The synthesis and crystal structure of ethyl (E)-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-5-((2-methoxybenzylidene)amino)-4-((trifluoromethyl)sulfinyl)-1H-pyrazole-3-carboxylate, C22H15N3Cl2F6O4S
Abstract
C22H15N3Cl2F6O4S, monoclinic, P1̄ (no. 14), a = 13.6099(10) Å, b = 12.0571(9) Å, c = 16.2351(13) Å, α = 90°, β = 112.714(2)°, γ = 90°, V = 1146.7(7) Å3, Z = 4, Rgt(F) = 0.0419, wRref(F2) = 0.1087, T = 173 K.
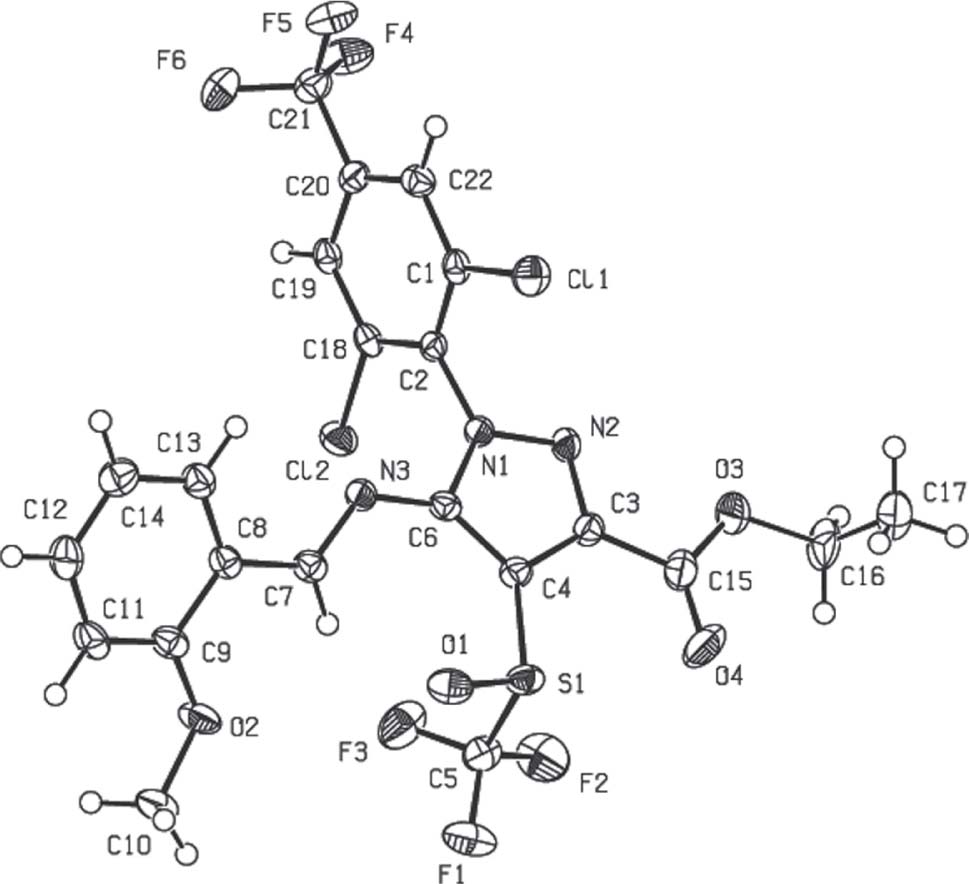
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.11 × 0.10 × 0.08 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.43 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| θmax, completeness: | 26.4°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 32747, 5048, 0.065 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 3776 |
| N(param)refined: | 365 |
| Programs: | CrysAlisPRO [1], SHELX [2], [3], Diamond [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| Cl1 | 0.64428(5) | 0.40984(5) | 0.32028(4) | 0.03194(16) |
| Cl2 | 0.95717(5) | 0.60071(5) | 0.59897(4) | 0.02916(16) |
| S1 | 0.66553(5) | 0.32083(5) | 0.67938(4) | 0.02626(16) |
| F1 | 0.73916(14) | 0.34879(15) | 0.85167(11) | 0.0457(4) |
| F2 | 0.85658(13) | 0.28402(16) | 0.80652(12) | 0.0503(5) |
| F3 | 0.81536(15) | 0.45606(14) | 0.78981(11) | 0.0504(5) |
| F4 | 0.98330(11) | 0.68408(15) | 0.27405(11) | 0.0405(4) |
| F5 | 0.81942(12) | 0.70765(14) | 0.18918(10) | 0.0382(4) |
| F6 | 0.89606(15) | 0.82160(13) | 0.29631(12) | 0.0483(5) |
| O1 | 0.58367(13) | 0.40576(16) | 0.67148(12) | 0.0318(4) |
| O2 | 0.63617(14) | 0.69185(16) | 0.76076(11) | 0.0329(4) |
| O3 | 0.87976(17) | 0.12062(15) | 0.56810(13) | 0.0404(5) |
| O4 | 0.78788(18) | 0.12097(16) | 0.65830(15) | 0.0469(5) |
| N1 | 0.78450(15) | 0.43550(16) | 0.51083(13) | 0.0205(4) |
| N2 | 0.82589(15) | 0.33135(16) | 0.52064(13) | 0.0223(4) |
| N3 | 0.67546(15) | 0.55701(16) | 0.55107(13) | 0.0219(4) |
| C1 | 0.74978(18) | 0.50129(18) | 0.36041(16) | 0.0207(5) |
| C2 | 0.80615(18) | 0.50982(18) | 0.45202(15) | 0.0191(5) |
| C3 | 0.79293(18) | 0.28412(19) | 0.57945(16) | 0.0224(5) |
| C4 | 0.73337(18) | 0.3582(2) | 0.61005(15) | 0.0218(5) |
| C5 | 0.7774(2) | 0.3549(2) | 0.78808(17) | 0.0303(6) |
| C6 | 0.72765(18) | 0.45682(19) | 0.56288(15) | 0.0204(5) |
| C7 | 0.65873(18) | 0.5996(2) | 0.61674(16) | 0.0227(5) |
| H7 | 0.689646 | 0.565645 | 0.673842 | 0.027* |
| C8 | 0.59356(18) | 0.6986(2) | 0.60634(16) | 0.0219(5) |
| C9 | 0.58016(19) | 0.7441(2) | 0.68174(16) | 0.0250(5) |
| C10 | 0.6125(2) | 0.7209(2) | 0.83648(17) | 0.0335(6) |
| H10A | 0.536661 | 0.708144 | 0.822756 | 0.050* |
| H10B | 0.629255 | 0.799336 | 0.850946 | 0.050* |
| H10C | 0.655270 | 0.675105 | 0.887649 | 0.050* |
| C11 | 0.51599(19) | 0.8354(2) | 0.67261(18) | 0.0285(6) |
| H11 | 0.505572 | 0.864701 | 0.722962 | 0.034* |
| C12 | 0.4666(2) | 0.8843(2) | 0.58930(19) | 0.0325(6) |
| H12 | 0.423308 | 0.947963 | 0.583402 | 0.039* |
| C13 | 0.4793(2) | 0.8421(2) | 0.51434(19) | 0.0331(6) |
| H13 | 0.445741 | 0.876718 | 0.457762 | 0.040* |
| C14 | 0.54167(19) | 0.7487(2) | 0.52376(17) | 0.0260(5) |
| H14 | 0.549181 | 0.718008 | 0.472594 | 0.031* |
| C15 | 0.8192(2) | 0.1672(2) | 0.60689(18) | 0.0311(6) |
| C16a | 0.9164(5) | 0.0072(4) | 0.5912(4) | 0.0423(13) |
| H16Aa | 0.923694 | −0.009488 | 0.652981 | 0.051* |
| H16Ba | 0.986786 | −0.002806 | 0.587521 | 0.051* |
| C17a | 0.8362(4) | −0.0697(3) | 0.5267(3) | 0.0511(15) |
| H17Aa | 0.766875 | −0.059512 | 0.530912 | 0.077* |
| H17Ba | 0.859659 | −0.146665 | 0.541466 | 0.077* |
| H17Ca | 0.829945 | −0.053125 | 0.465797 | 0.077* |
| C18 | 0.88701(18) | 0.58849(19) | 0.48496(16) | 0.0204(5) |
| C19 | 0.91300(18) | 0.65671(19) | 0.42771(16) | 0.0219(5) |
| H19 | 0.967452 | 0.710998 | 0.450399 | 0.026* |
| C20 | 0.85733(18) | 0.64378(19) | 0.33603(16) | 0.0218(5) |
| C21 | 0.8882(2) | 0.7143(2) | 0.27387(18) | 0.0284(6) |
| C22 | 0.77605(18) | 0.5667(2) | 0.30133(16) | 0.0234(5) |
| H22 | 0.739137 | 0.558829 | 0.238643 | 0.028* |
| C16Ab | 0.874(2) | −0.005(2) | 0.5890(18) | 0.040(5) |
| H16Cb | 0.914532 | −0.021518 | 0.653052 | 0.048* |
| H16Db | 0.799679 | −0.030574 | 0.571399 | 0.048* |
| C17Ab | 0.9251(18) | −0.0546(15) | 0.5324(11) | 0.049(6) |
| H17Db | 0.874905 | −0.053826 | 0.469972 | 0.074* |
| H17Eb | 0.945612 | −0.131309 | 0.551305 | 0.074* |
| H17Fb | 0.988649 | −0.011698 | 0.538435 | 0.074* |
aOccupancy: 0.812(9), bOccupancy: 0.188(9).
Source of material
Doubly distilled water was used throughout all experiments. All chemical solvents and reagents were of analytical grade quality, which were obtained from commercial suppliers and used directly without further purification (Wu Han Guoyao Chemical Reagent Co., Ltd.).
The synthesis of the target product is divided into two processes. In the first step, 5 mmol of fipronil was placed into a 100 mL round bottom flask, 1 mmol of anhydrous FeCl3 and 30.0 mL of ethanol were added and refluxed at 90 °C for 10 h. After the reaction was completed, the device was cooled to room temperature, and the reaction solution was transferred to a 100 mL rotary distillation bottle. The solvent was removed to obtain a brown-red thick liquid. We extacted with 30.0 mL of ethyl acetate and 80.0 mL of water, collected the organic phase, transferred the organic phase to a rotary flask, and added 3.0 g of silica gel for drying sample preparation. Finally, different proportions of ethyl acetate and petroleum ether were used as developing agents and separated by chromatography to obtain the intermediate product esterified fipronil: ethyl 5-amino-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-(trifluoromethylsulfinyl)-1H-pyrazole-3-carboxylate. Yield: 3.60 g (82.4%), 1H NMR (CDCl3, 400 MHz, ppm), δ 1.42–1.46 (t, J = 16.0 Hz, 3H, CH2—H), 3.89 (s, J = 12.0 Hz, 3H, O—CH3), 4.47–4.49 (q, J = 7.2 Hz, 2H, CH—H), 6.91–6.93 (t, J = 7.2 Hz, 2H, Ar—H), 7.72–7.74 (m, 4H, Ar—H), 9.39 (s, 1H, CH=N). IR (KBr, ν/cm−1): 3446–3328 (N—H), 2993 (Ar—H), 1729 (C=O), 1619 (C=N), 1400–1325 (C—F), 1137–1059 (C—O—C). MS (FAB): m/e, 484 (M+).
In the second step, 5.0 mmol of ethyl 5-amino-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-(trifluoromethylsulfinyl)-1H-pyrazole-3-carboxylate were added to 5.0 mmol of an aromatic aldehyde, and 1.0 g of 4 Å molecular sieve. Finally, 0.1 g of methylbenzenesulfonic acid was used as a catalyst, which was dissolved in 20.0 mL of toluene and heated under reflux at 120 °C for 20 h. After the reaction was completed, the reaction solution was cooled to 70 °C with suction filtration, and the filtrate was collected in a rotary flask, and 2.0 g of silica gel was added. Finally, different proportions of ethyl acetate and petroleum ether were used as developing agents. Dry loading and chromatography were performed to obtain the title compound. Yield: 1.25 g (76.0%). 1H NMR (CDCl3, 400 MHz, ppm) δ 3.75 (s, J = 7.2 Hz, 3H), 4.11 (d, J = 8.4 Hz, 2H), 6.44 (s, J = 6.8 Hz, 1H), 6.51 (s, J = 7.2 Hz, 2H), 6.76 (s, J = 7.2 Hz, 1H), 7.12 (s, J = 8.4 Hz, 1H), 7.55 (s, J = 7.2 Hz, 1H), 7.61 (s, J = 6.4 Hz, 1H). IR (KBr, ν/cm−1): 3063 (bezene ring C—H), 1729 (—C=O), 1570–1394 (benzene ring skeleton vibration), 1311 (C—F), 815 (aromatic ring C—H). MS (FAB): m/e, 602 (M+).
After allowing the Vethyl acetate/Vpetroleum ether (1:4) to stand in air for 14 days, transparent colorless crystals were formed by slow evaporation of the solvent. The crystals of the title compound were isolated, washed with light petroleum and dried in vacuum (yield 82.4%).
Experimental details
All H atoms were placed in geometrically idealized positions and constrained to ride on their parent atoms with C—H distances in the range 0.93–0.98 Å, and with Uiso(H) = 1.2 Ueq for aryl H atoms and 1.5 Ueq for the methyl H atoms. All H atoms were allowed to rotate to best fit the experimental electron density. The ethyl group was refined with a disorder model. Atom C16 and C17 of the ethyl moiety were found to be disordered over two positions (C16/C16A and C17/C17A).
Comment
Schiff base compounds are carbon-nitrogen double bond imine compounds, which are widely used in the fields of medicine and biology, and have antiviral, bactericidal and antibacterial activities. They also play a huge role in the field of fluorescent probes and organic optoelectronics [5], [6]. Some new fipronil Schiff base derivatives have been synthesized in the early stage of this experiment and achieved good insecticidal effects. Because the ester group occupies an important position in the fields of pesticides and medicines, such as pyrethroid pesticides and pre-ester compounds, it proves that the ester compounds have good activity. Phenylpyrazoles are widely used in pesticides, such as fipronil and acetonitrile. The toxicity of phenylpyrazoles to insects and mammals can be attributed to their role as non-competitive blockers on GABA receptors, effecting the structure of GABA-gated chloride channels [7], [8], [9], [10]. Many useful organic reactions are often used in pesticide synthesis, and reasonable and innovative methods are hoped for in the “acetation, phthalamidation, and sulfophthalation” [11]. In this experiment, esterification was used to synthesize ethyl 5-amino-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-(trifluoromethylsulfinyl)-1H-pyrazole-3-carboxylate with high yield, which is beneficial to the synthesis of title product.
The crystal structure of the title compound belongs to the space group P1̄, each unit cell is composed of 4 molecules. According to the data, in the ester group of the pyridine ring, the O(4)-C(15) bond length is with 1.210(3) Å shorter than the O(2)-C(9) bond length of 1.367(3) Å. This is because O(4)-C(15) is a carbon-oxygen double bond. N(3)-C(7) bond length is 1.280(3) Å, which is typical for a double bond. From the crystal structure, it is seen that the aryl and pyrazolyl moieties are not coplanar.
The packing of the title structure is partially facilitated by Y—X⋯π interactions between aromatic rings in neighboring molecules. The two most prominent interactions are given in the Y-X⋯Cg(π-ring) interactions table (Cg1 represents the centroid of ring N1/N2/C4/C2/C3, Cg2 that of C7/C12/C11/C10/C9/C8). The first of these interactions, C8—Cl(2)⋯C(g)1π which acts in centrosymmetric pairs between each two molecules, connects the molecules to infinite chains along the c axis of the unit cell. The second slightly weaker type of C19—F6A⋯C(g)2π interaction connects these chains with each other.
The bioactivities of the title compound against the 3rd instar larvae of Plutella xylostella were determined by the leaf disc-dipping assay. Leaves of Chinese cabbage grown in the greenhouse were collected, and discs (5 cm diameter) were punched from each leaf. The compounds were dissolved in acetone and suspended in distilled water containing Triton X-100. Leaf discs were dipped in each test solution for 60 s and allowed to dry for 3 h. The treated leaf discs were placed into Petri dishes (10 cm diameter). Then, ten Plutella xylostella larvae were introduced into each dish. Doubly distilled water containing acetone-Triton X-100 solution was used as the control. Petri dishes were kept in incubator at 20 °C and 80% relative humidity under a photoperiod of 18:10 h light: dark. All treatments were replicated three times. Mortalities were determined 24 h after treatment. The death rate of each treatment group was confirmed. LC50 value was calculated by the SPSS. Bioactivity result showed that the activities of the title compound against Plutella xylostella after 24 h is 13.86 mg ⋅ L−1 better than that of fipronil 26.97 mg ⋅ L−1. This approach proposes a novel insight to provide a great number of novel phenylpyrazole Schiff base fluorescent insecticide by a general green method.
It is noted that the absorption and photoluminescence spectra of the title compound in CH2Cl2 solution were investigated. In the absorption spectrum, intense absorptions are observed in the ultraviolet region of the spectrum. Strong absorption peak near 221 nm and 286 nm, belonging to the conjugated absorption peak of benzene ring and pyrazole ring, in the title compound. The aryl moiety C8—C13 forms a larger conjugated structure with the pyrazolyl moiety, resulting in a red shift in UV absorption and a medium-intensity absorption peak at 332–398 nm. Its UV absorption is mainly attributed to the p-π* transition of the compound conjugated system. The fluorescene spectrum of the title compound shows a strong peak at 456 nm. Thus phenylpyrazole heterocycle are good candidates to design and develop new fluorescent pesticides, which lays a foundation for the natural degradation and fluorescence detection of pesticide residues based on the phenylpyrazole Schiff base structure.
Acknowledgements
The authors thank the National Natural Science Foundation of China (Grant Nos. 20702064, 21177161 and 31402137); the Natural Science Foundation of Hubei province for Distinguished Yong Scholars (No. 2013CFA034); the Program for Excellent Talents in Hubei Province (RCJH15001) and the Fundamental Research Funds for the Central University, South-Central University for Nationalities.
References
1. Oxford Diffraction Ltd: CrysAlisPRO. Abingdon, Oxfordshire, England (2006).Search in Google Scholar
2. Sheldrick, G. M.: SHELXT – integrated space-group and crystal-structure determination. Acta Crystallogr. A71 (2015) 3–8.10.1107/S2053273314026370Search in Google Scholar PubMed PubMed Central
3. Sheldrick, G. M.: Crystal structure refinement with SHELXL. Acta Crystallogr. C71 (2015) 3–8.10.1107/S2053229614024218Search in Google Scholar PubMed PubMed Central
4. Brandenburg, K.: DIAMOND. Visual Crystal Structure Information System. Ver. 4.0. Crystal Impact, Bonn, Germany (2015).Search in Google Scholar
5. Abu-Dief, A. M.; Mohamed, I. M. A.: A review on versatile applications of transition metal complexes incorporating Schiff bases. Beni-suef Univ. J. Basic Appl. Sci. 4 (2015) 119–133.10.1016/j.bjbas.2015.05.004Search in Google Scholar PubMed PubMed Central
6. Ma, S. F.; Cui, Y.; Su, R. F.: Synthesis of triphenylamine imine compounds. Synthetic Method 20 (2012) 241–243.Search in Google Scholar
7. Cole, L. M.; Nicholson, R. A.; Casida, J. E.: Action of phenylpyrazole insecticides at the GABA-gated chloride channel. Pestic. Biochem. Physiol. 46 (1993) 47–54.10.1006/pest.1993.1035Search in Google Scholar
8. Bekhit, A. A.; Abdel-Aziem T.: Design, synthesis and biological evaluation of some pyrazole derivatives as anti-inflammatory-antimicrobial agents. Bioorg. Med. Chem. 22 (1993) 13–23.10.1016/j.bmc.2004.01.037Search in Google Scholar PubMed
9. Gant, D. B.; Chalmers, A. E.; Wolff, M. A.; Hoffman, H. B.; Bushey, D. F.: Fipronil: action at the GABA receptor. Rev. Toxicol. 2 (1998) 147–156.Search in Google Scholar
10. Caboni, P.; Sammelson, R. E.; Casida, J. E.: Phenylpyrazole insecticide photochemistry, metabolism, and GABAergic action: ethiprole compared with fipronil. J. Agric. Food Chem. 51 (2003) 7055–7061.10.1021/jf030439lSearch in Google Scholar PubMed
11. Cheng, Z. M.: Several organic reactions useful in pesticide synthesis: lipidation, acidification and sulfonation. World Pesticide 24 (2002) 6–7.Search in Google Scholar
©2020 Lianqing Chen et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Frontmatter
- Crystal structure of catena-poly[(μ2-3-(benzo[d]thiazol-2-yl)-5-carboxybenzoato-κ2N:O)silver(I)], C15H8AgNO4S
- Crystal structure of bis(4-phenylpiperazin-1-ium) bis(2-(4-phenylpiperazin-1-yl)succinato-κ2O,O′)copper(II) tetrahydrate, C48H70CuN8O12, [C10H14N2]2[Cu(C14H17N2O4)2] ⋅ 4 H2O
- Crystal structure of triaqua-bis(2-(6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylenedecahydronaphthalen-1-yl)-1-(2-oxo-2,5-dihydrofuran-3-yl)ethane-1-sulfonato-κ2O,O′)calcium(II) – ethanol (1/2), C44H76CaO19S2
- The crystal structure of ethyl 5-(4-(diphenylamino)phenyl)thiophene-2-carboxylate, C25H21NO2S
- The crystal structure of 5-bromo-2-(2-methyl-2H-tetrazol-5-yl)pyridine, C7H6BrN5
- The crystal structure of (E)-5-chloro-2-hydroxy-N′-(2-hydroxy-4-methoxybenzylidene)benzohydrazide, C15H13ClN2O4
- The crystal structure of (2Z,2′Z)-N′,N′′′′-(pyridine-2,6-dicarbonyl)dipicolinohydrazonamide, C19H17N9O2
- Photochromic properties and crystal structure of 3,3′-(perfluorocyclopent-1-ene-1,2-diyl)bis(5-(4-(azidomethyl)phenyl)-2-methylthiophene), C29H20F6N6S2
- Crystal structure of aqua-dichlorido-(4-(((3-ethoxy-2-oxidobenzylidene)hydrazono)(oxido)methyl)pyridin-1-ium-κ3N,O,O′)iron(III), C15H16Cl2N3O4Fe
- Crystal structure of catena-poly[diaqua-(μ2-4,4′-bipyridine-κ2N:N′)-bis(2,3,4,5-tetrabromo-6-carboxybenzoato-κ1O)-nickel(II)], C26H14Br8NiN2O10
- Crystal structure of diethanol-κ1O-bis(μ2-N-((2-oxidonaphthalen-1-yl)methylene)pyrazine-2-carbohydrazonato-κ5N,O,O′:O′:N′)-bis(nitrato-κ2O,O′)dieuropium(III), C36H32N10O12Eu2
- The crystal structure of 2-aminoisophthalic acid, C8H7NO4
- Crystal structure of (E)-2-(4-((3,4-difluorobenzyl)oxy)styryl)-4,6-dimethoxybenzaldehyde, C24H20F2O4
- Crystal structure of 2-benzoylpyrene, C23H14O
- Crystal structure of chlorido-(η6-p-cymene)-(N-(2-fluorophenyl)-1-(pyridin-2-yl)methanimine-κ2N,N′)ruthenium(II) – acetone (1/1), C22H23ClN2F7OPRu
- The crystal structure of 2-bromoisonicotinic acid, C6H4BrNO2
- Crystal structure of 1,3,5,7-tetraphenyl-8-(N-phenylformamido)-2-oxa-5-azabicyclo[4.2.0]oct -3-en-7-yl benzoate, C44H34N2O4
- Synthesis and crystal structure of 4-(3-acetyl-5-(thiophen-2-yl)-2,3-dihydro-1,3,4-oxadiazol-2-yl)-7-(diethylamino)-2H-chromen-2-one, C21H21N3O4S
- Crystal structure of poly[diaqua-(μ2-4,4′-bipyridine-κ2N:N′)-(μ2-3,4,5,6-tetrafluorophthalato-κ2O:O′)nickel(II)], C18H12F4NiN2O6
- Crystal structure of 4-hydroxynaphtho[2,3-b]benzofuran-6,11-dione, C16H8O4
- The crystal structure of 3,10-bis(4-methoxyphenyl)-6,12-dibenzyl-2,9-acetyl-6,12-diazapentacyclo[6.3.1.02,7.04,11.05,9]dodecane – acetone (1/1), C45H48N2O5
- The crystal structure of (E)-2-(((2-(1H-indol-3-yl)ethyl)iminio)methyl)-6-bromophenolate, C17H15N2BrO
- Crystal structure of catena-poly[diaqua-(μ2-oxalyl dihydrazide-κ4N,O:N′,O′)-bis(μ2-pyridine-2,3-dicarboxylato-κ3N,O,O′)dicadmium(II)] hexahydrate, C16H28O18N6Cd2
- Crystal structure of poly[tetra-(μ4-naphthalene-1,8-dicarboxylato-κ4O:O,O′: O′′:O′′,O′′′)-(μ4-oxo-κ4O:O:O:O) penta-lead(II)], C48H24O17Pb5
- Crystal structure of 5H-dibenzo[c,f][1,5]oxabismocin-12 (7H)-yl acetate, C16H15O3Bi
- The crystal structure of 2-(4-chloro-6-nitrophenyl)-1-(4-chloro-3-nitrophenyl)diazene 1-oxide, C12H6Cl2N4O5
- Crystal structure of bis(3-methyl-N-(1-(pyrazin-2-yl)ethylidene)benzohydrazonato-κ3O,N,N′)nickel(II), C28H26N8O2Ni
- Crystal structure of 3,10-bis(4-chlorophenyl)-6,12-dibenzyl-2,9-acetyl-6,12-diazapentacyclo[6.3.1.02,7.04,11.05,9]-dodecane, C40H36Cl2N2O2
- Crystal structure of bis[(μ2-4⋯O,O′:O′)-(4-hydroxybenzoato-κ2O,O′)-(1,10-phenanthroline-κ2N,N′)]-di-lead(II)μ-4-hydroxybenzoato-κ3O,O′:O′;κ3O,O′:O′-bis-[(4-hydroxybenzoato-κ2O,O′)bis(1,10-phenanthroline-κ2N,N′)di-lead(II)] monohydrate, C52H36N4O12Pb2 ⋅ H2O
- Crystal structure of poly[diaqua-(μ3-3,4,5,6-tetrafluoro-phthalato-κ3O:O′:O′′)-(μ2-1,2-bis(4-pyridyl)ethene-κ2N:N′)cobalt(II)], C14H9CoF4NO6
- Crystal structure of 7-hydroxy-4-phenyl-2H-chromen-2-one, C15H10O3
- Crystal structure of 3,7-dimethyl-1-(5-oxohexyl)-3,7-dihydro-1H-purine-2,6-dione 4-hydroxybenzoic acid, C20H24N4O6
- Crystal structure of catena-poly[(μ2-1,5-dimethyl-2-phenyl-4-((pyridin-4-ylmethylene)amino)-1,2-dihydro-3H-pyrazol-3-one-κ2N:O)-bis(nitrato-κ1O)zinc(II)], C17H16N6O7Zn
- The crystal structure of diaqua-bis(6-aminopicolinato-κ2N,O)magnesium(II), C12H14O6N4Mg
- Crystal structure of (pyridine-2-carboxamide-κ2N,O)-[tris((1H-benzo[d]imidazol-2-yl)methyl)amine-κ4N,N′,N′′,N′′′]nickel(II) diperchlorate — methanol (1/3), C33H39Cl2N9NiO12
- Crystal structure of catena-poly[diaqua-bis(3-(4-trifluoromethyl-phenyl)-acrylato-κO1)-(μ2-1,4-bis(1-imidazolyl)benzene-κ2N3:N3′)cobalt(II)], C32H26CoF6N4O6
- Crystal structure of (E)-3-(2-(2-hydroxy-4-methoxystyryl)-3,3-dimethyl-3H-indol-1-ium-1-yl)propane-1-sulfonate monohydrate, C22H25NO5S⋅H2O
- The crystal structure of bis(N-oxy-2-(1H-tetrazol-1-yl) acetamide κ2O,O′)-diaqua-zinc(II), C6H12ZnN10O6
- Crystal structure of (E)-4-((4-chlorophenylimino)methyl)pyridinium 3,5-dinitrobenzoate, C19H13ClN4O6
- Crystal structure of dichlorido-bis((E)-2-((pyridin-4-ylmethylene)amino)phenol)zinc(II), C24H20Cl2N4O2Zn
- Crystal structure of cyclo-[tetrachlorido-bis(μ2-p-xylylenediamine-κ2N:N′)dipalladium(II)] dimethyl sulfoxide solvate, C20H36Cl4N4O2Pd2S2
- Crystal structure of 4-(3-fluorophenyl)-7-hydroxy-2H-chromen-2-one, C15H9FO3
- Crystal structure of (E)-2-((2-(pyrimidin-2-yl)hydrazono)methyl)quinolin-1-ium perchlorate – methanol (1/1), C15H16N5O5Cl
- The crystal structure of bis(N-(amino(pyridin-2-yl)methylene)-5-chloro-2-hydroxybenzohydrazonato-κ3N,N′,O)zinc(II) – methanol (2/5), C57H60Cl2N16O13Zn2
- Synthesis and crystal structure of 4,4′-di(4-pyridyl)-6,6′-di(tert-butyl)-2,2′-[propylenedioxybis(nitrilomethylidyne)]diphenol, C35H40N4O4
- Crystal structure of (3E,3′E)-3,3′-((1,3,4-thiadiazole-2,5-diyl)bis(sulfanediyl))bis(4-hydroxy-4-phenylbut-3-en-2-one), C22H18N2O4S3
- Crystal structure of (N-benzyl-N-methyl-dithiocarbamato-κ2S,S′)di(4-chlorobenzyl)chloridotin(IV), C23H22Cl3NS2Sn
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ6O6) sodium bromide hydrate, [Na(18-crown-6)]Br ⋅ H2O, C12H26BrNaO7
- Crystal structure of 7-ethoxyl-6,8-difluoro-4-oxo-1-phenyl-1,4-dihydro-quinoline-3-carboxylic acid, C18H13F2N1O4
- Crystal structure of chlorido (2-(4-ethylphenyl)pyrimidine-k2C,N)(triphenylphosphane-kP) palladium(II), C30H26ClN2PPd
- Crystal structure of 18-crown-6 – 1,4-diiodotetrafluorobenzene – acetonitrile (1/1/2), C22H30F4I2N2O6
- Crystal structure of diisobutyl 2,5-dihydroxycyclohexa-1,4-diene-1,4-dicarboxylate, C16H24O6
- Crystal structure of poly[[tris(μ2-cis-1,2-cyclohexanedicarboxylato)-κ2O, O′]-bis[μ3-1,3,5-tris[(1H-imidazol-1-yl)methyl]benzene-κ3N, N′,N′′]-trizinc(II)] – water (1/20), C60H106N12O32Zn3
- The synthesis and crystal structure of 5-amino-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-((trifluoromethyl)sulfinyl)-1H-pyrazole-3-carboxamide–tetrahydrofuran (1/1), C16H14N4Cl2F6O3S
- Crystal structure of dimethylbis(diisopropyldithiocarbamato-κ2S,S′)tin(IV), C16H34N2S4Sn
- Crystal structure of diisopropyl 2,5-dihydroxycyclohexa-1,4-diene-1,4-dicarboxylate, C14H20O6
- The synthesis and crystal structure of ethyl (E)-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-5-((2-methoxybenzylidene)amino)-4-((trifluoromethyl)sulfinyl)-1H-pyrazole-3-carboxylate, C22H15N3Cl2F6O4S
- The crystal structure of a matrine derivative, 13-(methylamine-1-yl) carbodithioate matrine, C17H27N3OS2
- Crystal structure of bis(2-hydroxy-6-((phenylimino)methyl)phenolato-κ2N,O)copper(II), C26H20CuN2O4
- The crystal structure of 2-p-fluorophenyl-5-dihydroxymethyl-1,3,4-oxadiazole, C9H7FN2O3
- Crystal structure of dichloridobis(4-chlorophenyl-κC1)(1,10-phenanthroline-κ2N,N′)tin(IV), C24H16Cl4N2Sn
- Crystal structure of bis{bromido-triphenyltin(IV)}(μ2-[4,4′-bipyridine]1,1′-dioxide-κ2O:O′), C46H38Br2N2O2Sn2
- Crystal structure of 2-(5-chloro-quinolin-8-yloxy)-N-quinolin-8-yl-acetamide, C20H14N3O2Cl
- Crystal structure of bis(N-(1-(3-ethylpyrazin-2-yl)ethylidene)-3-hydroxy-2-naphthohydrazonato-κ3N,N′,O)cobalt(II) — dimethylformamide (1/1), C41H41N9O5Co
- Crystal structure of bis[2-(1-(3-ethylpyrazin-2-yl)ethylidene)-1-tosylhydrazin-1-ido-κ3-N,N′,O]copper(II), C30H34N8O4S2Cu
- Crystal structure of (2-p-tolylpyrimidine-κ2C,N)(triphenylphosphane-κP) palladium(II), C29H24ClN2PPd
- Halogen bonding in crystal structure of bis(1,4,7,10-tetraoxacyclododecane-κ4O,O′,O′′,O′′′)cesium triiodide, C16H32CsI3O8
- The synthesis and crystal structure of N-(3-cyano-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-(trifluoromethylsulfinyl)-1H-pyrazol-5-yl)-2-phenylacetamide, C20H10N4Cl2F6O2S
- The crystal structure of 4-(trifluoromethyl)nicotinic acid, C7H4F3NO2
- Crystal structure of 3-(2-methylbenzyl)thiazolidin-2-one, C11H13ONS
- The crystal structure of 2,2,2-trifluoro-1-(isoquinolin-1-yl)ethane-1,1-diol, C11H8F3NO2
- The crystal structure of 3-bromoisonicotinic acid, C6H4BrNO2
- The crystal structure of 5-nitropicolinic acid monohydrate, C6H6N2O5
- The crystal structure of 3-(4-hydroxybenzyl)-1,5-dioxaspiro[5.5]undecane-2,4-dione, C16H18O5
- Crystal structure of [[Mo3Se7(S2CNEt2)3]2(μ-Se)] ⋅ 2(C6H4Cl2), C42H68Cl4Mo6N6S12Se15
- Crystal structure of (E)-4-hydroxy-3-((5-phenyl-1,3,4-oxadiazol-2-yl)thio)pent-3-en-2-one, C13H12N2O3S
- The crystal structure of (2,3-dioxo-5,6:13,14-dibenzo-9,10-benzo-1,4,8,11-7, 11-diene-κ4N,N′,N′′,N′′′)-nickel(II), Ni(C22H14N4O2)
- Crystal structure of 3-(1-benzyl-2-ethyl-4-nitro-1H-imidazol-5-ylthio)-propanoic acid, C15H17N3O4S
- The crystal structure of dichlorobis(2-(dicyclohexylphosphino)-2′,4′,6′-tri-i-propyl-1,1′-biphenyl) palladium(II)-dichloroform, C68H100Cl8P2Pd
- Crystal structure and antimicrobial properties of (1,4,7,10-tetraoxacyclododecane-κ4O,O′,O′′,O′′′)cesium(I) pentaiodide, C16H32CsI5O8
Articles in the same Issue
- Frontmatter
- Crystal structure of catena-poly[(μ2-3-(benzo[d]thiazol-2-yl)-5-carboxybenzoato-κ2N:O)silver(I)], C15H8AgNO4S
- Crystal structure of bis(4-phenylpiperazin-1-ium) bis(2-(4-phenylpiperazin-1-yl)succinato-κ2O,O′)copper(II) tetrahydrate, C48H70CuN8O12, [C10H14N2]2[Cu(C14H17N2O4)2] ⋅ 4 H2O
- Crystal structure of triaqua-bis(2-(6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylenedecahydronaphthalen-1-yl)-1-(2-oxo-2,5-dihydrofuran-3-yl)ethane-1-sulfonato-κ2O,O′)calcium(II) – ethanol (1/2), C44H76CaO19S2
- The crystal structure of ethyl 5-(4-(diphenylamino)phenyl)thiophene-2-carboxylate, C25H21NO2S
- The crystal structure of 5-bromo-2-(2-methyl-2H-tetrazol-5-yl)pyridine, C7H6BrN5
- The crystal structure of (E)-5-chloro-2-hydroxy-N′-(2-hydroxy-4-methoxybenzylidene)benzohydrazide, C15H13ClN2O4
- The crystal structure of (2Z,2′Z)-N′,N′′′′-(pyridine-2,6-dicarbonyl)dipicolinohydrazonamide, C19H17N9O2
- Photochromic properties and crystal structure of 3,3′-(perfluorocyclopent-1-ene-1,2-diyl)bis(5-(4-(azidomethyl)phenyl)-2-methylthiophene), C29H20F6N6S2
- Crystal structure of aqua-dichlorido-(4-(((3-ethoxy-2-oxidobenzylidene)hydrazono)(oxido)methyl)pyridin-1-ium-κ3N,O,O′)iron(III), C15H16Cl2N3O4Fe
- Crystal structure of catena-poly[diaqua-(μ2-4,4′-bipyridine-κ2N:N′)-bis(2,3,4,5-tetrabromo-6-carboxybenzoato-κ1O)-nickel(II)], C26H14Br8NiN2O10
- Crystal structure of diethanol-κ1O-bis(μ2-N-((2-oxidonaphthalen-1-yl)methylene)pyrazine-2-carbohydrazonato-κ5N,O,O′:O′:N′)-bis(nitrato-κ2O,O′)dieuropium(III), C36H32N10O12Eu2
- The crystal structure of 2-aminoisophthalic acid, C8H7NO4
- Crystal structure of (E)-2-(4-((3,4-difluorobenzyl)oxy)styryl)-4,6-dimethoxybenzaldehyde, C24H20F2O4
- Crystal structure of 2-benzoylpyrene, C23H14O
- Crystal structure of chlorido-(η6-p-cymene)-(N-(2-fluorophenyl)-1-(pyridin-2-yl)methanimine-κ2N,N′)ruthenium(II) – acetone (1/1), C22H23ClN2F7OPRu
- The crystal structure of 2-bromoisonicotinic acid, C6H4BrNO2
- Crystal structure of 1,3,5,7-tetraphenyl-8-(N-phenylformamido)-2-oxa-5-azabicyclo[4.2.0]oct -3-en-7-yl benzoate, C44H34N2O4
- Synthesis and crystal structure of 4-(3-acetyl-5-(thiophen-2-yl)-2,3-dihydro-1,3,4-oxadiazol-2-yl)-7-(diethylamino)-2H-chromen-2-one, C21H21N3O4S
- Crystal structure of poly[diaqua-(μ2-4,4′-bipyridine-κ2N:N′)-(μ2-3,4,5,6-tetrafluorophthalato-κ2O:O′)nickel(II)], C18H12F4NiN2O6
- Crystal structure of 4-hydroxynaphtho[2,3-b]benzofuran-6,11-dione, C16H8O4
- The crystal structure of 3,10-bis(4-methoxyphenyl)-6,12-dibenzyl-2,9-acetyl-6,12-diazapentacyclo[6.3.1.02,7.04,11.05,9]dodecane – acetone (1/1), C45H48N2O5
- The crystal structure of (E)-2-(((2-(1H-indol-3-yl)ethyl)iminio)methyl)-6-bromophenolate, C17H15N2BrO
- Crystal structure of catena-poly[diaqua-(μ2-oxalyl dihydrazide-κ4N,O:N′,O′)-bis(μ2-pyridine-2,3-dicarboxylato-κ3N,O,O′)dicadmium(II)] hexahydrate, C16H28O18N6Cd2
- Crystal structure of poly[tetra-(μ4-naphthalene-1,8-dicarboxylato-κ4O:O,O′: O′′:O′′,O′′′)-(μ4-oxo-κ4O:O:O:O) penta-lead(II)], C48H24O17Pb5
- Crystal structure of 5H-dibenzo[c,f][1,5]oxabismocin-12 (7H)-yl acetate, C16H15O3Bi
- The crystal structure of 2-(4-chloro-6-nitrophenyl)-1-(4-chloro-3-nitrophenyl)diazene 1-oxide, C12H6Cl2N4O5
- Crystal structure of bis(3-methyl-N-(1-(pyrazin-2-yl)ethylidene)benzohydrazonato-κ3O,N,N′)nickel(II), C28H26N8O2Ni
- Crystal structure of 3,10-bis(4-chlorophenyl)-6,12-dibenzyl-2,9-acetyl-6,12-diazapentacyclo[6.3.1.02,7.04,11.05,9]-dodecane, C40H36Cl2N2O2
- Crystal structure of bis[(μ2-4⋯O,O′:O′)-(4-hydroxybenzoato-κ2O,O′)-(1,10-phenanthroline-κ2N,N′)]-di-lead(II)μ-4-hydroxybenzoato-κ3O,O′:O′;κ3O,O′:O′-bis-[(4-hydroxybenzoato-κ2O,O′)bis(1,10-phenanthroline-κ2N,N′)di-lead(II)] monohydrate, C52H36N4O12Pb2 ⋅ H2O
- Crystal structure of poly[diaqua-(μ3-3,4,5,6-tetrafluoro-phthalato-κ3O:O′:O′′)-(μ2-1,2-bis(4-pyridyl)ethene-κ2N:N′)cobalt(II)], C14H9CoF4NO6
- Crystal structure of 7-hydroxy-4-phenyl-2H-chromen-2-one, C15H10O3
- Crystal structure of 3,7-dimethyl-1-(5-oxohexyl)-3,7-dihydro-1H-purine-2,6-dione 4-hydroxybenzoic acid, C20H24N4O6
- Crystal structure of catena-poly[(μ2-1,5-dimethyl-2-phenyl-4-((pyridin-4-ylmethylene)amino)-1,2-dihydro-3H-pyrazol-3-one-κ2N:O)-bis(nitrato-κ1O)zinc(II)], C17H16N6O7Zn
- The crystal structure of diaqua-bis(6-aminopicolinato-κ2N,O)magnesium(II), C12H14O6N4Mg
- Crystal structure of (pyridine-2-carboxamide-κ2N,O)-[tris((1H-benzo[d]imidazol-2-yl)methyl)amine-κ4N,N′,N′′,N′′′]nickel(II) diperchlorate — methanol (1/3), C33H39Cl2N9NiO12
- Crystal structure of catena-poly[diaqua-bis(3-(4-trifluoromethyl-phenyl)-acrylato-κO1)-(μ2-1,4-bis(1-imidazolyl)benzene-κ2N3:N3′)cobalt(II)], C32H26CoF6N4O6
- Crystal structure of (E)-3-(2-(2-hydroxy-4-methoxystyryl)-3,3-dimethyl-3H-indol-1-ium-1-yl)propane-1-sulfonate monohydrate, C22H25NO5S⋅H2O
- The crystal structure of bis(N-oxy-2-(1H-tetrazol-1-yl) acetamide κ2O,O′)-diaqua-zinc(II), C6H12ZnN10O6
- Crystal structure of (E)-4-((4-chlorophenylimino)methyl)pyridinium 3,5-dinitrobenzoate, C19H13ClN4O6
- Crystal structure of dichlorido-bis((E)-2-((pyridin-4-ylmethylene)amino)phenol)zinc(II), C24H20Cl2N4O2Zn
- Crystal structure of cyclo-[tetrachlorido-bis(μ2-p-xylylenediamine-κ2N:N′)dipalladium(II)] dimethyl sulfoxide solvate, C20H36Cl4N4O2Pd2S2
- Crystal structure of 4-(3-fluorophenyl)-7-hydroxy-2H-chromen-2-one, C15H9FO3
- Crystal structure of (E)-2-((2-(pyrimidin-2-yl)hydrazono)methyl)quinolin-1-ium perchlorate – methanol (1/1), C15H16N5O5Cl
- The crystal structure of bis(N-(amino(pyridin-2-yl)methylene)-5-chloro-2-hydroxybenzohydrazonato-κ3N,N′,O)zinc(II) – methanol (2/5), C57H60Cl2N16O13Zn2
- Synthesis and crystal structure of 4,4′-di(4-pyridyl)-6,6′-di(tert-butyl)-2,2′-[propylenedioxybis(nitrilomethylidyne)]diphenol, C35H40N4O4
- Crystal structure of (3E,3′E)-3,3′-((1,3,4-thiadiazole-2,5-diyl)bis(sulfanediyl))bis(4-hydroxy-4-phenylbut-3-en-2-one), C22H18N2O4S3
- Crystal structure of (N-benzyl-N-methyl-dithiocarbamato-κ2S,S′)di(4-chlorobenzyl)chloridotin(IV), C23H22Cl3NS2Sn
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ6O6) sodium bromide hydrate, [Na(18-crown-6)]Br ⋅ H2O, C12H26BrNaO7
- Crystal structure of 7-ethoxyl-6,8-difluoro-4-oxo-1-phenyl-1,4-dihydro-quinoline-3-carboxylic acid, C18H13F2N1O4
- Crystal structure of chlorido (2-(4-ethylphenyl)pyrimidine-k2C,N)(triphenylphosphane-kP) palladium(II), C30H26ClN2PPd
- Crystal structure of 18-crown-6 – 1,4-diiodotetrafluorobenzene – acetonitrile (1/1/2), C22H30F4I2N2O6
- Crystal structure of diisobutyl 2,5-dihydroxycyclohexa-1,4-diene-1,4-dicarboxylate, C16H24O6
- Crystal structure of poly[[tris(μ2-cis-1,2-cyclohexanedicarboxylato)-κ2O, O′]-bis[μ3-1,3,5-tris[(1H-imidazol-1-yl)methyl]benzene-κ3N, N′,N′′]-trizinc(II)] – water (1/20), C60H106N12O32Zn3
- The synthesis and crystal structure of 5-amino-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-((trifluoromethyl)sulfinyl)-1H-pyrazole-3-carboxamide–tetrahydrofuran (1/1), C16H14N4Cl2F6O3S
- Crystal structure of dimethylbis(diisopropyldithiocarbamato-κ2S,S′)tin(IV), C16H34N2S4Sn
- Crystal structure of diisopropyl 2,5-dihydroxycyclohexa-1,4-diene-1,4-dicarboxylate, C14H20O6
- The synthesis and crystal structure of ethyl (E)-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-5-((2-methoxybenzylidene)amino)-4-((trifluoromethyl)sulfinyl)-1H-pyrazole-3-carboxylate, C22H15N3Cl2F6O4S
- The crystal structure of a matrine derivative, 13-(methylamine-1-yl) carbodithioate matrine, C17H27N3OS2
- Crystal structure of bis(2-hydroxy-6-((phenylimino)methyl)phenolato-κ2N,O)copper(II), C26H20CuN2O4
- The crystal structure of 2-p-fluorophenyl-5-dihydroxymethyl-1,3,4-oxadiazole, C9H7FN2O3
- Crystal structure of dichloridobis(4-chlorophenyl-κC1)(1,10-phenanthroline-κ2N,N′)tin(IV), C24H16Cl4N2Sn
- Crystal structure of bis{bromido-triphenyltin(IV)}(μ2-[4,4′-bipyridine]1,1′-dioxide-κ2O:O′), C46H38Br2N2O2Sn2
- Crystal structure of 2-(5-chloro-quinolin-8-yloxy)-N-quinolin-8-yl-acetamide, C20H14N3O2Cl
- Crystal structure of bis(N-(1-(3-ethylpyrazin-2-yl)ethylidene)-3-hydroxy-2-naphthohydrazonato-κ3N,N′,O)cobalt(II) — dimethylformamide (1/1), C41H41N9O5Co
- Crystal structure of bis[2-(1-(3-ethylpyrazin-2-yl)ethylidene)-1-tosylhydrazin-1-ido-κ3-N,N′,O]copper(II), C30H34N8O4S2Cu
- Crystal structure of (2-p-tolylpyrimidine-κ2C,N)(triphenylphosphane-κP) palladium(II), C29H24ClN2PPd
- Halogen bonding in crystal structure of bis(1,4,7,10-tetraoxacyclododecane-κ4O,O′,O′′,O′′′)cesium triiodide, C16H32CsI3O8
- The synthesis and crystal structure of N-(3-cyano-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-(trifluoromethylsulfinyl)-1H-pyrazol-5-yl)-2-phenylacetamide, C20H10N4Cl2F6O2S
- The crystal structure of 4-(trifluoromethyl)nicotinic acid, C7H4F3NO2
- Crystal structure of 3-(2-methylbenzyl)thiazolidin-2-one, C11H13ONS
- The crystal structure of 2,2,2-trifluoro-1-(isoquinolin-1-yl)ethane-1,1-diol, C11H8F3NO2
- The crystal structure of 3-bromoisonicotinic acid, C6H4BrNO2
- The crystal structure of 5-nitropicolinic acid monohydrate, C6H6N2O5
- The crystal structure of 3-(4-hydroxybenzyl)-1,5-dioxaspiro[5.5]undecane-2,4-dione, C16H18O5
- Crystal structure of [[Mo3Se7(S2CNEt2)3]2(μ-Se)] ⋅ 2(C6H4Cl2), C42H68Cl4Mo6N6S12Se15
- Crystal structure of (E)-4-hydroxy-3-((5-phenyl-1,3,4-oxadiazol-2-yl)thio)pent-3-en-2-one, C13H12N2O3S
- The crystal structure of (2,3-dioxo-5,6:13,14-dibenzo-9,10-benzo-1,4,8,11-7, 11-diene-κ4N,N′,N′′,N′′′)-nickel(II), Ni(C22H14N4O2)
- Crystal structure of 3-(1-benzyl-2-ethyl-4-nitro-1H-imidazol-5-ylthio)-propanoic acid, C15H17N3O4S
- The crystal structure of dichlorobis(2-(dicyclohexylphosphino)-2′,4′,6′-tri-i-propyl-1,1′-biphenyl) palladium(II)-dichloroform, C68H100Cl8P2Pd
- Crystal structure and antimicrobial properties of (1,4,7,10-tetraoxacyclododecane-κ4O,O′,O′′,O′′′)cesium(I) pentaiodide, C16H32CsI5O8


