenyl-1H-imidazol-3-ium-3-yl)propane-1-sulfonate, C8H12N2O3S
- Synthesis and crystal structure of di-tert-butyl 1″-acetyl-2,2″,9′-trioxo-4a′,9a′-dihydro-1′H,3′H,9′H-dispiro[indoline-3,2′-xanthene-4′,3″-indoline]-1,3′-dicarboxylate, C39H38N2O9
- The crystal structure of 4-chloro-2-(quinolin-8-yl)isoindoline-1,3-dione, C17H9ClN2O2
- The crystal structure of 1-fluoro-4-(p-tolylethynyl)benzene, C15H11F
- The crystal structure of bis[4-bromo-2-(1H-pyrazol-3-yl) phenolato-κ2N,O] copper(II), C18H12Br2CuN4O2
- The crystal structure of poly[(μ 3-imidazolato-κ 3 N:N:N′)(tetrahydrofuran- κ 1 O)lithium(I)], C7H11LiN2O
- Crystal structure of N′,N′′′-((1E,1′E)-(propane-2,2-diylbis(1H-pyrrole-5,2diyl))bis(methaneylylidene))di(nicotinohydrazide) pentahydrate, C25H24N8O2·5H2O
- Crystal structure of 3-(2-ethoxy-2-oxoethyl)-1-ethyl-1H-imidazol-3-ium hexafluoridophos-phate(V), C9H15F6N2O2P
- Crystal structure of (1,10-phenanthroline-κ2N,N′)-bis(3-thiophenecarboxylato-κ2O,O′)copper(II), C22H14N2O4S2Cu
- The crystal structure of 2-amino-3-carboxypyridin-1-ium iodide hemihydrate, C6H8IN2O2.5
- Crystal structure of (E)-7-methoxy-2-((6-methoxypyridin-2-yl)methylene)-tetralone, C18H17NO3
- The crystal structure of [μ-hydroxido-bis[(5,5′-dimethyl-2,2′-bipyridine-κ2N,N′)-tricarbonylrhenium(I)] bromide hemihydrate, C30H26N4O9Re2Br
- The crystal structure of 2,5-bis(3,5-dimethylphenyl)thiazolo[5,4-d]thiazole, C20H18N2S2
- The crystal structure of 5-benzoyl-1-[(E)-(4-fluorobenzylidene)amino]-4-phenylpyrimidin-2(1H)-one, C24H16FN3O2
- Crystal structure of monocarbonyl(N-nitroso-N-oxido-phenylamine-κ 2 O,O′)(tricyclohexylphosphine-κP)rhodium(I), C25H39N2O3PRh
- Crystal structure of poly[bis[μ3-1,3,5-tris[(1H-imidazol-1-yl)methyl]benzene-κ3N:N′:N″]nickel(II)] hexafluorosilicate, C36H36N12NiSiF6
- The crystal structure of 13-(pyrazole-1-yl-4-carbonitrile)-matrine, C19H25N5O
- Crystal structure of 3,5-bis((E)-4-methoxy-2-(trifluoromethyl)benzylidene)-1-methylpiperidin-4-one, C24H21F6NO3
- The crystal structure of N,N′-(Disulfanediyldi-2,1-phenylene)di(6′-methylpyridine)-2-carboxamide, C26H22N4O2S2
- Crystal structure of (E)-7-fluoro-2-(4-methoxy-2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C19H14F4O2
- Crystal structure of ethyl 1-(4-fluorophenyl)-4-phenyl-1H-pyrrole-3-carboxylate, C19H16FNO2
- The crystal structure of cis-diaqua-bis (N-butyl-N-(pyridin-2-yl)pyridin-2-amine-κ2N,N′)cobalt(II)] dichloride trihydrate, C28H44Cl2N6O5Co
- Crystal structure of (E)-7-methoxy-2-((6-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C18H17NO3
- Crystal structure of (E)-2-((3-fluoropyridin-4-yl)methylene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- The crystal structure of 6-bromohexanoic acid, C6H11BrO2
- The crystal structure of 4-chloro-thiophenol, C6H5ClS
- The crystal structure of 4-bromobenzyl chloride, C7H6BrCl
- The crystal structure of di-tert-butyl dicarbonate, C10H18O5
- The crystal structure of (2-(4-chlorophenyl)-5-methyl-1,3-dioxan-5-yl)methanol, C12H15ClO3
- The crystal structure of the co-crystal: 2-hydroxybenzoic acid – N′-(butan-2-ylidene)pyridine-4-carbohydrazide, C10H13N3O·C7H6O3
- Crystal structure and anti-inflammatory activity of (E)-7-fluoro-2-((5-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- Crystal structure of (E)-7-fluoro-2-((6-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- Crystal structure of 1,1′-(butane-1,4-diyl)bis(3-propyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C32H56F24N8P4
- The crystal structure of dichlorido-bis(3-methyl-3-imidazolium-1-ylpropionato-κ2)-cadmium(II), C14H20CdCl2N4O4
- Crystal structure of 1-(2-cyanobenzyl)-3-cyano-4-phenyl-4-(2-cyanobenzyl)-1,4-dihydropyridine monohydrate, C56H42N8O
- The crystal structure of 3-(carboxymethyl)-1-ethenyl-1H-imidazol-3-ium chloride, C7H9N2O2Cl
- The crystal structure of adamantylmethoxydiphenylsilane, C23H28OSi
- Redetermination of the crystal structure of (2E,4Z,13E,15Z)-3,5,14,16-tetramethyl-2,6,13,17-tetraazatricyclo[16.4.0.07,12]docosa-1(22),2,4,7,9,11,13,15,18,20-decaene, C22H24N4
- Crystal structure of (E)-7-hydroxy-2-((6-methoxypyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H15NO3
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2 N:N′)cobalt(II)] dinitrate, C18H28N10O8Co
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of [aqua-(4-iodopyridine-2,6-dicarboxylato-κ3 O,N,O′)-(1,10-phenanothroline-κ2 N,N′)copper(II)] dihydrate, C19H16O7N3CuI
- The crystal structure of tetrakis(1-isopropyl-1H-imidazolium) octamolybdate, C24H44Mo8N8O26
- Crystal structure of catena-poly[bis(µ2-3,5-bis(1-imidazolyl)pyridine-κ2 N:N′)-(µ2-3-nitrophthalato-k3 O,O′:O″)cadmium(II)] dihydrate, C30H25N11O8Cd
- The crystal structure of diaqua-bis(2-(3-(1H-pyrazol-4-yl)-1H-1,2,4-triazol-5-yl)pyridine-κ2 N:N′)-bis(3,5-dicarboxybenzoato-κ1 O)cobalt(II), C38H30CoN12O14
- Crystal structure of the nickel(II) complex aqua-(2,6-di(pyrazin-2-yl)-4,4′-bipyridine-κ3 N,N′,N′′)-(phthalato-κ2 O,O′)nickel(II) tetrahydrate, C26H26N6O9Ni
- The crystal structure of 1-[5-(2-fluorophenyl)-1-(pyridine-3-sulfonyl)-1H-pyrrol-3-yl]-N-methylmethanaminium 3-carboxyprop-2-enoate, C21H20FN3O6S
- The crystal structure of 1,2-bis(4-pyridyl)ethane - 4,4-dihydroxydiphenylmethane (1/1), C25H21N2O2
- Crystal structure of bis(2-((E)-5-chloro-2-hydroxybenzylidene)hydrazineyl)methaniminium trifluoroacetate dihydrate, C34H36Cl4N10O12
- Crystal structure of 1-cyclopropyl-7-ethoxy-6,8-difluoro-4-oxo-1,4-dihydro-quinoline-3-carboxylic acid, C15H13F2NO4
- Crystal structure of methyl 3-(1H-naphtho[1,8-de][1,3,2]diazaborinin-2(3H)-yl)benzoate, C18H15BN2O2
- Crystal structure of (E)-N′-(2-chloro-6-hydroxybenzylidene)-2-hydroxybenzohydrazide, C14H11ClN2O3
- Crystal structure of Al-rich fluorophlogopite, K1.0(Mg2.8Al0.2)(Si2.8Al1.2)O10F2
- The crystal structure of 4,5-diiodo-1,3-dimesityl-1H-1,2,3-triazol-3-ium hexafluoridoantimonate(V), C20H22F6I2N3Sb
- Crystal structure of tris(3-iodopyridin-1-ium) catena-poly[(hexachlorido-κ1 Cl)-(μ2-trichlorido-κ2 Cl:Cl)diantimony(III)], C15H15Cl9I3N3Sb2
- Crystal structure of methyl 2-(1H-naphtho[1,8-de][1.3.2]diazaborinin-2(3H-yl)benzoate C18H15BN2O2
- The crystal structure of 1,8-bis(4-methoxybenzoyl)naphthalene-2,7-diyl dibenzoate, C40H28O8
- Crystal structure of 2-bromo-1,3,6,8-tetramethylBOPHY (BOPHY = bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl)methylene)hydrazine), C14H15B2BrF4N4
- The crystal structure of (E)-3-chloro-2-(2-(2-fluorobenzylidene)hydrazinyl)pyridine, C12H9ClFN3
- Crystal structure of bis(µ2- 4-iodopyridine-2,6-dicarboxylato-κ3O:N:O′)-bis(4-iodopyridine-2,6-dicarboxylato-κ3O:N:O′)-bis(µ2-1-(4-pyridyl)piperazine-κ2N:N′)-hexa-aqua-tetra-copper(II), C46H46Cu4I4N10O22
- Crystal structure of poly[diaqua-(μ2-2,5-dihydroxyterephthalato-κ2O:O′)(μ2-bis(4-pyridylformyl)piperazine-κ2N:N′)cadmium(II)] dihydrate, C24H28CdN4O12
- Crystal structure of poly[aqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)-(μ3-2,3,5,6-tetrafluoroterephthalato-κ3O:O′:O′′)cadmium(II)], C17H14N4O5F4Cd
- Crystal structure of 6-(quinolin-8-yl)benzo[a]phenanthridin-5(6H)-one, C26H16N2O
- The crystal structure of aqua-bis(6-chloropicolinato-κ2N,O)copper(II), C12H8Cl2N2O5Cu
- Crystal structure of catena-poly[diaqua-bis(μ2-4,4′-bipyridyl-κ2N:N′) disilver(I)] 4-oxidopyridine-3-sulfonate trihydrate, C25H29Ag2N5O9S
- The crystal structure of 4-(3-bromophenyl)pyrimidin-2-amine, C10H8BrN3
- Crystal structure of 6-oxo-4-phenyl-1-propyl-1,6-dihydropyridine-3-carbonitrile, C15H14N2O
- Crystal structure of 4-(2,2-difluoroethyl)-2,4-dimethyl-6-(trifluoromethyl)isoquinoline-1,3(2H,4H)-dione, C14H12F5NO2
- Crystal structure of dibromido-(1-methyl-1H-imidazole-κ1N)-(3-(3-methyl-1H-imidazol-3-ium-1-yl)propanoato-κ1O)zinc(II), C11H16Br2N4O2Zn
- The crystal structure of 1,1′-(((2 (dimethylamino)ethyl)azanediyl)bis(methylene)) bis(naphthalen-2-olato-κ4 N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)- titanium(IV) ─ dichloromethane (2/1), C33H29N3O6Ti
- The layered crystal structure of bis(theophyllinium) hexachloridostannate (IV), C14H18N8O8SnCl6
- The crystal structre of 3-(1-ethenyl-1H-imidazol-3-ium-3-yl)propane-1-sulfonate, C8H12N2O3S
- Synthesis and crystal structure of di-tert-butyl 1″-acetyl-2,2″,9′-trioxo-4a′,9a′-dihydro-1′H,3′H,9′H-dispiro[indoline-3,2′-xanthene-4′,3″-indoline]-1,3′-dicarboxylate, C39H38N2O9
- The crystal structure of 4-chloro-2-(quinolin-8-yl)isoindoline-1,3-dione, C17H9ClN2O2
- The crystal structure of 1-fluoro-4-(p-tolylethynyl)benzene, C15H11F
- The crystal structure of bis[4-bromo-2-(1H-pyrazol-3-yl) phenolato-κ2N,O] copper(II), C18H12Br2CuN4O2
- The crystal structure of poly[(μ 3-imidazolato-κ 3 N:N:N′)(tetrahydrofuran- κ 1 O)lithium(I)], C7H11LiN2O
- Crystal structure of N′,N′′′-((1E,1′E)-(propane-2,2-diylbis(1H-pyrrole-5,2diyl))bis(methaneylylidene))di(nicotinohydrazide) pentahydrate, C25H24N8O2·5H2O
- Crystal structure of 3-(2-ethoxy-2-oxoethyl)-1-ethyl-1H-imidazol-3-ium hexafluoridophos-phate(V), C9H15F6N2O2P
- Crystal structure of (1,10-phenanthroline-κ2N,N′)-bis(3-thiophenecarboxylato-κ2O,O′)copper(II), C22H14N2O4S2Cu
- The crystal structure of 2-amino-3-carboxypyridin-1-ium iodide hemihydrate, C6H8IN2O2.5
- Crystal structure of (E)-7-methoxy-2-((6-methoxypyridin-2-yl)methylene)-tetralone, C18H17NO3
- The crystal structure of [μ-hydroxido-bis[(5,5′-dimethyl-2,2′-bipyridine-κ2N,N′)-tricarbonylrhenium(I)] bromide hemihydrate, C30H26N4O9Re2Br
- The crystal structure of 2,5-bis(3,5-dimethylphenyl)thiazolo[5,4-d]thiazole, C20H18N2S2
- The crystal structure of 5-benzoyl-1-[(E)-(4-fluorobenzylidene)amino]-4-phenylpyrimidin-2(1H)-one, C24H16FN3O2
- Crystal structure of monocarbonyl(N-nitroso-N-oxido-phenylamine-κ 2 O,O′)(tricyclohexylphosphine-κP)rhodium(I), C25H39N2O3PRh
- Crystal structure of poly[bis[μ3-1,3,5-tris[(1H-imidazol-1-yl)methyl]benzene-κ3N:N′:N″]nickel(II)] hexafluorosilicate, C36H36N12NiSiF6
- The crystal structure of 13-(pyrazole-1-yl-4-carbonitrile)-matrine, C19H25N5O
- Crystal structure of 3,5-bis((E)-4-methoxy-2-(trifluoromethyl)benzylidene)-1-methylpiperidin-4-one, C24H21F6NO3
- The crystal structure of N,N′-(Disulfanediyldi-2,1-phenylene)di(6′-methylpyridine)-2-carboxamide, C26H22N4O2S2
- Crystal structure of (E)-7-fluoro-2-(4-methoxy-2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C19H14F4O2
- Crystal structure of ethyl 1-(4-fluorophenyl)-4-phenyl-1H-pyrrole-3-carboxylate, C19H16FNO2
- The crystal structure of cis-diaqua-bis (N-butyl-N-(pyridin-2-yl)pyridin-2-amine-κ2N,N′)cobalt(II)] dichloride trihydrate, C28H44Cl2N6O5Co
- Crystal structure of (E)-7-methoxy-2-((6-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C18H17NO3
- Crystal structure of (E)-2-((3-fluoropyridin-4-yl)methylene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- The crystal structure of 6-bromohexanoic acid, C6H11BrO2
- The crystal structure of 4-chloro-thiophenol, C6H5ClS
- The crystal structure of 4-bromobenzyl chloride, C7H6BrCl
- The crystal structure of di-tert-butyl dicarbonate, C10H18O5
- The crystal structure of (2-(4-chlorophenyl)-5-methyl-1,3-dioxan-5-yl)methanol, C12H15ClO3
- The crystal structure of the co-crystal: 2-hydroxybenzoic acid – N′-(butan-2-ylidene)pyridine-4-carbohydrazide, C10H13N3O·C7H6O3
- Crystal structure and anti-inflammatory activity of (E)-7-fluoro-2-((5-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- Crystal structure of (E)-7-fluoro-2-((6-methoxypyridin-3-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- Crystal structure of 1,1′-(butane-1,4-diyl)bis(3-propyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C32H56F24N8P4
- The crystal structure of dichlorido-bis(3-methyl-3-imidazolium-1-ylpropionato-κ2)-cadmium(II), C14H20CdCl2N4O4
- Crystal structure of 1-(2-cyanobenzyl)-3-cyano-4-phenyl-4-(2-cyanobenzyl)-1,4-dihydropyridine monohydrate, C56H42N8O
- The crystal structure of 3-(carboxymethyl)-1-ethenyl-1H-imidazol-3-ium chloride, C7H9N2O2Cl
- The crystal structure of adamantylmethoxydiphenylsilane, C23H28OSi
- Redetermination of the crystal structure of (2E,4Z,13E,15Z)-3,5,14,16-tetramethyl-2,6,13,17-tetraazatricyclo[16.4.0.07,12]docosa-1(22),2,4,7,9,11,13,15,18,20-decaene, C22H24N4
- Crystal structure of (E)-7-hydroxy-2-((6-methoxypyridin-2-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one, C17H15NO3
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2 N:N′)cobalt(II)] dinitrate, C18H28N10O8Co

