Abstract
C42H38N4O7, triclinic,
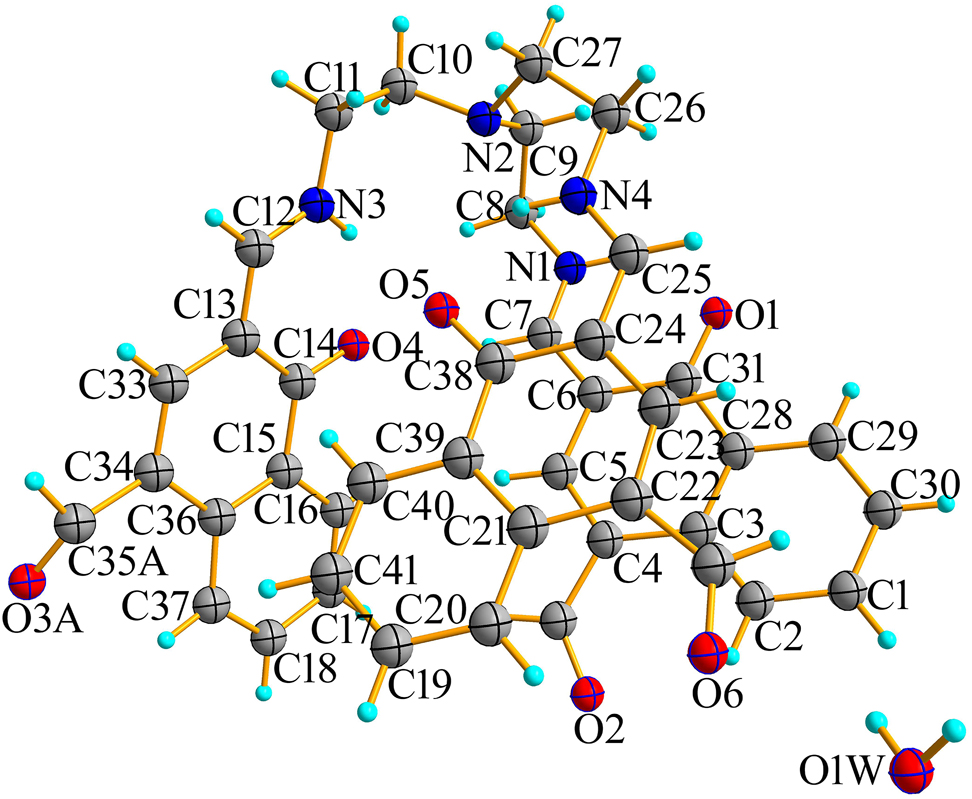
The molecular structure is shown in the Figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Yellow block |
| Size: | 0.24 × 0.23 × 0.21 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.09 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| θmax, completeness: | 26.0°, 99% |
| N(hkl)measured, N(hkl)unique, Rint: | 47,868, 6780, 0.040 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2σ(Iobs), 5360 |
| N(param)refined: | 495 |
| Programs: | Olex2 [1], Bruker [2], Shelx [3], Diamond [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| O1 | 0.67282 (11) | 0.17824 (11) | −0.05327 (10) | 0.0549 (3) |
| O2 | 0.6140 (2) | −0.15380 (18) | 0.20935 (17) | 0.1136 (7) |
| O4 | 0.56490 (10) | 0.41825 (11) | 0.38665 (9) | 0.0540 (3) |
| O5 | 0.85768 (14) | 0.40747 (13) | 0.36701 (11) | 0.0694 (4) |
| O6 | 1.01414 (17) | −0.07558 (14) | 0.23454 (16) | 0.0928 (5) |
| N1 | 0.59053 (11) | 0.34105 (11) | 0.08463 (11) | 0.0437 (3) |
| H1 | 0.6117 | 0.3148 | 0.0225 | 0.052* |
| N2 | 0.76003 (11) | 0.59836 (11) | 0.22108 (10) | 0.0415 (3) |
| N3 | 0.74550 (12) | 0.61887 (12) | 0.45091 (11) | 0.0451 (3) |
| H3 | 0.6848 | 0.5629 | 0.3989 | 0.054* |
| N4 | 0.90252 (13) | 0.43894 (13) | 0.19194 (12) | 0.0516 (3) |
| H4 | 0.8802 | 0.4655 | 0.2534 | 0.062* |
| C1 | 0.73993 (17) | −0.20695 (16) | −0.08058 (17) | 0.0616 (5) |
| H1A | 0.7592 | −0.2785 | −0.1001 | 0.074* |
| C2 | 0.70537 (15) | −0.15823 (15) | 0.01654 (16) | 0.0526 (4) |
| H2 | 0.7016 | −0.1971 | 0.0626 | 0.063* |
| C3 | 0.67525 (13) | −0.04999 (13) | 0.04830 (13) | 0.0402 (3) |
| C4 | 0.63472 (14) | 0.00249 (15) | 0.14884 (13) | 0.0446 (4) |
| C5 | 0.61104 (14) | 0.10929 (14) | 0.17492 (13) | 0.0431 (3) |
| H5 | 0.5863 | 0.1427 | 0.2404 | 0.052* |
| C6 | 0.62166 (12) | 0.17220 (13) | 0.10878 (12) | 0.0375 (3) |
| C7 | 0.59061 (13) | 0.27939 (13) | 0.14202 (12) | 0.0406 (3) |
| H7 | 0.5686 | 0.3081 | 0.2096 | 0.049* |
| C8 | 0.55727 (15) | 0.45134 (15) | 0.11765 (15) | 0.0504 (4) |
| H8A | 0.5375 | 0.4724 | 0.1896 | 0.060* |
| H8B | 0.4867 | 0.4367 | 0.0633 | 0.060* |
| C9 | 0.65926 (15) | 0.55664 (14) | 0.12552 (14) | 0.0498 (4) |
| H9A | 0.6868 | 0.5310 | 0.0572 | 0.060* |
| H9B | 0.6301 | 0.6240 | 0.1326 | 0.060* |
| C10 | 0.75461 (17) | 0.70437 (14) | 0.31734 (14) | 0.0506 (4) |
| H10A | 0.6712 | 0.6981 | 0.3161 | 0.061* |
| H10B | 0.7928 | 0.7781 | 0.3110 | 0.061* |
| C11 | 0.81524 (17) | 0.71464 (15) | 0.42733 (14) | 0.0531 (4) |
| H11A | 0.8948 | 0.7082 | 0.4247 | 0.064* |
| H11B | 0.8241 | 0.7939 | 0.4869 | 0.064* |
| C12 | 0.76748 (14) | 0.61120 (14) | 0.54377 (13) | 0.0440 (4) |
| H12 | 0.8319 | 0.6725 | 0.6009 | 0.053* |
| C13 | 0.70084 (14) | 0.51669 (14) | 0.56429 (12) | 0.0411 (3) |
| C14 | 0.60062 (13) | 0.41909 (14) | 0.47886 (12) | 0.0419 (3) |
| C15 | 0.54290 (14) | 0.31796 (14) | 0.50257 (13) | 0.0445 (4) |
| C16 | 0.45017 (16) | 0.21765 (17) | 0.41887 (15) | 0.0571 (4) |
| H16 | 0.4248 | 0.2179 | 0.3509 | 0.069* |
| C17 | 0.39626 (19) | 0.11893 (19) | 0.43580 (18) | 0.0708 (6) |
| H17 | 0.3345 | 0.0527 | 0.3798 | 0.085* |
| C18 | 0.4344 (2) | 0.11853 (19) | 0.53676 (18) | 0.0728 (6) |
| H18 | 0.3981 | 0.0512 | 0.5479 | 0.087* |
| C19 | 0.87199 (16) | 0.10065 (18) | 0.50848 (18) | 0.0615 (5) |
| H19 | 0.8667 | 0.0538 | 0.5474 | 0.074* |
| C20 | 0.91004 (15) | 0.06424 (15) | 0.41139 (17) | 0.0543 (4) |
| H20 | 0.9301 | −0.0071 | 0.3854 | 0.065* |
| C21 | 0.91929 (13) | 0.13297 (14) | 0.35022 (14) | 0.0437 (4) |
| C22 | 0.95756 (14) | 0.09777 (14) | 0.24645 (14) | 0.0467 (4) |
| C23 | 0.95906 (14) | 0.16782 (14) | 0.19058 (14) | 0.0466 (4) |
| H23 | 0.9816 | 0.1425 | 0.1231 | 0.056* |
| C24 | 0.92879 (13) | 0.27546 (14) | 0.22882 (13) | 0.0432 (3) |
| C25 | 0.93273 (14) | 0.34164 (15) | 0.16545 (14) | 0.0477 (4) |
| H25 | 0.9589 | 0.3132 | 0.1001 | 0.057* |
| C26 | 0.90287 (18) | 0.50816 (18) | 0.12679 (16) | 0.0585 (5) |
| H26A | 0.8428 | 0.4589 | 0.0564 | 0.070* |
| H26B | 0.9808 | 0.5284 | 0.1105 | 0.070* |
| C27 | 0.87601 (16) | 0.62363 (16) | 0.19195 (15) | 0.0527 (4) |
| H27A | 0.9392 | 0.6747 | 0.2600 | 0.063* |
| H27B | 0.8753 | 0.6684 | 0.1478 | 0.063* |
| C28 | 0.68638 (13) | 0.00916 (13) | −0.02121 (12) | 0.0394 (3) |
| C29 | 0.72153 (16) | −0.04322 (15) | −0.12058 (14) | 0.0523 (4) |
| H29 | 0.7280 | −0.0048 | −0.1670 | 0.063* |
| C30 | 0.74651 (18) | −0.15052 (17) | −0.15024 (16) | 0.0632 (5) |
| H30 | 0.7679 | −0.1855 | −0.2173 | 0.076* |
| C31 | 0.66041 (13) | 0.12407 (13) | 0.00769 (12) | 0.0391 (3) |
| C32 | 0.6099 (2) | −0.0547 (2) | 0.22167 (19) | 0.0730 (6) |
| H32 | 0.5881 | −0.0097 | 0.2857 | 0.088* |
| C33 | 0.73386 (15) | 0.51555 (15) | 0.66757 (12) | 0.0454 (4) |
| H33 | 0.7973 | 0.5810 | 0.7223 | 0.054* |
| C34 | 0.67810 (15) | 0.42366 (15) | 0.69214 (13) | 0.0469 (4) |
| C36 | 0.58160 (15) | 0.31893 (15) | 0.60675 (13) | 0.0468 (4) |
| C37 | 0.52430 (18) | 0.21533 (18) | 0.62054 (17) | 0.0626 (5) |
| H37 | 0.5481 | 0.2128 | 0.6877 | 0.075* |
| C38 | 0.88971 (15) | 0.31522 (15) | 0.33144 (14) | 0.0471 (4) |
| C39 | 0.88716 (14) | 0.23985 (15) | 0.39186 (14) | 0.0450 (4) |
| C40 | 0.84899 (17) | 0.27474 (18) | 0.49113 (16) | 0.0580 (4) |
| H40 | 0.8283 | 0.3457 | 0.5183 | 0.070* |
| C41 | 0.84143 (17) | 0.20636 (19) | 0.54918 (18) | 0.0632 (5) |
| H41 | 0.8160 | 0.2308 | 0.6152 | 0.076* |
| C42 | 0.99614 (17) | −0.00763 (16) | 0.19591 (18) | 0.0621 (5) |
| H42 | 1.0087 | −0.0255 | 0.1248 | 0.074* |
| O3a | 0.7964 (2) | 0.5211 (2) | 0.87907 (15) | 0.0734 (8) |
| C35Ab | 0.724 (3) | 0.425 (2) | 0.800 (2) | 0.0602 (18) |
| H35Ab | 0.6984 | 0.3542 | 0.8096 | 0.072* |
| O3Ab | 0.6662 (6) | 0.3918 (5) | 0.8569 (4) | 0.096 (2) |
| C35a | 0.7162 (12) | 0.4418 (9) | 0.8064 (11) | 0.0602 (18) |
| H35a | 0.7905 | 0.5011 | 0.8467 | 0.072* |
| O1Wc | 1.1141 (3) | −0.2607 (3) | 0.1164 (3) | 0.0865 (7) |
| H1WAc | 1.1767 | −0.2286 | 0.1001 | 0.130* |
| H1WBc | 1.0670 | −0.2215 | 0.1104 | 0.130* |
| O1Xd | 1.1651 (4) | −0.2285 (4) | 0.1835 (4) | 0.0865 (7) |
| H1XAd | 1.1426 | −0.1661 | 0.2104 | 0.130* |
| H1XBd | 1.2338 | −0.2080 | 0.1707 | 0.130* |
aOccupancy: 0.672(4), boccupancy: 0.328(4), coccupancy: 0.561(4), doccupancy: 0.439(4).
Source of material
To a solution of 1-naphthol (1 g, 6.9 mmol) in trifluoroacetic acid (10 mL) was added hexamethylenetetramine (1.94 g, 13.8 mmol). The above mixture was heated and stirred for about 1 h. Then concentrated sulfuric acid with a concentration of 33% (10 mL) was added to the stirred solution. The reaction mixture was then stirred and heated for 1 h. Then water was added and the whole solution was extracted by ethyl acetate (30 mL × 2). The mixture was washed with brine and dried and filtered by adding anhydrous magnesium sulfate. The reaction mixture is further purified by column chromatography on silica gel, using ethyl acetate: n-hexane (3:7) as the eluant to afford 1-hydroxy-2,4-diformylnaphthalene as yellow oil. To the solution, 1-hydroxy-2,4-diformylnaphthalene (0.60 g, 3 mmol) in 50 mL ethanol was added tri (2-aminoethyl) amine (0.15 g, 1 mmol). The reaction mixture was refluxed for 8 h, then the solution was cooled to room temperature. The yellow precipitated residue was removed from the solution by filtration and then washed with ethanol and trichloromethane three times. Then a share of the product (0.15 mmol) was dissolved in 20 mL DMF solution. Single crystals were obtained from DMF by slow evaporation at room temperature.
Experimental details
Using Olex2 [1], the structure was solved using Charge Flipping and refined with the ShelXL [3] refinement. All hydrogen atoms were positioned geometrically, with the d (C–H) = 0.97–0.99 Å, Uiso(H) = 1.2 times Ueq(C) and Uiso(H) = 1.5 times Ueq(O).
Comment
Naphthaldehyde based nitrogen rich Schiff bases bear excellent coordinating abilities to act as either multidentate ligands or bridging blocks in supramolecular chemistry and have been highlighted in the literature due to novel structural architectures as well as potential applications of their complexes [5]. In the crystal structure of the title compound, the asymmetric unit of the title compound consists of one formula unit of the target molecule and one water molecule. The bond lengths and bond angles are in the normal ranges [6]. The crystal structure shows that the hydrogen on the hydroxyl oxygen atom of phenol moiety is transferred to the nitrogen atom of imine to form keto-enamine [6]. There are intramolecular hydrogen bonds (for example: N1–H1⋯O1, N3–H3⋯O4, N4–H4⋯O5, C7–H7⋯O4) in the molecules. Through the hydrogen bond of O1W–H1WA⋯O1, O1W–H1WB⋯O6 the interaction between the target and water molecules extends into a dimer structure. The water molecule and one of the aldehyde groups show a disorder.
Funding source: National Natural Science Foundation of China
Award Identifier / Grant number: 22065009
Award Identifier / Grant number: 22066007
Funding source: Preventive Medicine
Award Identifier / Grant number: 2017[85]
Funding source: Science and Technology Foundation of Guizhou Province
Award Identifier / Grant number: [2019]2792
Award Identifier / Grant number: [2018]5779-14
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: This work was supported by and the National Natural Science Foundation of China (22065009, 22066007) and Preventive Medicine (No. 2017[85]), Science and Technology Foundation of Guizhou Province (grant numbers [2019]2792, [2018]5779-14).
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Suche in Google Scholar
2. Bruker. APEX2, SAINT and SADABS; Bruker AXS Inc.: Madison, Wisconsin, USA, 2012.Suche in Google Scholar
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar
4. Brandenburg, K. DIAMOND. Visual Crystal Structure Information System. Ver. 4.0; Crystal Impact: Bonn, Germany, 2015.Suche in Google Scholar
5. Naskar, B., Modak, R., Mait, D. K., Drew, M. G. B., Bauza, A., Frontera, A., Mukhopadhyay, C. D., Mishra, S., Saha, K. D., Goswami, S. A Schiff base platform: structures, sensing of Zn(II) and PPi in aqueous medium and anticancer activity. Dalton Trans. 2017, 46, 9498–9510; https://doi.org/10.1039/c7dt01932g.Suche in Google Scholar
6. Park, H., Kim, J., Kwon, E., Kim, T. H. Crystal structure of flucetosulfuron. Acta Crystallogr. 2017, E73, 1439–1442; https://doi.org/10.1107/s2056989017012737.Suche in Google Scholar
© 2021 Qing Shi et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of poly[(μ2-aqua-tetraaqua-(μ3-glutarato-κ4O,O′:O′:O′′)-(μ5-glutarato-κ6O:O,O′:O′:O′′:O′′′)distrontium(II)], C10H22O13Sr2
- The crystal structure of acetato-κ1O-{(2-(2-(2-aminophenoxy)ethoxy)phenyl)(4-oxo-4-phenylbut-2-en-2-yl)amido-κ2N,N′,O}copper(II), C26H26CuN2O5
- Crystal structure of dimethanolato-k2O:O-bis(1-((2-methyl-1H-benzo[d]imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κN)-bis(thiocyanato-κN)dicopper(II), C34H32Cu2N12O2S2
- Crystal structure of poly[diaqua-bis(μ2-3-(pyrimidin-5-yl)benzoato-κ2N:O)cobalt(II)] dihydrate, [Co(C11H11O2N2)2(H2O)2]
- Crystal structure of bis(3,3-dimethyl-1-phenylbut-1-en-2-yl)(trimethylsilyl)amido-k1N)zinc(II), Zn(C15H24NSi)2
- Crystal structure of catena-poly[(μ2-methanolato-κ2O:O)-(μ2-1-((2-methyl-1H-benzo[d]imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κ2N:N′)-(thiocyanato-κ1N)copper(II)] 0.25 hydrate, C17H16CuN6OS ⋅ 0.5H2O
- The crystal structure of 2-amino-5-nitroanilinium iodide monohydrate, C6H8IN3O2
- The crystal structure of 3-amino-5-carboxypyridin-1-ium perchlorate monohydrate, C6H9ClN2O7
- Crystal structure of 7-hydroxy-2,4-dimethoxy-9,10-dihydrophenanthrene from Arundina graminifolia, C16H16O3
- Crystal structure of 6,6′-((1E, 1′E)-(((1R, 2R)-1,2-diphenylethane-1,2-diyl) bis(azanylylidene))bis(methanylylidene))bis(2-ethylphenol), C32H32N2O2
- The crystal structure of 2-amino-5-carboxypyridin-1-ium iodide monohydrate, C6H9IN2O3
- The crystal structure of 2-(3,5-difluorophenyl)-2,3-dihydro-1H-naphtho[1,8-de][1,3,2]diazaborinine, C16H11BF2N2
- Crystal structure of bis{(2-pyridinyl)-1-phenyl-1-isopropylmethanolato-κ2N,O}nickel, C30H32N2NiO2
- Crystal structure of poly[(m3-3-carboxyadamantane-1-carboxylato-κ3O:O′:O″)-(phenanthroline-κ2N,N′)sodium(II)], C24H23N2NaO4
- Crystal structure of 2-phenylethynyl-1,3,6,8-tetramethylBOPHY (BOPHY = bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl)methylene)hydrazine), C22H20B2F4N4
- Crystal structure of 4-tert-butyl-2-N-(2-pyridylmethyl)aminophenol, C16H20N2O
- The crystal structure of (3Z,3′Z)-4,4′-((1,4-phenylenebis(methylene))bis(azanediyl))bis(pent-3-en-2-one), C18H24N2O2
- Crystal structure of (morpholine-1-carbodithioato-κ2-S,S′)bis(triphenylphosphine-κ-P)gold(I), C41H38AuNOP2S2
- Crystal structure of 1,4-bis(4-bromobenzyl)-4-(4-chlorophenyl)-1,4-dihydropyridine-3-carbonitrile, C26H19Br2ClN2
- The crystal structure of fac-tricarbonyl (N′-benzoyl-N,N-diphenylcarbamimidothioato-κ2S,O)-(pyrazole-κN)rhenium(I) — methanol (1/1) C26H23O4N4SRe
- The crystal structure of Ba2Mn(SeO3)2Cl2 containing 1∞[Mn(SeO3)2Cl2]4− chains
- Crystal structure of 3,3′,3″-((1E,1′E,1″E)-((nitrilotris(ethane-2,1-diyl))tris(azaneylylidene)) tris(methaneylylidene))tris(4-hydroxy-1-naphthaldehyde) monohydrate, C42H36N4O6·H2O
- The crystal structure of 4-(6-acetyl-5-methyl-4,7-dihydrotetrazolo[1,5-a]pyrimidin-7-yl)benzonitrile, C14H12N6O
- Crystal structure of benzo[d][1,3]dioxol-5-yl-2-(6-methoxynaphthalen-2-yl)propanoate, C21H18O5
- The crystal structure of ethyl 5-methyl-7-(4-(phenylthio)phenyl)-4,7-dihydrotetrazolo[1,5-a]pyrimidine-6-carboxylate, C20H19N5O2S
- Crystal structure of N′,N‴-((propane-2,2-diylbis(1H-pyrrole-5,2-diyl))bis(methaneylylidene))-di(isonicotinohydrazide)– water – dimethylformamide (1/4/2), C25H24N8O2·4H2O·2C3H7NO
- Synthesis and crystal structure of 4-(2,4-dinitrophenoxy)benzaldehyde, C13H8N2O6
- The crystal structure of 1-dodecylpyridin-1-ium bromide monohydrate, C17H32BrNO
- Crystal structure of (E)-amino(2-(4-(dimethylamino)benzylidene)hydrazineyl)methaniminium nitrate, C10H16N6O3
- Crystal structure of (E)-(2-((1H-pyrrol-2-yl)methylene)hydrazineyl)(amino)methaniminium nitrate monohydrate, C6H12N6O4
- The crystal structure of hexakis(1-propylimidazole-κ1N)copper(II) dichloride, C36H60Cl2CuN12
- The crystal structure of bis{(μ2-3,3-dimethyl-1-phenylbut-1-en-2-yl)((dimethylamino)dimethylsilyl)amido-κ3N,N′:N′}dilithium, C32H54Li2N4Si2
- The crystal structure of methyl 4-(1H-naphtho[1,8-de][1,3,2]diazaborinin-2(3H)-yl)benzoate, C18H15BN2O2
- Crystal structure of (E)-N-(1-((2-chlorothiazol-5-yl)methyl)pyridin-2(1H)-ylidene)-2,2,2-trifluoroacetamide, C11H7ClF3N3OS
- Crystal structure of N′, N‴-((propane-2,2-diylbis(1H-pyrrole-5,2-diyl))bis (methaneylylidene))di(picolinohydrazide) – water – methanol (1/1/1), C25H24N8O2·H2O·CH3OH
- Crystal structure of 3-(2-chloro-benzyl)-7-[4-(2-chloro-benzyl)-piperazin-1-yl]-5,6,8-trifluoro-3H-quinazolin-4-one, C26H21Cl2F3N4O
- Crystal structure of N1,N2-bis(2-fluorobenzyl)benzene-1,2-diamine,C20H18F2N2
- The crystal structure of 2-(benzo[d][1,3]dioxol-5-yl)-2,3-dihydro-1H-naphtho[1,8-de][1,3,2]diazaborinine, C17H13BN2O2
- The crystal structure of 6,6′-((1E,1′E)-hydrazine-1,2-diylidenebis(methaneylylidene)) bis(2-bromo-4-nitrophenol) — dimethylsulfoxide (1/2), C14H8Br2N4O6⋅2(C2H6OS)
- Selective biocatalytic synthesis and crystal structure of (2R,6R)-hydroxyketaminium chloride, C13H17Cl2NO2
- Crystal structure of bis{tetraaqua-[μ3-1-(4-carboxylatophenyl)-5-methyl-1H-pyrazole-3-carboxylate-κ4N,O,O′,O″] [μ2-1-methyl-1H-pyrazole-3,5-dicarboxylate-κ3N,O:O]dicobalt(II)} dihydrate, C36H44Co4N8O26
- Crystal structure of diethyl-2,2′-naphthalene-2,3-diylbis(oxy)diacetate, C18H20O6
- Synthesis and crystal structure of poly[(μ3-2-(2-carboxylatophenyl)-1H-benzo[d]imidazole-5-carboxylato-κO,O′:O′;:O″, O″′)-(μ2-1-(4-(1Himidazol-1-yl)phenyl)-1H-imidazole-κ2N:N′)cadmium(II)], C27H18CdN6O4
- The crystal structure of catena-poly[diaqua-bis(μ2-2-((2-(2-phenylacetyl)hydrazineylidene)methyl)benzoato-κ2O:O')zinc(II)], C32H30N4O8Zn
- The crystal structure of 2-(3,4-dimethoxyphenyl)-2,3-dihydro-1H-naphtho [1,8-de][1,3,2]diazaborinine, C18H17BN2O2
- The crystal structure of hexakis(1-ethylimidazole-κ1N)nickel(II) dichloride – 1-ethylimidazole (1/2), C40H64Cl2NiN16
- Crystal structure of diaqua-bis(2,4-dinitrophenolato-κ2O,O′)copper(II) 1.5 hydrate, C12H13CuN4O13.5
- Crystal structure of N′,N‴-((1E,1′E)-((decane-1,10-diylbis(oxy))bis(2,1-phenylene)) bis(methaneylylidene))di(isonicotinohydrazide), C36H40N6O4
- The crystal structure of 2-[(R)-1-(naphthalen-1-yl)ethyl]-2,3,7,7a-tetrahydro-3a,6-epoxyisoindol-1(6H)-one, C19H20NO2
- Synthesis and crystal structure of (1E,2E)-3-(anthracen-9-yl)-1-(4-methoxyphenyl)prop-2-en-1-one oxime, C24H19NO2
- Synthesis and crystal structure of (2E,2′E)-3,3′-(1,3-phenylene)bis(1-(3-bromophenyl)prop-2-en-1-one), C24H16Br2O2
- The crystal structure of catena-poly[bis(µ2-1,2-bis((1H-imidazol-1-yl)methyl)benzene- κ2N:N′)-bis(nitrato-κO)copper(II)], C28H28N10O6Cu
- Synthesis and crystal structure of the novel chiral acetyl-3-thiophene-5-(9-anthryl)-2-pyrazoline, C23H18N2OS
- Crystal structure of (E)-3-(dimethylamino)-1-(thiophen-3-yl)prop-2-en-1-one, C9H11NOS
- Crystal structure of catena-poly[aqua-(4-iodopyridine-2,6-dicarboxylato-κ3N,O,O′)-(μ2-4-amino-4H-1,2,4-triazole-κ2N:N′) copper(II)], C9H8N5O5CuI
- Crystal structure of cyclopropane-1,2,3-triyltris(phenylmethanone), C24H18O3
- Crystal structure of bis(amino(thioureido)methaniminium) terephthalate, C12H18N8O4S2
- A three-dimensional Eu(III) framework in the crystal structure of dimethylaminium poly[dimethylformamide-κ1N)bis(μ4-terephthalato-κ4O:O′:O′′:O′′′)europium(III)] monohydrate, C21H25EuN2O10
- Crystal structure of 2-methoxyphenyl 2-(6-methoxynaphthalen-2-yl)propanoate, C21H20O4
- The crystal structure of Hexakis(diethylamido)dimolybdenum, Mo2(NEt2)6
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of poly[(μ2-aqua-tetraaqua-(μ3-glutarato-κ4O,O′:O′:O′′)-(μ5-glutarato-κ6O:O,O′:O′:O′′:O′′′)distrontium(II)], C10H22O13Sr2
- The crystal structure of acetato-κ1O-{(2-(2-(2-aminophenoxy)ethoxy)phenyl)(4-oxo-4-phenylbut-2-en-2-yl)amido-κ2N,N′,O}copper(II), C26H26CuN2O5
- Crystal structure of dimethanolato-k2O:O-bis(1-((2-methyl-1H-benzo[d]imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κN)-bis(thiocyanato-κN)dicopper(II), C34H32Cu2N12O2S2
- Crystal structure of poly[diaqua-bis(μ2-3-(pyrimidin-5-yl)benzoato-κ2N:O)cobalt(II)] dihydrate, [Co(C11H11O2N2)2(H2O)2]
- Crystal structure of bis(3,3-dimethyl-1-phenylbut-1-en-2-yl)(trimethylsilyl)amido-k1N)zinc(II), Zn(C15H24NSi)2
- Crystal structure of catena-poly[(μ2-methanolato-κ2O:O)-(μ2-1-((2-methyl-1H-benzo[d]imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κ2N:N′)-(thiocyanato-κ1N)copper(II)] 0.25 hydrate, C17H16CuN6OS ⋅ 0.5H2O
- The crystal structure of 2-amino-5-nitroanilinium iodide monohydrate, C6H8IN3O2
- The crystal structure of 3-amino-5-carboxypyridin-1-ium perchlorate monohydrate, C6H9ClN2O7
- Crystal structure of 7-hydroxy-2,4-dimethoxy-9,10-dihydrophenanthrene from Arundina graminifolia, C16H16O3
- Crystal structure of 6,6′-((1E, 1′E)-(((1R, 2R)-1,2-diphenylethane-1,2-diyl) bis(azanylylidene))bis(methanylylidene))bis(2-ethylphenol), C32H32N2O2
- The crystal structure of 2-amino-5-carboxypyridin-1-ium iodide monohydrate, C6H9IN2O3
- The crystal structure of 2-(3,5-difluorophenyl)-2,3-dihydro-1H-naphtho[1,8-de][1,3,2]diazaborinine, C16H11BF2N2
- Crystal structure of bis{(2-pyridinyl)-1-phenyl-1-isopropylmethanolato-κ2N,O}nickel, C30H32N2NiO2
- Crystal structure of poly[(m3-3-carboxyadamantane-1-carboxylato-κ3O:O′:O″)-(phenanthroline-κ2N,N′)sodium(II)], C24H23N2NaO4
- Crystal structure of 2-phenylethynyl-1,3,6,8-tetramethylBOPHY (BOPHY = bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl)methylene)hydrazine), C22H20B2F4N4
- Crystal structure of 4-tert-butyl-2-N-(2-pyridylmethyl)aminophenol, C16H20N2O
- The crystal structure of (3Z,3′Z)-4,4′-((1,4-phenylenebis(methylene))bis(azanediyl))bis(pent-3-en-2-one), C18H24N2O2
- Crystal structure of (morpholine-1-carbodithioato-κ2-S,S′)bis(triphenylphosphine-κ-P)gold(I), C41H38AuNOP2S2
- Crystal structure of 1,4-bis(4-bromobenzyl)-4-(4-chlorophenyl)-1,4-dihydropyridine-3-carbonitrile, C26H19Br2ClN2
- The crystal structure of fac-tricarbonyl (N′-benzoyl-N,N-diphenylcarbamimidothioato-κ2S,O)-(pyrazole-κN)rhenium(I) — methanol (1/1) C26H23O4N4SRe
- The crystal structure of Ba2Mn(SeO3)2Cl2 containing 1∞[Mn(SeO3)2Cl2]4− chains
- Crystal structure of 3,3′,3″-((1E,1′E,1″E)-((nitrilotris(ethane-2,1-diyl))tris(azaneylylidene)) tris(methaneylylidene))tris(4-hydroxy-1-naphthaldehyde) monohydrate, C42H36N4O6·H2O
- The crystal structure of 4-(6-acetyl-5-methyl-4,7-dihydrotetrazolo[1,5-a]pyrimidin-7-yl)benzonitrile, C14H12N6O
- Crystal structure of benzo[d][1,3]dioxol-5-yl-2-(6-methoxynaphthalen-2-yl)propanoate, C21H18O5
- The crystal structure of ethyl 5-methyl-7-(4-(phenylthio)phenyl)-4,7-dihydrotetrazolo[1,5-a]pyrimidine-6-carboxylate, C20H19N5O2S
- Crystal structure of N′,N‴-((propane-2,2-diylbis(1H-pyrrole-5,2-diyl))bis(methaneylylidene))-di(isonicotinohydrazide)– water – dimethylformamide (1/4/2), C25H24N8O2·4H2O·2C3H7NO
- Synthesis and crystal structure of 4-(2,4-dinitrophenoxy)benzaldehyde, C13H8N2O6
- The crystal structure of 1-dodecylpyridin-1-ium bromide monohydrate, C17H32BrNO
- Crystal structure of (E)-amino(2-(4-(dimethylamino)benzylidene)hydrazineyl)methaniminium nitrate, C10H16N6O3
- Crystal structure of (E)-(2-((1H-pyrrol-2-yl)methylene)hydrazineyl)(amino)methaniminium nitrate monohydrate, C6H12N6O4
- The crystal structure of hexakis(1-propylimidazole-κ1N)copper(II) dichloride, C36H60Cl2CuN12
- The crystal structure of bis{(μ2-3,3-dimethyl-1-phenylbut-1-en-2-yl)((dimethylamino)dimethylsilyl)amido-κ3N,N′:N′}dilithium, C32H54Li2N4Si2
- The crystal structure of methyl 4-(1H-naphtho[1,8-de][1,3,2]diazaborinin-2(3H)-yl)benzoate, C18H15BN2O2
- Crystal structure of (E)-N-(1-((2-chlorothiazol-5-yl)methyl)pyridin-2(1H)-ylidene)-2,2,2-trifluoroacetamide, C11H7ClF3N3OS
- Crystal structure of N′, N‴-((propane-2,2-diylbis(1H-pyrrole-5,2-diyl))bis (methaneylylidene))di(picolinohydrazide) – water – methanol (1/1/1), C25H24N8O2·H2O·CH3OH
- Crystal structure of 3-(2-chloro-benzyl)-7-[4-(2-chloro-benzyl)-piperazin-1-yl]-5,6,8-trifluoro-3H-quinazolin-4-one, C26H21Cl2F3N4O
- Crystal structure of N1,N2-bis(2-fluorobenzyl)benzene-1,2-diamine,C20H18F2N2
- The crystal structure of 2-(benzo[d][1,3]dioxol-5-yl)-2,3-dihydro-1H-naphtho[1,8-de][1,3,2]diazaborinine, C17H13BN2O2
- The crystal structure of 6,6′-((1E,1′E)-hydrazine-1,2-diylidenebis(methaneylylidene)) bis(2-bromo-4-nitrophenol) — dimethylsulfoxide (1/2), C14H8Br2N4O6⋅2(C2H6OS)
- Selective biocatalytic synthesis and crystal structure of (2R,6R)-hydroxyketaminium chloride, C13H17Cl2NO2
- Crystal structure of bis{tetraaqua-[μ3-1-(4-carboxylatophenyl)-5-methyl-1H-pyrazole-3-carboxylate-κ4N,O,O′,O″] [μ2-1-methyl-1H-pyrazole-3,5-dicarboxylate-κ3N,O:O]dicobalt(II)} dihydrate, C36H44Co4N8O26
- Crystal structure of diethyl-2,2′-naphthalene-2,3-diylbis(oxy)diacetate, C18H20O6
- Synthesis and crystal structure of poly[(μ3-2-(2-carboxylatophenyl)-1H-benzo[d]imidazole-5-carboxylato-κO,O′:O′;:O″, O″′)-(μ2-1-(4-(1Himidazol-1-yl)phenyl)-1H-imidazole-κ2N:N′)cadmium(II)], C27H18CdN6O4
- The crystal structure of catena-poly[diaqua-bis(μ2-2-((2-(2-phenylacetyl)hydrazineylidene)methyl)benzoato-κ2O:O')zinc(II)], C32H30N4O8Zn
- The crystal structure of 2-(3,4-dimethoxyphenyl)-2,3-dihydro-1H-naphtho [1,8-de][1,3,2]diazaborinine, C18H17BN2O2
- The crystal structure of hexakis(1-ethylimidazole-κ1N)nickel(II) dichloride – 1-ethylimidazole (1/2), C40H64Cl2NiN16
- Crystal structure of diaqua-bis(2,4-dinitrophenolato-κ2O,O′)copper(II) 1.5 hydrate, C12H13CuN4O13.5
- Crystal structure of N′,N‴-((1E,1′E)-((decane-1,10-diylbis(oxy))bis(2,1-phenylene)) bis(methaneylylidene))di(isonicotinohydrazide), C36H40N6O4
- The crystal structure of 2-[(R)-1-(naphthalen-1-yl)ethyl]-2,3,7,7a-tetrahydro-3a,6-epoxyisoindol-1(6H)-one, C19H20NO2
- Synthesis and crystal structure of (1E,2E)-3-(anthracen-9-yl)-1-(4-methoxyphenyl)prop-2-en-1-one oxime, C24H19NO2
- Synthesis and crystal structure of (2E,2′E)-3,3′-(1,3-phenylene)bis(1-(3-bromophenyl)prop-2-en-1-one), C24H16Br2O2
- The crystal structure of catena-poly[bis(µ2-1,2-bis((1H-imidazol-1-yl)methyl)benzene- κ2N:N′)-bis(nitrato-κO)copper(II)], C28H28N10O6Cu
- Synthesis and crystal structure of the novel chiral acetyl-3-thiophene-5-(9-anthryl)-2-pyrazoline, C23H18N2OS
- Crystal structure of (E)-3-(dimethylamino)-1-(thiophen-3-yl)prop-2-en-1-one, C9H11NOS
- Crystal structure of catena-poly[aqua-(4-iodopyridine-2,6-dicarboxylato-κ3N,O,O′)-(μ2-4-amino-4H-1,2,4-triazole-κ2N:N′) copper(II)], C9H8N5O5CuI
- Crystal structure of cyclopropane-1,2,3-triyltris(phenylmethanone), C24H18O3
- Crystal structure of bis(amino(thioureido)methaniminium) terephthalate, C12H18N8O4S2
- A three-dimensional Eu(III) framework in the crystal structure of dimethylaminium poly[dimethylformamide-κ1N)bis(μ4-terephthalato-κ4O:O′:O′′:O′′′)europium(III)] monohydrate, C21H25EuN2O10
- Crystal structure of 2-methoxyphenyl 2-(6-methoxynaphthalen-2-yl)propanoate, C21H20O4
- The crystal structure of Hexakis(diethylamido)dimolybdenum, Mo2(NEt2)6

