Abstract
C10H22O13Sr2, monoclinic,
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.13 × 0.10 × 0.06 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 6.08 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, |
| θmax, completeness: | 27.5°, 99% |
| N(hkl)measured, N(hkl)unique, Rint: | 23,735, 4032, 0.069 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 3720 |
| N(param)refined: | 226 |
| Programs: | Bruker [1], SHELX [2], WinGX/ORTEP [3], Diamond [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1 | 0.51616 (17) | 0.19246 (16) | 0.6658 (3) | 0.0159 (4) |
| C2 | 0.42909 (17) | 0.19170 (18) | 0.5253 (3) | 0.0180 (5) |
| H2A | 0.4198 | 0.2538 | 0.4829 | 0.022* |
| H2B | 0.3692 | 0.1746 | 0.554 | 0.022* |
| C3 | 0.44446 (19) | 0.1242 (2) | 0.4047 (3) | 0.0230 (5) |
| H3A | 0.5052 | 0.1399 | 0.3773 | 0.028* |
| H3B | 0.4513 | 0.0615 | 0.4452 | 0.028* |
| C4 | 0.35708 (18) | 0.12804 (19) | 0.2644 (3) | 0.0202 (5) |
| H4A | 0.2962 | 0.1163 | 0.2941 | 0.024* |
| H4B | 0.3528 | 0.1902 | 0.222 | 0.024* |
| C5 | 0.36496 (17) | 0.05896 (16) | 0.1441 (3) | 0.0159 (5) |
| C6 | −0.06624 (18) | −0.12153 (17) | 0.1609 (3) | 0.0176 (5) |
| C7 | −0.16274 (18) | −0.1385 (2) | 0.2035 (3) | 0.0243 (5) |
| H7A | −0.2056 | −0.1761 | 0.1253 | 0.029* |
| H7B | −0.1956 | −0.0793 | 0.2057 | 0.029* |
| C8 | −0.1512 (2) | −0.18666 (19) | 0.3554 (3) | 0.0245 (5) |
| H8A | −0.2165 | −0.1937 | 0.373 | 0.029* |
| H8B | −0.1248 | −0.2485 | 0.3492 | 0.029* |
| C9 | −0.0848 (2) | −0.1375 (2) | 0.4901 (3) | 0.0317 (7) |
| H9A | −0.0297 | −0.1101 | 0.4586 | 0.038* |
| H9B | −0.0573 | −0.1837 | 0.5665 | 0.038* |
| C10 | −0.13254 (18) | −0.06194 (18) | 0.5629 (3) | 0.0195 (5) |
| O1 | 0.59173 (13) | 0.23877 (13) | 0.6605 (2) | 0.0235 (4) |
| O2 | 0.50993 (14) | 0.14622 (14) | 0.7787 (2) | 0.0277 (4) |
| O1W | 0.05383 (13) | −0.16005 (13) | −0.1429 (2) | 0.0240 (4) |
| H11W | 0.0156 | −0.1311 | −0.1881 | 0.036* |
| H12W | 0.0372 | −0.2114 | −0.1714 | 0.036* |
| O3 | 0.29968 (13) | −0.00266 (13) | 0.1063 (2) | 0.0242 (4) |
| O2W | 0.37484 (15) | 0.00523 (16) | −0.2703 (2) | 0.0382 (5) |
| H21W | 0.3267 | 0.0183 | −0.3427 | 0.057* |
| H22W | 0.4119 | 0.049 | −0.2576 | 0.057* |
| O4 | 0.43685 (13) | 0.06463 (13) | 0.0838 (2) | 0.0215 (4) |
| O3W | 0.21968 (14) | −0.11833 (16) | −0.2416 (2) | 0.0348 (5) |
| H31W | 0.206 | −0.0829 | −0.2932 | 0.052* |
| H32W | 0.1653 | −0.1289 | −0.2213 | 0.052* |
| O5 | −0.06442 (14) | −0.05736 (14) | 0.0690 (2) | 0.0259 (4) |
| O4W | 0.26228 (13) | −0.20336 (12) | 0.0631 (2) | 0.0222 (4) |
| H41W | 0.2893 | −0.2233 | 0.1305 | 0.033* |
| H42W | 0.2454 | −0.2437 | 0.0114 | 0.033* |
| O6 | 0.00898 (13) | −0.17032 (13) | 0.2169 (2) | 0.0250 (4) |
| O5W | 0.23792 (14) | −0.13547 (14) | 0.3716 (2) | 0.0288 (4) |
| H51W | 0.2915 | −0.1455 | 0.3775 | 0.043* |
| H52W | 0.2364 | −0.0931 | 0.419 | 0.043* |
| O7 | −0.19957 (15) | −0.01268 (15) | 0.4795 (2) | 0.0331 (5) |
| O8 | −0.10218 (15) | −0.05047 (14) | 0.7027 (2) | 0.0282 (4) |
| Sr1 | 0.13186 (2) | −0.07267 (2) | 0.10847 (2) | 0.01443 (8) |
| Sr2 | 0.39088 (2) | −0.10281 (2) | −0.05032 (2) | 0.01555 (8) |
Source of material
Block colourless single crystals of the title compound were obtained by a hydrothermal reaction of an equimolar ratio (0.5 mmol) of strontium chloride and glutaric acid (2 mmol) of imidazole and 6 ml of water in a 23 ml Teflon-lined acid digestion bomb (Parr), which was heated for three days at 180 °C under autogeneous pressure and then cooled down to room temperature. The product obtained were collected by filtration, thoroughly washed with distilled water and ethanol, and finally dried at room temperature.
Experimental details
The H atoms of water molecules were refined freely. All other hydrogen atoms bonded to C atoms were placed in idealized positions using the standard riding models of the SHELX System (with C—H = 0.97 Å) [2].
Comment
The synthesis of coordination polymers requires polyfunctional organic ligands (linkers), which bind metal atoms together to form structures of different dimensionalities. Rational design and synthesis of metal-organic coordination polymers has become one of the most active areas of chemical research and materials science [5]. The anions of polycarboxylic acids, and dicarboxylic acid in particular, are used quite often as linkers [6], [7], [8], [9], [10]. Over the past decades glutaric acid which is an aliphatic dicarboxylic acid was used to obtain coordination polymers [11], [12], [13]. It is not only balancing the charge of the metal organic hybrid species, but it also plays a crucial role in deriving structural diversity.
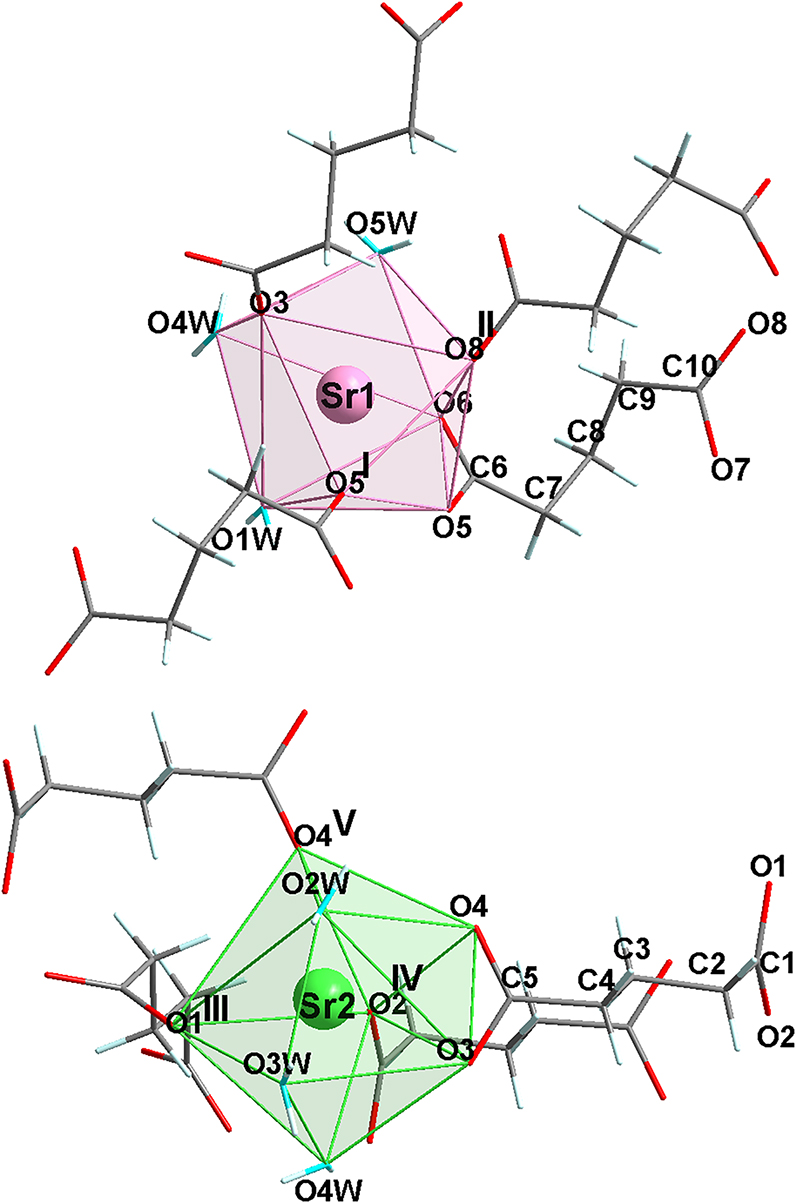
The asymmetric unit of the title compound contains two Sr2+ ions, two glutarate ligands and five H2O molecules, connected together to form a three-dimensional framework. Each Sr polyhedron shares two edges through either two O5 atoms, two O4 atoms or O3 and O4W atoms to form zig-zag chains running along the a axis with Sr1 ⃛ Sr1, Sr2 ⃛ Sr2 and Sr1 ⃛ Sr2 distances of 4.2494(3), 4.1719(3) and 4.2475(3) Å, respectively. The connexion between adjacent chains is ensured by the organic ligands. The coordination geometry of each Sr2+ ion can be described as a dodecahedral fashion comprised of five O atoms from glutarate anions involved in a mono- and bidentate mode, and water molecules.
In the Sr1 bisdisphenoid, the standard mean deviation from planarity of the first face [O3, O6, O8II, O4W] is 0.1147 Å (for symmetry operation (II) −x, −y, 1−z). Also, the distances from the central Sr1 atom to the centre of each face differ from each other [0.6547 Å against 1.8548 Å for the second plane [O5, O5I, O1W] (for symmetry operation (I) −x, −y, −z)]. The Sr1–O distances fall in the range 2.4966(1) − 2.7205(1) Å (av. = 2.61 Å), in good accordance with the value calculated with the bond valence program VALENCE [14] for an eightfold-coordinated Sr2+ cation, i.e., 2.62 Å. Then, the sum of the bond valences around the strontium atom, i.e., 2.1 v.u. must be compared with the +2 oxidation state of Sr. In the Sr2 bisdisphenoid, the standard mean deviation from planarity of the first face [O1III, O2IV, O3, O3W] is 0.039 Å (for symmetry operation (III) 1−x, −1/2 + y, 1/2−z and (IV) 1−x, −y, 1−z). Also, the distances from the central Sr2 atom to the centre of each face differ from each other (0.7669 Å against 1.8571 Å for the second plane [O4, O4V, O2W] [for symmetry operation (V) 1−x, −y, −z]). The Sr-O distances fall in the range 2.5078(1) − 2.7100(1) Å (av. = 2.60 Å), in good accordance with the value calculated with the bond valence program VALENCE [14] for eightfold-coordinated Sr2+ cation, i.e., 2.62 Å. Then, the sum of the bond valences around the strontium atom, i.e., 2.2 v.u. must be compared with the +2 oxidation state of Sr.
There are two crystallographically distinct glutarate ligands, which exhibit two different conformations, namely anti–anti and gauche–gauche. One adopts anti–anti conformation, illustrated by the C1—C2—C3—C4 [178.205(5)°] and C2—C3—C4—C5 [176.774(5)°] torsion angles, the other adopt gauche–gauche conformation demonstrated by the C6—C7—C8—C9 [57.498 (8)°] and C7—C8—C9—C10 [84.859(7)°] torsion angles, respectively. The first carboxylate group binds five metal cations through bidentate and monodentate chelation, the three Csp3–Csp3–Csp3 angles of the glutarate anion C1–C2–C3, C2–C3–C4 and C3–C4–C5 are 112.664(5), 110.804(5) and 113.460(5)°. While the second carboxylate group binds three metal cations through bidentate and monodentate chelation, the three Csp3–Csp3–Csp3 angles of the glutarate anion C6–C7–C8, C7–C8–C9 and C8–C9–C10 are 114.597(5), 115.425(5) and 116.646(5)°. But they are considerably greater than the tetrahedral angle 109°.
Acknowledgements
Thanks are also due to MESRS (Ministère de l’Enseignement Supérieur et de la Recherche Scientifique).
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: This work is supported by the Unité de recherche de Chimie de l’Environnement et Moléculaire Structurale, CHEMS, Université des Frères Mentouri Constantine 1, Algeria.
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. APEX2; Bruker AXS Inc.: Madison, Wisconsin, USA, 2011.Search in Google Scholar
2. Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Search in Google Scholar
3. Farrugia, L. J. WinGX and ORTEP for Windows: an update. J. Appl. Crystallogr. 2012, 45, 849–854; https://doi.org/10.1107/s0021889812029111.Search in Google Scholar
4. Brandenburg, K., Berndt, M. DIAMOND. Visual crystal structure information system; Crystal Impact GbR: Bonn, Germany, 2001.Search in Google Scholar
5. Zheng, Y.-Q., Lin, J.-L., Xu, W., Xie, H.-Z., Sun, J., Wang, X.-W. A family of new glutarate compounds: synthesis, crystal structures of: Co(H2O)5L(1), Na2[CoL2] (2), Na2[L(H2L)4/2] (3), {[Co3(H2O)6L2](HL)2}⋅H2O (4), {[Co3(H2O)6L2](HL)2}.10H2O (5), {[Co3(H2O)6L2]L2/2}⋅4H2O (6), and Na{2[Co3(H2O)2]]L8/2]⋅6H2O (7), and magnetic properties of 1 and 2 with H2L = HOOC-(CH2)3–COOH. Inorg. Chem. 2008, 47, 10280–10287; https://doi.org/10.1021/ic801053p.Search in Google Scholar
6. Kitagawa, S., Kitaura, R., Noro, S. Functional porous coordination polymers. Angew. Chem. Int. Ed. 2004, 43, 2334–2375; https://doi.org/10.1002/anie.200300610.Search in Google Scholar
7. Férey, G. Hybrid porous solids: past, present, future. Chem. Soc. Rev. 2007, 37, 191–214.10.1039/B618320BSearch in Google Scholar PubMed
8. Grzesiak-Nowak, M., Nitek, W., Rafalska-Lasocha, A., Lasocha, W. Synthesis and investigations of new strontium dicarboxylates. Z. für Kristallogr.-Cryst. Mater. 2013, 228, 590–597; https://doi.org/10.1524/zkri.2013.1665.Search in Google Scholar
9. Bataille, T., Bouhali, A., Kouvatas, C., Trifa, C., Audebrand, N., Boudaren, C. Hydrates and polymorphs of lead squarate Pb(C4O4): structural transformations studied by in situ X-ray powder diffraction and solid state NMR. Polyhedron 2019, 164, 123–131; https://doi.org/10.1016/j.poly.2019.02.047.Search in Google Scholar
10. Bouhali, A., Trifa, C., Bouacida, S., Boudaren, C., Bataille, T. Poly [[μ-aqua-tetraaquabis (μ-2-hydroxy-4-oxocyclobut-1-ene-1,3-diolato)strontium] hemihydrate]. Acta Crystallogr. 2011, E67, m1130–m1131; https://doi.org/10.1107/s1600536811028704.Search in Google Scholar
11. Martin, D. P., Montney, M. R., Supkowski, R. M., LaDuca, R. L. Cadmium glutarate coordination polymers containing hydrogen-bonding capable tethering organodiimines: from double interpenetration to supramolecular cavities containing an unprecedented water tape morphology. Cryst. Growth Des. 2008, 8, 3091–3097; https://doi.org/10.1021/cg8003118.Search in Google Scholar
12. Wen, G. L., Wang, Y. Y., Zhang, W. H., Ren, C., Liu, R. T., Shi, Q. Z. Self-assembled coordination polymers of V-shaped bis(pyridyl)thiadiazole dependent upon the spacer length and flexibility of aliphatic dicarboxylate ligands. CrystEngComm 2010, 12, 1238–1251; https://doi.org/10.1039/b919381m.Search in Google Scholar
13. Ghosh, A. K., Ghoshal, D., Zangrando, E., Ribas, J., Chaudhuri, N. R. Syntheses, crystal structures, and magnetic properties of metal-organic hybrid materials of Cu(II): effect of a long chain dicarboxylate backbone, and counteranion in their structural diversity. Inorg. Chem. 2007, 46, 3057–3071; https://doi.org/10.1021/ic061720v.Search in Google Scholar
14. Brown, I. D. VALENCE: a program for calculating bond valences. J. Appl. Crystallogr. 1996, 29, 479–480; https://doi.org/10.1107/s002188989600163x.Search in Google Scholar
© 2021 Amira Bouhali et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of poly[(μ2-aqua-tetraaqua-(μ3-glutarato-κ4O,O′:O′:O′′)-(μ5-glutarato-κ6O:O,O′:O′:O′′:O′′′)distrontium(II)], C10H22O13Sr2
- The crystal structure of acetato-κ1O-{(2-(2-(2-aminophenoxy)ethoxy)phenyl)(4-oxo-4-phenylbut-2-en-2-yl)amido-κ2N,N′,O}copper(II), C26H26CuN2O5
- Crystal structure of dimethanolato-k2O:O-bis(1-((2-methyl-1H-benzo[d]imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κN)-bis(thiocyanato-κN)dicopper(II), C34H32Cu2N12O2S2
- Crystal structure of poly[diaqua-bis(μ2-3-(pyrimidin-5-yl)benzoato-κ2N:O)cobalt(II)] dihydrate, [Co(C11H11O2N2)2(H2O)2]
- Crystal structure of bis(3,3-dimethyl-1-phenylbut-1-en-2-yl)(trimethylsilyl)amido-k1N)zinc(II), Zn(C15H24NSi)2
- Crystal structure of catena-poly[(μ2-methanolato-κ2O:O)-(μ2-1-((2-methyl-1H-benzo[d]imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κ2N:N′)-(thiocyanato-κ1N)copper(II)] 0.25 hydrate, C17H16CuN6OS ⋅ 0.5H2O
- The crystal structure of 2-amino-5-nitroanilinium iodide monohydrate, C6H8IN3O2
- The crystal structure of 3-amino-5-carboxypyridin-1-ium perchlorate monohydrate, C6H9ClN2O7
- Crystal structure of 7-hydroxy-2,4-dimethoxy-9,10-dihydrophenanthrene from Arundina graminifolia, C16H16O3
- Crystal structure of 6,6′-((1E, 1′E)-(((1R, 2R)-1,2-diphenylethane-1,2-diyl) bis(azanylylidene))bis(methanylylidene))bis(2-ethylphenol), C32H32N2O2
- The crystal structure of 2-amino-5-carboxypyridin-1-ium iodide monohydrate, C6H9IN2O3
- The crystal structure of 2-(3,5-difluorophenyl)-2,3-dihydro-1H-naphtho[1,8-de][1,3,2]diazaborinine, C16H11BF2N2
- Crystal structure of bis{(2-pyridinyl)-1-phenyl-1-isopropylmethanolato-κ2N,O}nickel, C30H32N2NiO2
- Crystal structure of poly[(m3-3-carboxyadamantane-1-carboxylato-κ3O:O′:O″)-(phenanthroline-κ2N,N′)sodium(II)], C24H23N2NaO4
- Crystal structure of 2-phenylethynyl-1,3,6,8-tetramethylBOPHY (BOPHY = bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl)methylene)hydrazine), C22H20B2F4N4
- Crystal structure of 4-tert-butyl-2-N-(2-pyridylmethyl)aminophenol, C16H20N2O
- The crystal structure of (3Z,3′Z)-4,4′-((1,4-phenylenebis(methylene))bis(azanediyl))bis(pent-3-en-2-one), C18H24N2O2
- Crystal structure of (morpholine-1-carbodithioato-κ2-S,S′)bis(triphenylphosphine-κ-P)gold(I), C41H38AuNOP2S2
- Crystal structure of 1,4-bis(4-bromobenzyl)-4-(4-chlorophenyl)-1,4-dihydropyridine-3-carbonitrile, C26H19Br2ClN2
- The crystal structure of fac-tricarbonyl (N′-benzoyl-N,N-diphenylcarbamimidothioato-κ2S,O)-(pyrazole-κN)rhenium(I) — methanol (1/1) C26H23O4N4SRe
- The crystal structure of Ba2Mn(SeO3)2Cl2 containing 1∞[Mn(SeO3)2Cl2]4− chains
- Crystal structure of 3,3′,3″-((1E,1′E,1″E)-((nitrilotris(ethane-2,1-diyl))tris(azaneylylidene)) tris(methaneylylidene))tris(4-hydroxy-1-naphthaldehyde) monohydrate, C42H36N4O6·H2O
- The crystal structure of 4-(6-acetyl-5-methyl-4,7-dihydrotetrazolo[1,5-a]pyrimidin-7-yl)benzonitrile, C14H12N6O
- Crystal structure of benzo[d][1,3]dioxol-5-yl-2-(6-methoxynaphthalen-2-yl)propanoate, C21H18O5
- The crystal structure of ethyl 5-methyl-7-(4-(phenylthio)phenyl)-4,7-dihydrotetrazolo[1,5-a]pyrimidine-6-carboxylate, C20H19N5O2S
- Crystal structure of N′,N‴-((propane-2,2-diylbis(1H-pyrrole-5,2-diyl))bis(methaneylylidene))-di(isonicotinohydrazide)– water – dimethylformamide (1/4/2), C25H24N8O2·4H2O·2C3H7NO
- Synthesis and crystal structure of 4-(2,4-dinitrophenoxy)benzaldehyde, C13H8N2O6
- The crystal structure of 1-dodecylpyridin-1-ium bromide monohydrate, C17H32BrNO
- Crystal structure of (E)-amino(2-(4-(dimethylamino)benzylidene)hydrazineyl)methaniminium nitrate, C10H16N6O3
- Crystal structure of (E)-(2-((1H-pyrrol-2-yl)methylene)hydrazineyl)(amino)methaniminium nitrate monohydrate, C6H12N6O4
- The crystal structure of hexakis(1-propylimidazole-κ1N)copper(II) dichloride, C36H60Cl2CuN12
- The crystal structure of bis{(μ2-3,3-dimethyl-1-phenylbut-1-en-2-yl)((dimethylamino)dimethylsilyl)amido-κ3N,N′:N′}dilithium, C32H54Li2N4Si2
- The crystal structure of methyl 4-(1H-naphtho[1,8-de][1,3,2]diazaborinin-2(3H)-yl)benzoate, C18H15BN2O2
- Crystal structure of (E)-N-(1-((2-chlorothiazol-5-yl)methyl)pyridin-2(1H)-ylidene)-2,2,2-trifluoroacetamide, C11H7ClF3N3OS
- Crystal structure of N′, N‴-((propane-2,2-diylbis(1H-pyrrole-5,2-diyl))bis (methaneylylidene))di(picolinohydrazide) – water – methanol (1/1/1), C25H24N8O2·H2O·CH3OH
- Crystal structure of 3-(2-chloro-benzyl)-7-[4-(2-chloro-benzyl)-piperazin-1-yl]-5,6,8-trifluoro-3H-quinazolin-4-one, C26H21Cl2F3N4O
- Crystal structure of N1,N2-bis(2-fluorobenzyl)benzene-1,2-diamine,C20H18F2N2
- The crystal structure of 2-(benzo[d][1,3]dioxol-5-yl)-2,3-dihydro-1H-naphtho[1,8-de][1,3,2]diazaborinine, C17H13BN2O2
- The crystal structure of 6,6′-((1E,1′E)-hydrazine-1,2-diylidenebis(methaneylylidene)) bis(2-bromo-4-nitrophenol) — dimethylsulfoxide (1/2), C14H8Br2N4O6⋅2(C2H6OS)
- Selective biocatalytic synthesis and crystal structure of (2R,6R)-hydroxyketaminium chloride, C13H17Cl2NO2
- Crystal structure of bis{tetraaqua-[μ3-1-(4-carboxylatophenyl)-5-methyl-1H-pyrazole-3-carboxylate-κ4N,O,O′,O″] [μ2-1-methyl-1H-pyrazole-3,5-dicarboxylate-κ3N,O:O]dicobalt(II)} dihydrate, C36H44Co4N8O26
- Crystal structure of diethyl-2,2′-naphthalene-2,3-diylbis(oxy)diacetate, C18H20O6
- Synthesis and crystal structure of poly[(μ3-2-(2-carboxylatophenyl)-1H-benzo[d]imidazole-5-carboxylato-κO,O′:O′;:O″, O″′)-(μ2-1-(4-(1Himidazol-1-yl)phenyl)-1H-imidazole-κ2N:N′)cadmium(II)], C27H18CdN6O4
- The crystal structure of catena-poly[diaqua-bis(μ2-2-((2-(2-phenylacetyl)hydrazineylidene)methyl)benzoato-κ2O:O')zinc(II)], C32H30N4O8Zn
- The crystal structure of 2-(3,4-dimethoxyphenyl)-2,3-dihydro-1H-naphtho [1,8-de][1,3,2]diazaborinine, C18H17BN2O2
- The crystal structure of hexakis(1-ethylimidazole-κ1N)nickel(II) dichloride – 1-ethylimidazole (1/2), C40H64Cl2NiN16
- Crystal structure of diaqua-bis(2,4-dinitrophenolato-κ2O,O′)copper(II) 1.5 hydrate, C12H13CuN4O13.5
- Crystal structure of N′,N‴-((1E,1′E)-((decane-1,10-diylbis(oxy))bis(2,1-phenylene)) bis(methaneylylidene))di(isonicotinohydrazide), C36H40N6O4
- The crystal structure of 2-[(R)-1-(naphthalen-1-yl)ethyl]-2,3,7,7a-tetrahydro-3a,6-epoxyisoindol-1(6H)-one, C19H20NO2
- Synthesis and crystal structure of (1E,2E)-3-(anthracen-9-yl)-1-(4-methoxyphenyl)prop-2-en-1-one oxime, C24H19NO2
- Synthesis and crystal structure of (2E,2′E)-3,3′-(1,3-phenylene)bis(1-(3-bromophenyl)prop-2-en-1-one), C24H16Br2O2
- The crystal structure of catena-poly[bis(µ2-1,2-bis((1H-imidazol-1-yl)methyl)benzene- κ2N:N′)-bis(nitrato-κO)copper(II)], C28H28N10O6Cu
- Synthesis and crystal structure of the novel chiral acetyl-3-thiophene-5-(9-anthryl)-2-pyrazoline, C23H18N2OS
- Crystal structure of (E)-3-(dimethylamino)-1-(thiophen-3-yl)prop-2-en-1-one, C9H11NOS
- Crystal structure of catena-poly[aqua-(4-iodopyridine-2,6-dicarboxylato-κ3N,O,O′)-(μ2-4-amino-4H-1,2,4-triazole-κ2N:N′) copper(II)], C9H8N5O5CuI
- Crystal structure of cyclopropane-1,2,3-triyltris(phenylmethanone), C24H18O3
- Crystal structure of bis(amino(thioureido)methaniminium) terephthalate, C12H18N8O4S2
- A three-dimensional Eu(III) framework in the crystal structure of dimethylaminium poly[dimethylformamide-κ1N)bis(μ4-terephthalato-κ4O:O′:O′′:O′′′)europium(III)] monohydrate, C21H25EuN2O10
- Crystal structure of 2-methoxyphenyl 2-(6-methoxynaphthalen-2-yl)propanoate, C21H20O4
- The crystal structure of Hexakis(diethylamido)dimolybdenum, Mo2(NEt2)6
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of poly[(μ2-aqua-tetraaqua-(μ3-glutarato-κ4O,O′:O′:O′′)-(μ5-glutarato-κ6O:O,O′:O′:O′′:O′′′)distrontium(II)], C10H22O13Sr2
- The crystal structure of acetato-κ1O-{(2-(2-(2-aminophenoxy)ethoxy)phenyl)(4-oxo-4-phenylbut-2-en-2-yl)amido-κ2N,N′,O}copper(II), C26H26CuN2O5
- Crystal structure of dimethanolato-k2O:O-bis(1-((2-methyl-1H-benzo[d]imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κN)-bis(thiocyanato-κN)dicopper(II), C34H32Cu2N12O2S2
- Crystal structure of poly[diaqua-bis(μ2-3-(pyrimidin-5-yl)benzoato-κ2N:O)cobalt(II)] dihydrate, [Co(C11H11O2N2)2(H2O)2]
- Crystal structure of bis(3,3-dimethyl-1-phenylbut-1-en-2-yl)(trimethylsilyl)amido-k1N)zinc(II), Zn(C15H24NSi)2
- Crystal structure of catena-poly[(μ2-methanolato-κ2O:O)-(μ2-1-((2-methyl-1H-benzo[d]imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κ2N:N′)-(thiocyanato-κ1N)copper(II)] 0.25 hydrate, C17H16CuN6OS ⋅ 0.5H2O
- The crystal structure of 2-amino-5-nitroanilinium iodide monohydrate, C6H8IN3O2
- The crystal structure of 3-amino-5-carboxypyridin-1-ium perchlorate monohydrate, C6H9ClN2O7
- Crystal structure of 7-hydroxy-2,4-dimethoxy-9,10-dihydrophenanthrene from Arundina graminifolia, C16H16O3
- Crystal structure of 6,6′-((1E, 1′E)-(((1R, 2R)-1,2-diphenylethane-1,2-diyl) bis(azanylylidene))bis(methanylylidene))bis(2-ethylphenol), C32H32N2O2
- The crystal structure of 2-amino-5-carboxypyridin-1-ium iodide monohydrate, C6H9IN2O3
- The crystal structure of 2-(3,5-difluorophenyl)-2,3-dihydro-1H-naphtho[1,8-de][1,3,2]diazaborinine, C16H11BF2N2
- Crystal structure of bis{(2-pyridinyl)-1-phenyl-1-isopropylmethanolato-κ2N,O}nickel, C30H32N2NiO2
- Crystal structure of poly[(m3-3-carboxyadamantane-1-carboxylato-κ3O:O′:O″)-(phenanthroline-κ2N,N′)sodium(II)], C24H23N2NaO4
- Crystal structure of 2-phenylethynyl-1,3,6,8-tetramethylBOPHY (BOPHY = bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl)methylene)hydrazine), C22H20B2F4N4
- Crystal structure of 4-tert-butyl-2-N-(2-pyridylmethyl)aminophenol, C16H20N2O
- The crystal structure of (3Z,3′Z)-4,4′-((1,4-phenylenebis(methylene))bis(azanediyl))bis(pent-3-en-2-one), C18H24N2O2
- Crystal structure of (morpholine-1-carbodithioato-κ2-S,S′)bis(triphenylphosphine-κ-P)gold(I), C41H38AuNOP2S2
- Crystal structure of 1,4-bis(4-bromobenzyl)-4-(4-chlorophenyl)-1,4-dihydropyridine-3-carbonitrile, C26H19Br2ClN2
- The crystal structure of fac-tricarbonyl (N′-benzoyl-N,N-diphenylcarbamimidothioato-κ2S,O)-(pyrazole-κN)rhenium(I) — methanol (1/1) C26H23O4N4SRe
- The crystal structure of Ba2Mn(SeO3)2Cl2 containing 1∞[Mn(SeO3)2Cl2]4− chains
- Crystal structure of 3,3′,3″-((1E,1′E,1″E)-((nitrilotris(ethane-2,1-diyl))tris(azaneylylidene)) tris(methaneylylidene))tris(4-hydroxy-1-naphthaldehyde) monohydrate, C42H36N4O6·H2O
- The crystal structure of 4-(6-acetyl-5-methyl-4,7-dihydrotetrazolo[1,5-a]pyrimidin-7-yl)benzonitrile, C14H12N6O
- Crystal structure of benzo[d][1,3]dioxol-5-yl-2-(6-methoxynaphthalen-2-yl)propanoate, C21H18O5
- The crystal structure of ethyl 5-methyl-7-(4-(phenylthio)phenyl)-4,7-dihydrotetrazolo[1,5-a]pyrimidine-6-carboxylate, C20H19N5O2S
- Crystal structure of N′,N‴-((propane-2,2-diylbis(1H-pyrrole-5,2-diyl))bis(methaneylylidene))-di(isonicotinohydrazide)– water – dimethylformamide (1/4/2), C25H24N8O2·4H2O·2C3H7NO
- Synthesis and crystal structure of 4-(2,4-dinitrophenoxy)benzaldehyde, C13H8N2O6
- The crystal structure of 1-dodecylpyridin-1-ium bromide monohydrate, C17H32BrNO
- Crystal structure of (E)-amino(2-(4-(dimethylamino)benzylidene)hydrazineyl)methaniminium nitrate, C10H16N6O3
- Crystal structure of (E)-(2-((1H-pyrrol-2-yl)methylene)hydrazineyl)(amino)methaniminium nitrate monohydrate, C6H12N6O4
- The crystal structure of hexakis(1-propylimidazole-κ1N)copper(II) dichloride, C36H60Cl2CuN12
- The crystal structure of bis{(μ2-3,3-dimethyl-1-phenylbut-1-en-2-yl)((dimethylamino)dimethylsilyl)amido-κ3N,N′:N′}dilithium, C32H54Li2N4Si2
- The crystal structure of methyl 4-(1H-naphtho[1,8-de][1,3,2]diazaborinin-2(3H)-yl)benzoate, C18H15BN2O2
- Crystal structure of (E)-N-(1-((2-chlorothiazol-5-yl)methyl)pyridin-2(1H)-ylidene)-2,2,2-trifluoroacetamide, C11H7ClF3N3OS
- Crystal structure of N′, N‴-((propane-2,2-diylbis(1H-pyrrole-5,2-diyl))bis (methaneylylidene))di(picolinohydrazide) – water – methanol (1/1/1), C25H24N8O2·H2O·CH3OH
- Crystal structure of 3-(2-chloro-benzyl)-7-[4-(2-chloro-benzyl)-piperazin-1-yl]-5,6,8-trifluoro-3H-quinazolin-4-one, C26H21Cl2F3N4O
- Crystal structure of N1,N2-bis(2-fluorobenzyl)benzene-1,2-diamine,C20H18F2N2
- The crystal structure of 2-(benzo[d][1,3]dioxol-5-yl)-2,3-dihydro-1H-naphtho[1,8-de][1,3,2]diazaborinine, C17H13BN2O2
- The crystal structure of 6,6′-((1E,1′E)-hydrazine-1,2-diylidenebis(methaneylylidene)) bis(2-bromo-4-nitrophenol) — dimethylsulfoxide (1/2), C14H8Br2N4O6⋅2(C2H6OS)
- Selective biocatalytic synthesis and crystal structure of (2R,6R)-hydroxyketaminium chloride, C13H17Cl2NO2
- Crystal structure of bis{tetraaqua-[μ3-1-(4-carboxylatophenyl)-5-methyl-1H-pyrazole-3-carboxylate-κ4N,O,O′,O″] [μ2-1-methyl-1H-pyrazole-3,5-dicarboxylate-κ3N,O:O]dicobalt(II)} dihydrate, C36H44Co4N8O26
- Crystal structure of diethyl-2,2′-naphthalene-2,3-diylbis(oxy)diacetate, C18H20O6
- Synthesis and crystal structure of poly[(μ3-2-(2-carboxylatophenyl)-1H-benzo[d]imidazole-5-carboxylato-κO,O′:O′;:O″, O″′)-(μ2-1-(4-(1Himidazol-1-yl)phenyl)-1H-imidazole-κ2N:N′)cadmium(II)], C27H18CdN6O4
- The crystal structure of catena-poly[diaqua-bis(μ2-2-((2-(2-phenylacetyl)hydrazineylidene)methyl)benzoato-κ2O:O')zinc(II)], C32H30N4O8Zn
- The crystal structure of 2-(3,4-dimethoxyphenyl)-2,3-dihydro-1H-naphtho [1,8-de][1,3,2]diazaborinine, C18H17BN2O2
- The crystal structure of hexakis(1-ethylimidazole-κ1N)nickel(II) dichloride – 1-ethylimidazole (1/2), C40H64Cl2NiN16
- Crystal structure of diaqua-bis(2,4-dinitrophenolato-κ2O,O′)copper(II) 1.5 hydrate, C12H13CuN4O13.5
- Crystal structure of N′,N‴-((1E,1′E)-((decane-1,10-diylbis(oxy))bis(2,1-phenylene)) bis(methaneylylidene))di(isonicotinohydrazide), C36H40N6O4
- The crystal structure of 2-[(R)-1-(naphthalen-1-yl)ethyl]-2,3,7,7a-tetrahydro-3a,6-epoxyisoindol-1(6H)-one, C19H20NO2
- Synthesis and crystal structure of (1E,2E)-3-(anthracen-9-yl)-1-(4-methoxyphenyl)prop-2-en-1-one oxime, C24H19NO2
- Synthesis and crystal structure of (2E,2′E)-3,3′-(1,3-phenylene)bis(1-(3-bromophenyl)prop-2-en-1-one), C24H16Br2O2
- The crystal structure of catena-poly[bis(µ2-1,2-bis((1H-imidazol-1-yl)methyl)benzene- κ2N:N′)-bis(nitrato-κO)copper(II)], C28H28N10O6Cu
- Synthesis and crystal structure of the novel chiral acetyl-3-thiophene-5-(9-anthryl)-2-pyrazoline, C23H18N2OS
- Crystal structure of (E)-3-(dimethylamino)-1-(thiophen-3-yl)prop-2-en-1-one, C9H11NOS
- Crystal structure of catena-poly[aqua-(4-iodopyridine-2,6-dicarboxylato-κ3N,O,O′)-(μ2-4-amino-4H-1,2,4-triazole-κ2N:N′) copper(II)], C9H8N5O5CuI
- Crystal structure of cyclopropane-1,2,3-triyltris(phenylmethanone), C24H18O3
- Crystal structure of bis(amino(thioureido)methaniminium) terephthalate, C12H18N8O4S2
- A three-dimensional Eu(III) framework in the crystal structure of dimethylaminium poly[dimethylformamide-κ1N)bis(μ4-terephthalato-κ4O:O′:O′′:O′′′)europium(III)] monohydrate, C21H25EuN2O10
- Crystal structure of 2-methoxyphenyl 2-(6-methoxynaphthalen-2-yl)propanoate, C21H20O4
- The crystal structure of Hexakis(diethylamido)dimolybdenum, Mo2(NEt2)6

