Abstract
C24H16Br2O2, orthorhombic, Pnma (no. 62), a = 7.407(3) Å, b = 44.336(16) Å, c = 6.046(2) Å, V = 944.8(3) Å3, Z = 4, Rgt(F) = 0.0602, wRref(F2) = 0.1315, T = 293 K.
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
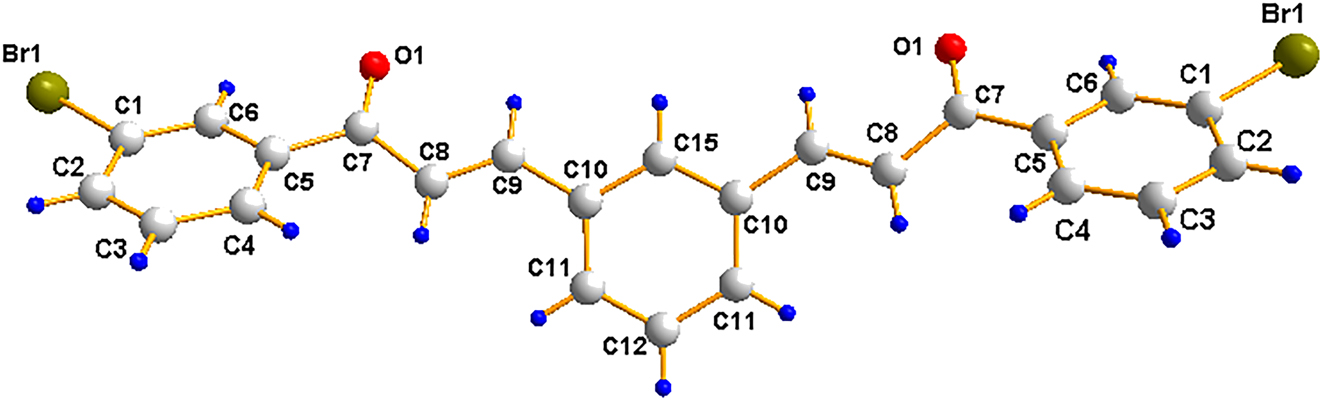
Data collection and handling.
| Crystal: | Colorless block |
| Size: | 0.20 × 0.14 × 0.10 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 4.10 mm−1 |
| Diffractometer, scan mode: | φ and ω |
| θmax, completeness: | 25.0°, 98% |
| N(hkl)measured, N(hkl)unique, Rint: | 7399, 1779, 0.109 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 1183 |
| N(param)refined: | 130 |
| Programs: | Bruker [1], SHELX [2], Diamond [3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | Y | z | Uiso*/Ueq |
|---|---|---|---|---|
| Br1 | 0.33707 (10) | 0.02317 (2) | 0.33389 (13) | 0.0622 (3) |
| O1 | 0.2911 (8) | 0.14332 (10) | 0.4304 (8) | 0.0741 (17) |
| C1 | 0.3569 (8) | 0.05838 (12) | 0.1574 (11) | 0.0453 (15) |
| C2 | 0.4218 (10) | 0.05592 (15) | −0.0586 (12) | 0.0562 (18) |
| H2 | 0.4492 | 0.0371 | −0.1185 | 0.067* |
| C3 | 0.4440 (10) | 0.08155 (16) | −0.1802 (12) | 0.062 (2) |
| H3 | 0.4919 | 0.0804 | −0.3221 | 0.074* |
| C4 | 0.3962 (10) | 0.10920 (14) | −0.0943 (11) | 0.0521 (18) |
| H4 | 0.4084 | 0.1264 | −0.1809 | 0.063* |
| C5 | 0.3299 (9) | 0.11171 (13) | 0.1196 (11) | 0.0474 (16) |
| C6 | 0.3108 (9) | 0.08564 (12) | 0.2468 (12) | 0.0467 (16) |
| H6 | 0.2672 | 0.0868 | 0.3908 | 0.056* |
| C7 | 0.2874 (10) | 0.14117 (13) | 0.2288 (13) | 0.0519 (18) |
| C8 | 0.2396 (10) | 0.16735 (13) | 0.0899 (12) | 0.0548 (18) |
| H8 | 0.2185 | 0.1647 | −0.0604 | 0.066* |
| C9 | 0.2267 (9) | 0.19450 (12) | 0.1772 (11) | 0.0479 (16) |
| H9 | 0.2527 | 0.1960 | 0.3273 | 0.057* |
| C10 | 0.1760 (9) | 0.22249 (12) | 0.0655 (11) | 0.0452 (15) |
| C11 | 0.0960 (9) | 0.22306 (13) | −0.1432 (11) | 0.0491 (16) |
| H11 | 0.0694 | 0.2050 | −0.2140 | 0.059* |
| C12 | 0.0554 (13) | 0.2500 | −0.2467 (17) | 0.054 (3) |
| H12 | 0.0010 | 0.2500 | −0.3853 | 0.065* |
| C15 | 0.2110 (12) | 0.2500 | 0.1673 (14) | 0.041 (2) |
| H15 | 0.2598 | 0.2500 | 0.3090 | 0.050* |
Source of material
The target compound (2E,2′E)-3,3′-(1,3-phenylene)bis (1-(3-bromophenyl)prop-2-en-1-one) (PBP) was synthesized according to the procedure reported in ref. [4]. A base catalyzed condensation of 3-bromoacetophenon (2 equivalents) and isophthalaldehyde (1 equivalent) in ethanol in presence of NaOH at room temperature resulted in the PBP in good yield (85%). Good quality colorless block crystals were obtained by slow evaporation of the ethyl acetate solution.
Experimental details
The hydrogen atoms bonded to the carbon atoms were placed in calculated positions and refined as riding mode, with C–H = 0.96 Å with Uiso(H) = 1.2 Ueq(C).
Comment
Chalcones are well known organic compounds and a major component of some natural products, which have attracted immense interest owing to their characteristic biological activities namely, anti-cancer [5], anti-microbial [6], anti-inflammatory [7], anti-protozoal [8], anti-oxidant [9] and anti-malarial [10] etc. The presence of the double bond believe to impart bioactive characteristic to the chalcone and removal of double bonds will make them biological inactive. Structural modification, biological active and facile preparation are some of the characteristics that differentiate chalcones from the plethora of other bioactive molecules. The crystals of the PBP were subjected to single crystal X-ray diffraction (SXRD) to determine the non-covalent forces and molecular stacking in the solid state. The crystal structure of the title compound is mainly established by the hydrogen bonds C4–H4…O1 (2.615 Å) between two neighboring molecules to form a sheet like structure along the a axis. Additionally, C12–H12…π interaction (2.740 Å) along c keeps the stack together. Thus, these two different interactions enhance each other, and in turn stabilize the overall crystal structure. All parameters are in expected ranges [11].
Funding source: National Key Research and Development Program of China
Award Identifier / Grant number: 2017YFA0205301
Funding source: NSFC
Award Identifier / Grant number: 61701176
Funding source: Taif University
Award Identifier / Grant number: TURSP-2020/01
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: This research was financially supported by the National Key Research and Development Program of China (2017YFA0205301), NSFC (61701176) & Taif University Researches Supporting Project number (TURSP-2020/01), Taif University, Taif, Saudi Arabia.
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. SAINT, APEX2 and SADABS; Bruker AXS Inc.: Madison, Wisconsin, USA, 2009.Search in Google Scholar
2. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
3. Brandenburg, K. DIAMOND. Visual Crystal Structure Information System, Ver. 4.0; Crystal Impact: Bonn, Germany, 2015.Search in Google Scholar
4. Bhale, P. S., Dongare, S. B., Chanshetti, U. B. Synthesis and antimicrobial screening of chalcones containing imidazo [1,2-a] pyridine nucleus. Res. J. Chem. Sci. 2013, 3, 38–42.Search in Google Scholar
5. Varmus, H. The new era in cancer research. Science 2006, 312, 1162–1165; https://doi.org/10.1126/science.1126758.Search in Google Scholar
6. Mai, C. W., Yaeghoobi, M., Abd–Rahman, N., Kang, Y. B., Pichika, M. R. Chalcones with electron-withdrawing and electron-donating substituents: anticancer activity against TRAIL resistant cancer cells, structure –activity relationship analysis and regulation of apoptotic proteins. Eur. J. Med. Chem. 2014, 77, 378–387; https://doi.org/10.1016/j.ejmech.2014.03.002.Search in Google Scholar
7. Kant, R., Kumar, D., Agarwal, D., Gupta, R. D., Tilak, R., Awasthi, S. K., Agarwal, A. Synthesis of newer 1,2,3-triazole linked chalcone and flavone hybrid compounds and evaluation of their antimicrobial and cytotoxic activities. Eur. J. Med. Chem. 2016, 113, 34–49; https://doi.org/10.1016/j.ejmech.2016.02.041.Search in Google Scholar
8. Bano, S., Javed, K., Ahmad, S., Rathish, I., Singh, S., Chaitanya, M., Arunasree, K., Alam, M. Synthesis of some novel chalcones, flavanones and flavones and evaluation of their anti-inflammatory activity. Eur. J. Med. Chem. 2013, 65, 51–59; https://doi.org/10.1016/j.ejmech.2013.04.056.Search in Google Scholar
9. Nazarian, Z., Emami, S., Heydari, S., Ardestani, S. K., Nakhjiri, M., Poorrajab, F., Shafiee, A., Foroumadi, A. Novel antileishmanial chalconoids: synthesis and biological activity of 1-or 3-(6-chloro-2H-chromen-3-yl) propen-1-ones. Eur. J. Med. Chem. 2010, 45, 1424–1429; https://doi.org/10.1016/j.ejmech.2009.12.046.Search in Google Scholar
10. Aly, M. R. E. S., Fodah, H. H. A. E. R., Saleh, S. Y. Antiobesity, antioxidant and cytotoxicity activities of newly synthesized chalcone derivatives and their metal complexes. Eur. J. Med. Chem. 2014, 76, 517–530.10.1016/j.ejmech.2014.02.021Search in Google Scholar PubMed
11. Yadav, N., Dixit, S. K., Bhattacharya, A., Mishra, L. C., Sharma, M., Awasthi, S. K., Bhasin, V. K. Antimalarial activity of newly synthesized chalcone derivatives in vitro. Chem. Biol. Drug Des. 2012, 80, 340–347; https://doi.org/10.1111/j.1747-0285.2012.01383.x.Search in Google Scholar
© 2021 Rabia Usman et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of poly[(μ2-aqua-tetraaqua-(μ3-glutarato-κ4O,O′:O′:O′′)-(μ5-glutarato-κ6O:O,O′:O′:O′′:O′′′)distrontium(II)], C10H22O13Sr2
- The crystal structure of acetato-κ1O-{(2-(2-(2-aminophenoxy)ethoxy)phenyl)(4-oxo-4-phenylbut-2-en-2-yl)amido-κ2N,N′,O}copper(II), C26H26CuN2O5
- Crystal structure of dimethanolato-k2O:O-bis(1-((2-methyl-1H-benzo[d]imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κN)-bis(thiocyanato-κN)dicopper(II), C34H32Cu2N12O2S2
- Crystal structure of poly[diaqua-bis(μ2-3-(pyrimidin-5-yl)benzoato-κ2N:O)cobalt(II)] dihydrate, [Co(C11H11O2N2)2(H2O)2]
- Crystal structure of bis(3,3-dimethyl-1-phenylbut-1-en-2-yl)(trimethylsilyl)amido-k1N)zinc(II), Zn(C15H24NSi)2
- Crystal structure of catena-poly[(μ2-methanolato-κ2O:O)-(μ2-1-((2-methyl-1H-benzo[d]imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κ2N:N′)-(thiocyanato-κ1N)copper(II)] 0.25 hydrate, C17H16CuN6OS ⋅ 0.5H2O
- The crystal structure of 2-amino-5-nitroanilinium iodide monohydrate, C6H8IN3O2
- The crystal structure of 3-amino-5-carboxypyridin-1-ium perchlorate monohydrate, C6H9ClN2O7
- Crystal structure of 7-hydroxy-2,4-dimethoxy-9,10-dihydrophenanthrene from Arundina graminifolia, C16H16O3
- Crystal structure of 6,6′-((1E, 1′E)-(((1R, 2R)-1,2-diphenylethane-1,2-diyl) bis(azanylylidene))bis(methanylylidene))bis(2-ethylphenol), C32H32N2O2
- The crystal structure of 2-amino-5-carboxypyridin-1-ium iodide monohydrate, C6H9IN2O3
- The crystal structure of 2-(3,5-difluorophenyl)-2,3-dihydro-1H-naphtho[1,8-de][1,3,2]diazaborinine, C16H11BF2N2
- Crystal structure of bis{(2-pyridinyl)-1-phenyl-1-isopropylmethanolato-κ2N,O}nickel, C30H32N2NiO2
- Crystal structure of poly[(m3-3-carboxyadamantane-1-carboxylato-κ3O:O′:O″)-(phenanthroline-κ2N,N′)sodium(II)], C24H23N2NaO4
- Crystal structure of 2-phenylethynyl-1,3,6,8-tetramethylBOPHY (BOPHY = bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl)methylene)hydrazine), C22H20B2F4N4
- Crystal structure of 4-tert-butyl-2-N-(2-pyridylmethyl)aminophenol, C16H20N2O
- The crystal structure of (3Z,3′Z)-4,4′-((1,4-phenylenebis(methylene))bis(azanediyl))bis(pent-3-en-2-one), C18H24N2O2
- Crystal structure of (morpholine-1-carbodithioato-κ2-S,S′)bis(triphenylphosphine-κ-P)gold(I), C41H38AuNOP2S2
- Crystal structure of 1,4-bis(4-bromobenzyl)-4-(4-chlorophenyl)-1,4-dihydropyridine-3-carbonitrile, C26H19Br2ClN2
- The crystal structure of fac-tricarbonyl (N′-benzoyl-N,N-diphenylcarbamimidothioato-κ2S,O)-(pyrazole-κN)rhenium(I) — methanol (1/1) C26H23O4N4SRe
- The crystal structure of Ba2Mn(SeO3)2Cl2 containing 1∞[Mn(SeO3)2Cl2]4− chains
- Crystal structure of 3,3′,3″-((1E,1′E,1″E)-((nitrilotris(ethane-2,1-diyl))tris(azaneylylidene)) tris(methaneylylidene))tris(4-hydroxy-1-naphthaldehyde) monohydrate, C42H36N4O6·H2O
- The crystal structure of 4-(6-acetyl-5-methyl-4,7-dihydrotetrazolo[1,5-a]pyrimidin-7-yl)benzonitrile, C14H12N6O
- Crystal structure of benzo[d][1,3]dioxol-5-yl-2-(6-methoxynaphthalen-2-yl)propanoate, C21H18O5
- The crystal structure of ethyl 5-methyl-7-(4-(phenylthio)phenyl)-4,7-dihydrotetrazolo[1,5-a]pyrimidine-6-carboxylate, C20H19N5O2S
- Crystal structure of N′,N‴-((propane-2,2-diylbis(1H-pyrrole-5,2-diyl))bis(methaneylylidene))-di(isonicotinohydrazide)– water – dimethylformamide (1/4/2), C25H24N8O2·4H2O·2C3H7NO
- Synthesis and crystal structure of 4-(2,4-dinitrophenoxy)benzaldehyde, C13H8N2O6
- The crystal structure of 1-dodecylpyridin-1-ium bromide monohydrate, C17H32BrNO
- Crystal structure of (E)-amino(2-(4-(dimethylamino)benzylidene)hydrazineyl)methaniminium nitrate, C10H16N6O3
- Crystal structure of (E)-(2-((1H-pyrrol-2-yl)methylene)hydrazineyl)(amino)methaniminium nitrate monohydrate, C6H12N6O4
- The crystal structure of hexakis(1-propylimidazole-κ1N)copper(II) dichloride, C36H60Cl2CuN12
- The crystal structure of bis{(μ2-3,3-dimethyl-1-phenylbut-1-en-2-yl)((dimethylamino)dimethylsilyl)amido-κ3N,N′:N′}dilithium, C32H54Li2N4Si2
- The crystal structure of methyl 4-(1H-naphtho[1,8-de][1,3,2]diazaborinin-2(3H)-yl)benzoate, C18H15BN2O2
- Crystal structure of (E)-N-(1-((2-chlorothiazol-5-yl)methyl)pyridin-2(1H)-ylidene)-2,2,2-trifluoroacetamide, C11H7ClF3N3OS
- Crystal structure of N′, N‴-((propane-2,2-diylbis(1H-pyrrole-5,2-diyl))bis (methaneylylidene))di(picolinohydrazide) – water – methanol (1/1/1), C25H24N8O2·H2O·CH3OH
- Crystal structure of 3-(2-chloro-benzyl)-7-[4-(2-chloro-benzyl)-piperazin-1-yl]-5,6,8-trifluoro-3H-quinazolin-4-one, C26H21Cl2F3N4O
- Crystal structure of N1,N2-bis(2-fluorobenzyl)benzene-1,2-diamine,C20H18F2N2
- The crystal structure of 2-(benzo[d][1,3]dioxol-5-yl)-2,3-dihydro-1H-naphtho[1,8-de][1,3,2]diazaborinine, C17H13BN2O2
- The crystal structure of 6,6′-((1E,1′E)-hydrazine-1,2-diylidenebis(methaneylylidene)) bis(2-bromo-4-nitrophenol) — dimethylsulfoxide (1/2), C14H8Br2N4O6⋅2(C2H6OS)
- Selective biocatalytic synthesis and crystal structure of (2R,6R)-hydroxyketaminium chloride, C13H17Cl2NO2
- Crystal structure of bis{tetraaqua-[μ3-1-(4-carboxylatophenyl)-5-methyl-1H-pyrazole-3-carboxylate-κ4N,O,O′,O″] [μ2-1-methyl-1H-pyrazole-3,5-dicarboxylate-κ3N,O:O]dicobalt(II)} dihydrate, C36H44Co4N8O26
- Crystal structure of diethyl-2,2′-naphthalene-2,3-diylbis(oxy)diacetate, C18H20O6
- Synthesis and crystal structure of poly[(μ3-2-(2-carboxylatophenyl)-1H-benzo[d]imidazole-5-carboxylato-κO,O′:O′;:O″, O″′)-(μ2-1-(4-(1Himidazol-1-yl)phenyl)-1H-imidazole-κ2N:N′)cadmium(II)], C27H18CdN6O4
- The crystal structure of catena-poly[diaqua-bis(μ2-2-((2-(2-phenylacetyl)hydrazineylidene)methyl)benzoato-κ2O:O')zinc(II)], C32H30N4O8Zn
- The crystal structure of 2-(3,4-dimethoxyphenyl)-2,3-dihydro-1H-naphtho [1,8-de][1,3,2]diazaborinine, C18H17BN2O2
- The crystal structure of hexakis(1-ethylimidazole-κ1N)nickel(II) dichloride – 1-ethylimidazole (1/2), C40H64Cl2NiN16
- Crystal structure of diaqua-bis(2,4-dinitrophenolato-κ2O,O′)copper(II) 1.5 hydrate, C12H13CuN4O13.5
- Crystal structure of N′,N‴-((1E,1′E)-((decane-1,10-diylbis(oxy))bis(2,1-phenylene)) bis(methaneylylidene))di(isonicotinohydrazide), C36H40N6O4
- The crystal structure of 2-[(R)-1-(naphthalen-1-yl)ethyl]-2,3,7,7a-tetrahydro-3a,6-epoxyisoindol-1(6H)-one, C19H20NO2
- Synthesis and crystal structure of (1E,2E)-3-(anthracen-9-yl)-1-(4-methoxyphenyl)prop-2-en-1-one oxime, C24H19NO2
- Synthesis and crystal structure of (2E,2′E)-3,3′-(1,3-phenylene)bis(1-(3-bromophenyl)prop-2-en-1-one), C24H16Br2O2
- The crystal structure of catena-poly[bis(µ2-1,2-bis((1H-imidazol-1-yl)methyl)benzene- κ2N:N′)-bis(nitrato-κO)copper(II)], C28H28N10O6Cu
- Synthesis and crystal structure of the novel chiral acetyl-3-thiophene-5-(9-anthryl)-2-pyrazoline, C23H18N2OS
- Crystal structure of (E)-3-(dimethylamino)-1-(thiophen-3-yl)prop-2-en-1-one, C9H11NOS
- Crystal structure of catena-poly[aqua-(4-iodopyridine-2,6-dicarboxylato-κ3N,O,O′)-(μ2-4-amino-4H-1,2,4-triazole-κ2N:N′) copper(II)], C9H8N5O5CuI
- Crystal structure of cyclopropane-1,2,3-triyltris(phenylmethanone), C24H18O3
- Crystal structure of bis(amino(thioureido)methaniminium) terephthalate, C12H18N8O4S2
- A three-dimensional Eu(III) framework in the crystal structure of dimethylaminium poly[dimethylformamide-κ1N)bis(μ4-terephthalato-κ4O:O′:O′′:O′′′)europium(III)] monohydrate, C21H25EuN2O10
- Crystal structure of 2-methoxyphenyl 2-(6-methoxynaphthalen-2-yl)propanoate, C21H20O4
- The crystal structure of Hexakis(diethylamido)dimolybdenum, Mo2(NEt2)6
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of poly[(μ2-aqua-tetraaqua-(μ3-glutarato-κ4O,O′:O′:O′′)-(μ5-glutarato-κ6O:O,O′:O′:O′′:O′′′)distrontium(II)], C10H22O13Sr2
- The crystal structure of acetato-κ1O-{(2-(2-(2-aminophenoxy)ethoxy)phenyl)(4-oxo-4-phenylbut-2-en-2-yl)amido-κ2N,N′,O}copper(II), C26H26CuN2O5
- Crystal structure of dimethanolato-k2O:O-bis(1-((2-methyl-1H-benzo[d]imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κN)-bis(thiocyanato-κN)dicopper(II), C34H32Cu2N12O2S2
- Crystal structure of poly[diaqua-bis(μ2-3-(pyrimidin-5-yl)benzoato-κ2N:O)cobalt(II)] dihydrate, [Co(C11H11O2N2)2(H2O)2]
- Crystal structure of bis(3,3-dimethyl-1-phenylbut-1-en-2-yl)(trimethylsilyl)amido-k1N)zinc(II), Zn(C15H24NSi)2
- Crystal structure of catena-poly[(μ2-methanolato-κ2O:O)-(μ2-1-((2-methyl-1H-benzo[d]imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κ2N:N′)-(thiocyanato-κ1N)copper(II)] 0.25 hydrate, C17H16CuN6OS ⋅ 0.5H2O
- The crystal structure of 2-amino-5-nitroanilinium iodide monohydrate, C6H8IN3O2
- The crystal structure of 3-amino-5-carboxypyridin-1-ium perchlorate monohydrate, C6H9ClN2O7
- Crystal structure of 7-hydroxy-2,4-dimethoxy-9,10-dihydrophenanthrene from Arundina graminifolia, C16H16O3
- Crystal structure of 6,6′-((1E, 1′E)-(((1R, 2R)-1,2-diphenylethane-1,2-diyl) bis(azanylylidene))bis(methanylylidene))bis(2-ethylphenol), C32H32N2O2
- The crystal structure of 2-amino-5-carboxypyridin-1-ium iodide monohydrate, C6H9IN2O3
- The crystal structure of 2-(3,5-difluorophenyl)-2,3-dihydro-1H-naphtho[1,8-de][1,3,2]diazaborinine, C16H11BF2N2
- Crystal structure of bis{(2-pyridinyl)-1-phenyl-1-isopropylmethanolato-κ2N,O}nickel, C30H32N2NiO2
- Crystal structure of poly[(m3-3-carboxyadamantane-1-carboxylato-κ3O:O′:O″)-(phenanthroline-κ2N,N′)sodium(II)], C24H23N2NaO4
- Crystal structure of 2-phenylethynyl-1,3,6,8-tetramethylBOPHY (BOPHY = bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl)methylene)hydrazine), C22H20B2F4N4
- Crystal structure of 4-tert-butyl-2-N-(2-pyridylmethyl)aminophenol, C16H20N2O
- The crystal structure of (3Z,3′Z)-4,4′-((1,4-phenylenebis(methylene))bis(azanediyl))bis(pent-3-en-2-one), C18H24N2O2
- Crystal structure of (morpholine-1-carbodithioato-κ2-S,S′)bis(triphenylphosphine-κ-P)gold(I), C41H38AuNOP2S2
- Crystal structure of 1,4-bis(4-bromobenzyl)-4-(4-chlorophenyl)-1,4-dihydropyridine-3-carbonitrile, C26H19Br2ClN2
- The crystal structure of fac-tricarbonyl (N′-benzoyl-N,N-diphenylcarbamimidothioato-κ2S,O)-(pyrazole-κN)rhenium(I) — methanol (1/1) C26H23O4N4SRe
- The crystal structure of Ba2Mn(SeO3)2Cl2 containing 1∞[Mn(SeO3)2Cl2]4− chains
- Crystal structure of 3,3′,3″-((1E,1′E,1″E)-((nitrilotris(ethane-2,1-diyl))tris(azaneylylidene)) tris(methaneylylidene))tris(4-hydroxy-1-naphthaldehyde) monohydrate, C42H36N4O6·H2O
- The crystal structure of 4-(6-acetyl-5-methyl-4,7-dihydrotetrazolo[1,5-a]pyrimidin-7-yl)benzonitrile, C14H12N6O
- Crystal structure of benzo[d][1,3]dioxol-5-yl-2-(6-methoxynaphthalen-2-yl)propanoate, C21H18O5
- The crystal structure of ethyl 5-methyl-7-(4-(phenylthio)phenyl)-4,7-dihydrotetrazolo[1,5-a]pyrimidine-6-carboxylate, C20H19N5O2S
- Crystal structure of N′,N‴-((propane-2,2-diylbis(1H-pyrrole-5,2-diyl))bis(methaneylylidene))-di(isonicotinohydrazide)– water – dimethylformamide (1/4/2), C25H24N8O2·4H2O·2C3H7NO
- Synthesis and crystal structure of 4-(2,4-dinitrophenoxy)benzaldehyde, C13H8N2O6
- The crystal structure of 1-dodecylpyridin-1-ium bromide monohydrate, C17H32BrNO
- Crystal structure of (E)-amino(2-(4-(dimethylamino)benzylidene)hydrazineyl)methaniminium nitrate, C10H16N6O3
- Crystal structure of (E)-(2-((1H-pyrrol-2-yl)methylene)hydrazineyl)(amino)methaniminium nitrate monohydrate, C6H12N6O4
- The crystal structure of hexakis(1-propylimidazole-κ1N)copper(II) dichloride, C36H60Cl2CuN12
- The crystal structure of bis{(μ2-3,3-dimethyl-1-phenylbut-1-en-2-yl)((dimethylamino)dimethylsilyl)amido-κ3N,N′:N′}dilithium, C32H54Li2N4Si2
- The crystal structure of methyl 4-(1H-naphtho[1,8-de][1,3,2]diazaborinin-2(3H)-yl)benzoate, C18H15BN2O2
- Crystal structure of (E)-N-(1-((2-chlorothiazol-5-yl)methyl)pyridin-2(1H)-ylidene)-2,2,2-trifluoroacetamide, C11H7ClF3N3OS
- Crystal structure of N′, N‴-((propane-2,2-diylbis(1H-pyrrole-5,2-diyl))bis (methaneylylidene))di(picolinohydrazide) – water – methanol (1/1/1), C25H24N8O2·H2O·CH3OH
- Crystal structure of 3-(2-chloro-benzyl)-7-[4-(2-chloro-benzyl)-piperazin-1-yl]-5,6,8-trifluoro-3H-quinazolin-4-one, C26H21Cl2F3N4O
- Crystal structure of N1,N2-bis(2-fluorobenzyl)benzene-1,2-diamine,C20H18F2N2
- The crystal structure of 2-(benzo[d][1,3]dioxol-5-yl)-2,3-dihydro-1H-naphtho[1,8-de][1,3,2]diazaborinine, C17H13BN2O2
- The crystal structure of 6,6′-((1E,1′E)-hydrazine-1,2-diylidenebis(methaneylylidene)) bis(2-bromo-4-nitrophenol) — dimethylsulfoxide (1/2), C14H8Br2N4O6⋅2(C2H6OS)
- Selective biocatalytic synthesis and crystal structure of (2R,6R)-hydroxyketaminium chloride, C13H17Cl2NO2
- Crystal structure of bis{tetraaqua-[μ3-1-(4-carboxylatophenyl)-5-methyl-1H-pyrazole-3-carboxylate-κ4N,O,O′,O″] [μ2-1-methyl-1H-pyrazole-3,5-dicarboxylate-κ3N,O:O]dicobalt(II)} dihydrate, C36H44Co4N8O26
- Crystal structure of diethyl-2,2′-naphthalene-2,3-diylbis(oxy)diacetate, C18H20O6
- Synthesis and crystal structure of poly[(μ3-2-(2-carboxylatophenyl)-1H-benzo[d]imidazole-5-carboxylato-κO,O′:O′;:O″, O″′)-(μ2-1-(4-(1Himidazol-1-yl)phenyl)-1H-imidazole-κ2N:N′)cadmium(II)], C27H18CdN6O4
- The crystal structure of catena-poly[diaqua-bis(μ2-2-((2-(2-phenylacetyl)hydrazineylidene)methyl)benzoato-κ2O:O')zinc(II)], C32H30N4O8Zn
- The crystal structure of 2-(3,4-dimethoxyphenyl)-2,3-dihydro-1H-naphtho [1,8-de][1,3,2]diazaborinine, C18H17BN2O2
- The crystal structure of hexakis(1-ethylimidazole-κ1N)nickel(II) dichloride – 1-ethylimidazole (1/2), C40H64Cl2NiN16
- Crystal structure of diaqua-bis(2,4-dinitrophenolato-κ2O,O′)copper(II) 1.5 hydrate, C12H13CuN4O13.5
- Crystal structure of N′,N‴-((1E,1′E)-((decane-1,10-diylbis(oxy))bis(2,1-phenylene)) bis(methaneylylidene))di(isonicotinohydrazide), C36H40N6O4
- The crystal structure of 2-[(R)-1-(naphthalen-1-yl)ethyl]-2,3,7,7a-tetrahydro-3a,6-epoxyisoindol-1(6H)-one, C19H20NO2
- Synthesis and crystal structure of (1E,2E)-3-(anthracen-9-yl)-1-(4-methoxyphenyl)prop-2-en-1-one oxime, C24H19NO2
- Synthesis and crystal structure of (2E,2′E)-3,3′-(1,3-phenylene)bis(1-(3-bromophenyl)prop-2-en-1-one), C24H16Br2O2
- The crystal structure of catena-poly[bis(µ2-1,2-bis((1H-imidazol-1-yl)methyl)benzene- κ2N:N′)-bis(nitrato-κO)copper(II)], C28H28N10O6Cu
- Synthesis and crystal structure of the novel chiral acetyl-3-thiophene-5-(9-anthryl)-2-pyrazoline, C23H18N2OS
- Crystal structure of (E)-3-(dimethylamino)-1-(thiophen-3-yl)prop-2-en-1-one, C9H11NOS
- Crystal structure of catena-poly[aqua-(4-iodopyridine-2,6-dicarboxylato-κ3N,O,O′)-(μ2-4-amino-4H-1,2,4-triazole-κ2N:N′) copper(II)], C9H8N5O5CuI
- Crystal structure of cyclopropane-1,2,3-triyltris(phenylmethanone), C24H18O3
- Crystal structure of bis(amino(thioureido)methaniminium) terephthalate, C12H18N8O4S2
- A three-dimensional Eu(III) framework in the crystal structure of dimethylaminium poly[dimethylformamide-κ1N)bis(μ4-terephthalato-κ4O:O′:O′′:O′′′)europium(III)] monohydrate, C21H25EuN2O10
- Crystal structure of 2-methoxyphenyl 2-(6-methoxynaphthalen-2-yl)propanoate, C21H20O4
- The crystal structure of Hexakis(diethylamido)dimolybdenum, Mo2(NEt2)6

