Abstract
C25H20BNO5, triclinic, P1̅ (no. 2), a = 7.0868(6) Å, b = 10.3522(6) Å, c = 15.3140(10) Å, α = 74.088(7)°, β = 89.996(7)°, γ = 79.190(1)°, V = 1059.71(13) Å3, Z = 2, Rgt(F) = 0.0445, wRref(F2) = 0.1105, T = 298 K.
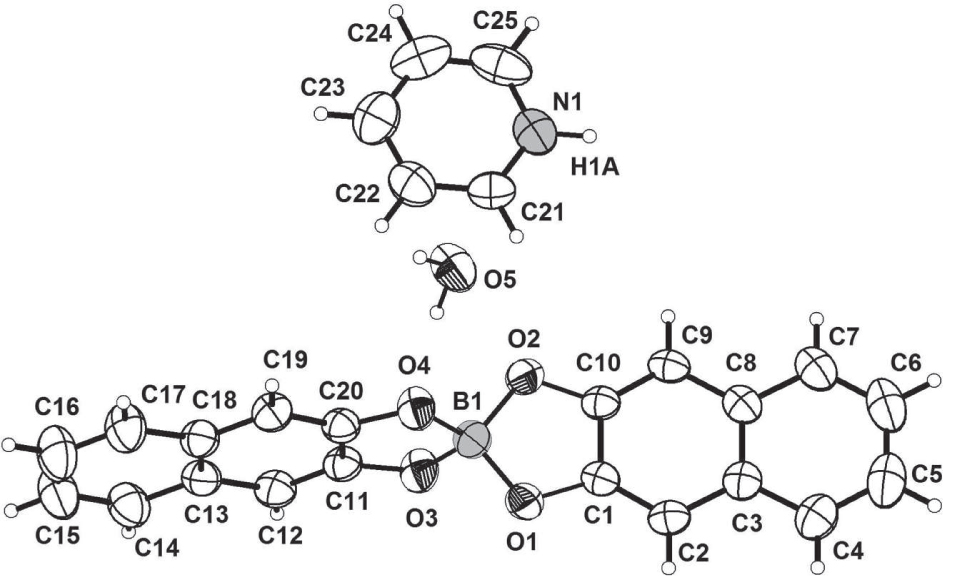
The asymmetric unit of the title crystal structure is shown in the figure. Tables 1 and 2 contain details of the measurement method and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless prism |
| Size: | 0.50 × 0.40 × 0.12 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.9 cm−1 |
| Diffractometer, scan mode: | Xcalibur, ω-scans |
| 2θmax, completeness: | 52.8°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 7281, 4305, 0.014 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 3102 |
| N(param)refined: | 299 |
| Programs: | CrysAlis [20], SHELX [21] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1 | 0.1529(2) | 0.18915(15) | −0.04570(11) | 0.0409(4) |
| C2 | 0.1050(2) | 0.17895(16) | −0.12874(12) | 0.0468(4) |
| H2 | 0.0982 | 0.0944 | −0.1373 | 0.056* |
| C3 | 0.0654(2) | 0.29984(17) | −0.20252(11) | 0.0457(4) |
| C4 | 0.0173(3) | 0.2979(2) | −0.29141(13) | 0.0628(5) |
| H4 | 0.0090 | 0.2148 | −0.3021 | 0.075* |
| C5 | −0.0174(3) | 0.4141(3) | −0.36221(14) | 0.0761(6) |
| H5 | −0.0480 | 0.4096 | −0.4202 | 0.091* |
| C6 | −0.0069(3) | 0.5396(2) | −0.34768(14) | 0.0731(6) |
| H6 | −0.0295 | 0.6188 | −0.3962 | 0.088* |
| C7 | 0.0361(3) | 0.54693(19) | −0.26290(13) | 0.0602(5) |
| H7 | 0.0410 | 0.6317 | −0.2541 | 0.072* |
| C8 | 0.0738(2) | 0.42871(16) | −0.18787(11) | 0.0453(4) |
| C9 | 0.1213(2) | 0.43532(16) | −0.09975(12) | 0.0454(4) |
| H9 | 0.1257 | 0.5188 | −0.0889 | 0.054* |
| C10 | 0.1603(2) | 0.31739(15) | −0.03158(11) | 0.0408(4) |
| C11 | 0.1930(2) | 0.05650(16) | 0.25597(11) | 0.0439(4) |
| C12 | 0.1266(2) | 0.00386(17) | 0.33792(12) | 0.0488(4) |
| H12 | −0.0048 | 0.0180 | 0.3465 | 0.059* |
| C13 | 0.2607(2) | −0.07369(16) | 0.41109(12) | 0.0477(4) |
| C14 | 0.2017(3) | −0.1357(2) | 0.49793(13) | 0.0623(5) |
| H14 | 0.0710 | −0.1272 | 0.5078 | 0.075* |
| C15 | 0.3318(3) | −0.2079(2) | 0.56764(15) | 0.0760(6) |
| H15 | 0.2893 | −0.2471 | 0.6245 | 0.091* |
| C16 | 0.5275(3) | −0.2232(2) | 0.55426(15) | 0.0787(6) |
| H16 | 0.6159 | −0.2719 | 0.6023 | 0.094* |
| C17 | 0.5899(3) | −0.1674(2) | 0.47154(13) | 0.0652(5) |
| H17 | 0.7214 | −0.1797 | 0.4633 | 0.078* |
| C18 | 0.4607(2) | −0.09099(16) | 0.39731(12) | 0.0481(4) |
| C19 | 0.5243(2) | −0.03233(17) | 0.31039(12) | 0.0489(4) |
| H19 | 0.6550 | −0.0419 | 0.3004 | 0.059* |
| C20 | 0.3919(2) | 0.03778(16) | 0.24223(11) | 0.0439(4) |
| C21 | 0.3748(3) | 0.5365(2) | 0.11457(14) | 0.0594(5) |
| H21 | 0.3493 | 0.4811 | 0.0792 | 0.071* |
| C22 | 0.4305(3) | 0.4804(2) | 0.20310(15) | 0.0677(5) |
| H22 | 0.4442 | 0.3864 | 0.2286 | 0.081* |
| C23 | 0.4661(3) | 0.5600(3) | 0.25446(16) | 0.0748(6) |
| H23 | 0.5026 | 0.5218 | 0.3158 | 0.090* |
| C24 | 0.4486(3) | 0.6961(3) | 0.21650(19) | 0.0825(7) |
| H24 | 0.4748 | 0.7518 | 0.2516 | 0.099* |
| C25 | 0.3927(3) | 0.7517(2) | 0.1270(2) | 0.0781(7) |
| H25 | 0.3800 | 0.8454 | 0.1004 | 0.094* |
| N1 | 0.3563(2) | 0.66954(17) | 0.07771(12) | 0.0611(4) |
| H1A | 0.321(3) | 0.707(2) | 0.0169(15) | 0.073* |
| O1 | 0.19842(17) | 0.08873(11) | 0.03352(8) | 0.0512(3) |
| O2 | 0.21094(17) | 0.30021(11) | 0.05712(8) | 0.0523(3) |
| O3 | 0.09247(15) | 0.12874(12) | 0.17660(8) | 0.0546(3) |
| O4 | 0.42123(15) | 0.09631(12) | 0.15389(8) | 0.0537(3) |
| O5 | 0.7172(2) | 0.23705(15) | 0.10376(11) | 0.0744(4) |
| H5A | 0.636(2) | 0.184(2) | 0.1239(14) | 0.089* |
| H5B | 0.823(2) | 0.192(2) | 0.1328(14) | 0.089* |
| B1 | 0.2307(3) | 0.1539(2) | 0.10424(14) | 0.0491(5) |
Source of material
A water solution (5 mL) of B(OH)3 (124 mg, 2.00 mmol,) was carefully added to a stirred ethanole (10 mL) solution of 2,3-naphthalenediol (673 mg, 4.2 mmol) at ambient temperature. The reaction mixture was stirred vigorously at 338 K for 30 min. The dropwise addition of pyridine (0.5 mL, 6.2 mmol) resulted in an immediate formation of a precipitate. A light pink coloured product was obtained after 40 min of stirring. The resulting solid was dissolved in the mixture of acetone/DMF (10 mL; 1:1) and allowed to stand at room temperature for a couple of days, thereupon transparent and fine crystals were harvested. Yield: (0.61 g, 70%) (based on B(OH)3); Elemental analysis: (Found): C 69.57, H 4.78, N 3.37%. Calculated for C25H20O5NB: C 70.61, H 4.74, N 3.29%. 1H-NMR (d6-DMSO-CDCl3, 298 K, TMS): δ (p.p.m.): 6.70 (m, 4H), 6.97 (m, 4H), 7.37 (m, 4H), 7.76 (m, 2H, pyridinium), 8.27 (m, 2H, pyridinium), 8.65 (m, 1H, pyridinium).
Experimental details
The H atoms of the water molecule were located in Fourier difference maps and refined freely. The remaining H atoms were positioned geometrically (C—H = 0.96–0.97Å) and refined using a riding model with Uiso(H) = 1.2 or 1.5Ueq(C).
Discussion
In recent years, attentions have been particularly paid to compounds containing borate groups on account of the fact that they possess extensive use as synthons in organic synthesis, especially in Suzuki cross coupling reactions [1]. Certain boron-containing species such as organoboronic acids [RB(OH)2] or boronate esters [RB(OR′)2] are exceedingly alluring for these carbon—carbon bond forming reactions as they are generally readily prepared, are air- and water-stable, and have relatively low toxicities [2]. The discovery of these simple boron compounds become significant owing to their displaying a wide range of biological properties [3]. The structures of borates are largely complex, and borates possessing novel structures that exhibit great variety potential applications notably in mineralogy and nonlinear optics (NLO) has been well documented [4], [5]. The coordination mode of boron in borate complexes is mostly either a BO3 triangle or a BO4 tetrahedron that linked together by the sharing of oxygen atoms. The resulting anionic entities may eventuate in isolated chains, rings, or cages, or may condense further to form polymeric chains, sheets or three-dimensional networks [6], [7]. The non-shared oxygen atoms will either bear a further negative charge (anhydrous borates) or will be protonated (hydrated borates). Hydrated borates are typically further hydrated by water molecules [8]. Most borates synthesized and studied to date have been prepared under the templating effect of cations, such as alkali-metal, alkaline-earth metal, transition metal, main group metal, rare earth metal, and inorganic-organic hybrid borates. Arylspiroboranate esters are a significant class of compounds being of special importance consisting of two catecholato groups linked to central boron atom. These compounds offers a number of advantageous such as being nontoxic, inexpensive and thermally, chemically and electrochemically stable and have found extensive usage in chemistry and industry. To set an example, lithium salts of arylspiroboranate esters are being considered for their potential use as electrolytes in batteries [9]. Other applications of these particular type of compounds involve a use as catalysts, or co-catalysts, for the Diels-Alder reaction [10], methoxycarbonylation reactions [11], and in amide and ester condensation reactions [12]. As part of our ongoing study of the synthesis and structure of organoborate complexes [13], [14], [15], [16], we have prepared a new organically templated borate using pyridine as the structure-directing agent.
The asymmetric unit of title compound consists of one [BO4(C10H6)2]− anion, one [C5H5NH)]+ cation and one water molecule. The [BO4(C10H6)2]− anion consists of one set of distorted [BO4] tetrahedra and two sets of [C20H12] planes with oxygen atoms as sharing vertexes. The boron atom is bonded to four oxygen atoms to form a tetrahedral environment (mean O—B—O bond angle of 109.5°). Bond distances and angles are similar to those reported in related arylspiroborate compounds [17], [18], [19]. Unsurprisingly, the cyclic ring structure induces a contraction of the O—B—O angles in the five membered rings, with O(1)—B(1)—O(2) 105.45(14)° and O(3)—B(1)—O(4) 103.58(14)°. The exocyclic angles of O(1)—B(1)—O(3) 111.46(14)°, O(2)—B(1)—O(3) 112.09(15)°, O(1)—B(1)—O(4) 112.70(15)°, and O(2)—B(1)—O(4) 111.75(14)° are substantially larger. In the crystal structure [BO4(C10H6)2]− anion and [C5H5NH)]+ cation are discrete units and they interact both electrostatically and via N—H ⋯O and O—H⋯O hydrogen bonds with N—O distance is 2.696(2) Å. The water molecule is also involved in a normal, slightly bent, hydrogen bond with the borate anion at a distance of 2.788(17) Å. The stabilization of the crystal structure arises from electrostatic interactions and is assisted by intermolecular O—H⋯O and N—H⋯O hydrogen bonds between the layers.
Acknowledgements
The authors gratefully acknowledge Kırıkkale University Scientific Research Centre for financial support of this work (grant No. 2007/49).
References
1 Miyaura, N.; Suzuki, A.: Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 95 (1995) 2457–2483.10.1021/cr00039a007Search in Google Scholar
2 Hébert, M. J. G.; Flewelling, A. J.; Clark, T. N.; Levesque, N. A.; Fran cois, J. J.; Surette, M. E.; Gray, C. A.; Vogels, C. M.; Touaibia, M.; Westcott, S. A.: Synthesis and biological activity of arylspiroborate salts derived from caffeic acid phenethyl ester. Int. J. Med. Chem. 2015 (2015) 1–9.10.1155/2015/418362Search in Google Scholar PubMed PubMed Central
3 Groziak, M. P.: Boron therapeutics on the horizon. Am. J. Ther. 8 (2001) 321–328.10.1097/00045391-200109000-00005Search in Google Scholar PubMed
4 Grice, J. D.; Burns, P. C.; Hawthorne, F. C.: Borate minerals. II. A hierarchy of structures based upon the borate fundamental building block. Can. Mineral. 37 (1999) 731–762.Search in Google Scholar
5 Knyrim, J. S.; Becker, P.; Johrendt, D.; Huppertz, H.: A new non-centrosymmetric modification of BiB3O6. Angew. Chem. Int. Ed. 45 (2006) 8239–8241.10.1002/anie.200602993Search in Google Scholar PubMed
6 Christ, C. L.; Clark, J. R.: A crystal-chemistry classification of borate structures with emphasis on hydrated borates. Phys. Chem. Mineral. 2 (1977) 59–87.10.1007/BF00307525Search in Google Scholar
7 Yu, Z. T.; Shi, Z.; Jiang, Y. S.; Yuan, H. M.; Chen, J. S.: A chiral lead borate containing infinite and finite chains built up from BO4 and BO3 units. Chem. Mater. 14 (2002) 1314–1318.10.1021/cm010387kSearch in Google Scholar
8 Beckett, M. A.: Recent advances in crystalline hydrated borates with non-metal or transition-metal complex cations. Coord. Chem. Rev. 323 (2016) 2–14.10.1016/j.ccr.2015.12.012Search in Google Scholar
9 Downard, A.; Niewenhuyzen, M.; Seddon, K. R.; Van den Berg, J. A.; Schmidt, Vaughan, J. F. S.; Biermann, U. W.: Structural Features of Lithium Organoborates. Cryst. Growth Des. 2 (2002) 111–119.10.1021/cg010035qSearch in Google Scholar
10 Kelly, R. T.; Whiting, A.; Chandrakumar, N. S.: A rationally designed, chiral lewis acid for the asymmetric induction of some Diels-Alder reactions. J. Am. Chem. Soc. 108 (1986) 3510–3512.10.1021/ja00272a058Search in Google Scholar
11 Vieira, T. O.; Green, M. J.; Alper, H.: Highly regioselective anti-markovnikov palladium-borate-catalyzed methoxycarbonylation reactions: unprecedented results for aryl olefins. Org. Lett. 8 (2006) 6143–6145.10.1021/ol062646nSearch in Google Scholar PubMed
12 Maki, T.; Ishihara, K.; Yamamoto, H.: New boron(III)-catalyzed amide and ester condensation reactions. Tetrahedron 63 (2007) 8645–8657.10.1016/j.tet.2007.03.157Search in Google Scholar
13 Errington, R. J.; Tombul, M.; Walker, G. L. P.; Clegg, W.; Heath, S. L.; Horsburgh, L. J.: Tetraphenoxoborate complexes of barium: crystal structures of the metalloborates [Ba(thf)4{B(OPh)4}2] and [Ba(dme)2{B(OPh)4}2]. Dalton Trans. (1999) 3533–3534.10.1039/a906089hSearch in Google Scholar
14 Tombul, M.; Errington, R. J.; Coxall, R. A.; Clegg, W.: An alkoxide cluster with 18 Li+ ions encapsulating two borate anions, [(tBuO)12Li18(BO3)2]. Acta Crystallogr. C59 (2003) m231–m233.10.1107/S0108270103009636Search in Google Scholar
15 Tombul, M.; Güven, K.; Büyükgüngör, O.; Aktas, H.; Durlu, T. N.: Dipotassium maleate with boric acid. Acta Crystallogr. C63 (2007) m430–m432.10.1107/S0108270107036165Search in Google Scholar PubMed
16 Tombul, M.; Güven, K.; Svoboda, I.: Dimethylammonium bis(3-oxidonaphthalene-2-carboxylato)borate hemihydrate. Acta Crystallogr. E64 (2008) o309–o310.10.1107/S1600536807066810Search in Google Scholar PubMed PubMed Central
17 Vogels, C. M.; Wescott, S. A.: Arylspiroboronate esters: From lithium batteries to wood preservatives to catalysis. Chem. Soc. Rev. 40 (2011) 1446–1458.10.1039/C0CS00023JSearch in Google Scholar
18 Mosseler, J. A.; Melanson, J. A.; Bowes, E. G.; Lee, G. M.; Vogels, C. M.; Decken, A.; Westcott, S. A.: Synthesis, characterization and antifungal studies of arylspiroborate esters derived from 4-nitrocatechol. J. Mol. Struct. 1002 (2011) 24–27.10.1016/j.molstruc.2011.06.034Search in Google Scholar
19 Geier, M. J.; Bowes, E. G.; Lee, G. M.; Li, H.; O’Neill, T.; Flewelling, A.; Vogels, C. M.; Decken, A.; Gray, C. A.; Westcott, S. A.: Synthesis and biological activities of arylspiroborates derived from 2,3-dihydroxynaphthalene. Heteroat. Chem. 2 (2013) 116–123.10.1002/hc.21072Search in Google Scholar
20 Agilent Technologies: CrysAlis Software system, version 1.171.34.40, Agilent Technologies UK Ltd, Oxford, UK, 2010.Search in Google Scholar
21 Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Search in Google Scholar PubMed
©2017 Mustafa Tombul et al., published by De Gruyter.
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Articles in the same Issue
- Cover and Frontmatter
- The crystal structure of triphenylphosphineoxide – 2,5-dichloro-3,6-dihydroxycyclohexa-2,5-diene-1,4-dione (2/1), C42H32Cl2O6P2
- Crystal structure of poly-[diaqua-[bis(μ2-hydroxy)-bis(μ4-3,4,5,6-tetrachlorophthalato-κ3O,O′:O′; κ2O′′:O′′′)dilanthanum(III)], C8H3Cl4LaO6
- Crystal structure of 1,1′-(3,4-diphenylthieno[2,3-b]thiophene-2,5-diyl)bis[1-phenyl-methanone], C32H20O2S2
- Crystal structure of 4a-hydroxy-9-(3,5-dibromo-phenyl)-3,4,4a,5,6,7,9,9a-octahydro-2H-xanthene-1,8-dione, C19H18Br2O4
- Crystal structure of 5-hydroxy-4,6,9,10-tetramethyl-1-oxo-6-vinyldecahydro-3a,9-propanocyclopenta[8]annulen-8-yl 2-((2-methyl-1-(3-methylbenzamido)propan-2-yl)thio)acetate, C34H49NO5S
- Crystal structure of pyridinium bis(naphthalane-2,3-diolato-κ2O,O′)borate monohydrate, C25H20BNO5
- Crystal structure of 1,1′-((1E,1′E)-(((ethane-1,2-diylbis(oxy))bis(2,1-phenylene))bis(azanylylidene))bis(methanylylidene))bis(naphthalen-2-olato)nickel(II), C72H52N4O8Ni2
- The crystal structure of 3-(2-acetyl-4-butyramido-phenoxy)-2-hydroxy-N-isopropylpropan-1-aminium tetraphenylborate, C42H49BN2O4
- Crystal structure of 4-bromobenzyl (Z)-N′-(adamantan-1-yl)-4-phenylpiperazine-1-carbothioimidate, C28H34BrN3S
- Crystal structure of poly-[(μ6-benzene-1,2,4,5-tetracarboxylato)-(μ2-1,2-bis(imidazol-1-ylmethyl)benzene)dicobalt(II)], Co2C24H16N4O8
- Crystal structure of catena-(bis(μ2-1, 2-bis(imidazole-1-ylmethyl)benzene-κN:N′)-dichlororido-nickel(II)), C28H28Cl2N8Ni
- Crystal structure of (Z)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-hydroxy-3-(4-methoxyphenyl)prop-2-en-1-one, C15H16N2O3
- Crystal structure of (Z)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-hydroxy-3-phenylprop-2-en-1-one, C14H14N2O2
- Crystal structure of (E)-2-(4-hydroxy-3-methoxybenzylidene)-6-methoxy-3,4-dihydronaphthalen-1(2H)-one, C19H18O4
- Crystal structure of (Z)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-(4-ethoxyphenyl)-3-hydroxyprop-2-en-1-one, C16H18N2O3
- Crystal structure of (Z)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-hydroxy-3-(p-toly)prop-2-en-1-one, C15H16N2O2
- Crystal structure of 1-acetyl-3-(3-chlorophenyl)-5-(4-isopropylphenyl)-4,5-dihydro-(1H)-pyrazole, C20H21ClN2O
- The crystal structure of 1-methyl-2,4-dinitro-5-iodoimidazole, C4H3IN4O4
- The crystal structure of 4-chloro-3,5-dinitroaniline, C6H4ClN3O4
- Crystal structure of N,N-dimethyl-N′-(2-methyl-4-oxo-5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidin-3(4H)-yl)formimidamide, C14H18N4OS
- Crystal structure of tetrakis(μ2-acetato-κ2O:O′)-bis[μ3-4-chloro-2,6-bis((methylimino)methyl)phenolato-κ2N,O:O,N′]-(μ4-oxido)tetracopper(II), C28H32Cl2Cu4N4O11
- Crystal structure of catena-poly[diaqua-bis(μ2-ethane-1,2-diyl-bis(pyridine-3-carboxylate-κ2N:N′))copper(II)] dinitrate, C28H28CuN6O16
- Synthesis and crystal structure of catena-poly[(μ2-nicotinato-κ2O,O′: κ1N)-(nitrato-κ1O)-(bis(2-benzimidazol-ylmethyl)amine-κ3N,N′,N′′)lead(II)], C22H18N7O5Pb
- The twinned crystal structure of (4SR)-7-benzyl-2,4,8,8-tetramethyl-7,8-dihydroimidazo[5,1-c][1,2,4]triazine-3,6(2H,4H)-dione, C16H20N4O2
- Crystal structure of (Z)-3-hydroxy-3-(4-methoxyphenyl)-1-(pyridin-2-yl)prop-2-en-1-one, C15H13NO3
- Crystal structure of 2-amino-4-(2,3-dichlorophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H12Cl2N2O2
- Crystal structure of catena-poly[(μ2-butane-1,4-diyl-bis(pyridine-3-carboxylato-κN))silver(I)] tetrafluoroborate, C16H16AgN2O4BF4
- Crystal structure of poly[diaqua-(1,10-phenanthroline-κ2N,N′)-(μ2-2,5-dihydroxytere-phthalato)-bis(μ4-2,5-dihydroxyterephthalato)dicerium(III)], C24H16CeN2O10
- Crystal structure of 5,7,4′-trihydroxy-3,8,3′-trymethoxyflavone, C18H16O8
- Crystal structure of N-(3,4-dichlorobenzylidene)-4-methylaniline, C14H11Cl2N
- Crystal structure of 4-(3-Methoxy-phenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylic acid ethyl ester, C22H27NO4
- Crystal structure of 2-amino-4-(3-fluorophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H13FN2O2
- Crystal structure of 1,1,(3,4-dihydroxythieno[2,3-b] thiophene-2,5-diyl)bis(2-bromoethanone), C10H6Br2O4S2
- The crystal structure of N,N′-(4,4′-oxydibenzyl)-bisisonicotinamide 3.5 hydrate, C24H24N4O6
- Crystal structure of catena-poly[hexakis(μ2-chlorido)-hexakis(4-(1H-pyrazol-5-yl)pyridine-κN)tricadmium(II)], Cd3C48H42Cl6N18
- Crystal structure of 2-(4-(dimethylamino)phenyl)-1,3-dimethyl-1H-perimidin-3-ium iodide, C21H22I1N3
- Crystal structure of 4-(1,3-dimethyl-2,3-dihydro-1H-perimidin-2-yl)benzonitrile, C20H17N3
- Crystal structure of tetrakis(μ2-acetato-κ2O:O′)-bis(2,2′-sulfonyldipyrazine-κ1N)dicopper(II), C24H24Cu2N8O12S2
- Crystal structure of 1-(4-chlorophenyl)-6,8-diphenyl-1H-pyrazolo[4,3-c]quinoline, C28H18ClN3
- Crystal structure of methyl 3-((1-(2-(methoxycarbonyl)benzyl)-1H-1,2,3-triazol-4-yl)methoxy)-2-naphthoate, C24H21N3O5
- Crystal structure of (tris(2-pyridylmethyl)amine-κ4N,N′,N′′,N′′′′)-chloranilato-κO,O′-zinc(II) – methanol (1/1), C25H22Cl2N4O5Zn
- Crystal structure of 1,1-dimethyl-3-(4-methoxyphenyl)urea, C10H14N2O2
- Crystal structure of 4a-Hydroxy-9-(2-nitro-phenyl)-3,4,4a,5,6,7,9,9a-octahydro-2H-xanthene-1,8-dione, C19H19NO6
- Crystal structure of chlorido-(η6–1-isopropyl-4-methyl benzene)-(1-(pyridin-2-yl)-N-(p-tolyl)methanimine-κ2N,N′)ruthenium(II) hexafluorophosphate(V), C23H26ClF6N2PRu
- Crystal structure of phenyl(2-phenyl-2,3-dihydro-1H-perimidin-2-yl)methanone, C24H18N2O
- Crystal structure of (E)-3-methyl-4-((3-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)methylene)-1-phenyl-1H-pyrazol-5(4H)-one, C29H23N7O
- Crystal structure of 2-(4-(2-butyl-1,3-dioxo-2,3-dihydro-1H-benzo[de]isoquinolin-6-yl)piperazin-1-yl)-2-oxoethyldiethylcarbamodithioate, C27H34N4O3S2
- Crystal structure of poly-[diaqua-bis(μ-4,4′-bipyridine-κ2N:N′)cobalt(II)] bis(4-chlorobenzenesulfonate) – 4,4′-bipyridine – water (1/1/2), C42H40Cl2CoN6O10S2
- Crystal structure of (η6-benzene)-(N-(2,6-dimethylphenyl)-1-(pyridin-2-yl)methanimine-κ2N,N′)ruthenium(II) perchlorate monohydrate, C20H20Cl2N2O5Ru
- Crystal structure of 4,10,16,22-tetrahydroxy-6,12,18,24-tetramethoxy-2,8,14,20-tetraethylphenylresorcin[4]arene – ethyl acetate (1/1), C68H72O10
- Crystal structure of chlorido-(N-(2,5-dichlorophenyl)-1-(pyridin-2-yl)methanimine-κ2N,N′)(η6-1-isopropyl-4-methyl benzene) ruthenium (II) tetrafluoroborate, C22H22Cl3N2BF4Ru
- Crystal structure of 3-(5-methyl-1-p-tolyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazole-4-carbaldehyde, a rare Z′ = 3 structure, C20H17N5O
- Crystal structure of 5-(5-(4-chlorophenyl)-1-phenyl-1H-pyrazol-3-yl)-N-phenyl-1,3,4-thiadiazol-2-amine, C23H16ClN5S
- Crystal structure of 7-hydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one-N,N-dimethylformamide (1/1), C18H17NO5
- Crystal structure of halogen-bonded 2-chloro-1,10-phenanthroline—1,4-diiodotetrafluorobenzene (2/1), C30H14Cl2F4I2N4
- Crystal structure of 1-(4,4-dimethyl-2,6-dithioxo-1,3,5-triazinan-1-yl)-3-(diethylaminocarbonyl)thiourea, C11H20N6OS3
- Crystal structure of methyl 1-(4-fluorobenzyl)-3-phenyl-1H-pyrazole-5-carboxylate, C18H15FN2O2
- Crystal structure of 1,1-dimethyl-3-(4-methylphenyl)urea, C10H14N2O
- Crystal structure of yttrium gallium antimonide, Y5Ga1.24Sb2.77
- Crystal structure of 2-(bis(4-methoxyphenyl)amino)-2-oxoacetic acid, C16H15NO5
Articles in the same Issue
- Cover and Frontmatter
- The crystal structure of triphenylphosphineoxide – 2,5-dichloro-3,6-dihydroxycyclohexa-2,5-diene-1,4-dione (2/1), C42H32Cl2O6P2
- Crystal structure of poly-[diaqua-[bis(μ2-hydroxy)-bis(μ4-3,4,5,6-tetrachlorophthalato-κ3O,O′:O′; κ2O′′:O′′′)dilanthanum(III)], C8H3Cl4LaO6
- Crystal structure of 1,1′-(3,4-diphenylthieno[2,3-b]thiophene-2,5-diyl)bis[1-phenyl-methanone], C32H20O2S2
- Crystal structure of 4a-hydroxy-9-(3,5-dibromo-phenyl)-3,4,4a,5,6,7,9,9a-octahydro-2H-xanthene-1,8-dione, C19H18Br2O4
- Crystal structure of 5-hydroxy-4,6,9,10-tetramethyl-1-oxo-6-vinyldecahydro-3a,9-propanocyclopenta[8]annulen-8-yl 2-((2-methyl-1-(3-methylbenzamido)propan-2-yl)thio)acetate, C34H49NO5S
- Crystal structure of pyridinium bis(naphthalane-2,3-diolato-κ2O,O′)borate monohydrate, C25H20BNO5
- Crystal structure of 1,1′-((1E,1′E)-(((ethane-1,2-diylbis(oxy))bis(2,1-phenylene))bis(azanylylidene))bis(methanylylidene))bis(naphthalen-2-olato)nickel(II), C72H52N4O8Ni2
- The crystal structure of 3-(2-acetyl-4-butyramido-phenoxy)-2-hydroxy-N-isopropylpropan-1-aminium tetraphenylborate, C42H49BN2O4
- Crystal structure of 4-bromobenzyl (Z)-N′-(adamantan-1-yl)-4-phenylpiperazine-1-carbothioimidate, C28H34BrN3S
- Crystal structure of poly-[(μ6-benzene-1,2,4,5-tetracarboxylato)-(μ2-1,2-bis(imidazol-1-ylmethyl)benzene)dicobalt(II)], Co2C24H16N4O8
- Crystal structure of catena-(bis(μ2-1, 2-bis(imidazole-1-ylmethyl)benzene-κN:N′)-dichlororido-nickel(II)), C28H28Cl2N8Ni
- Crystal structure of (Z)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-hydroxy-3-(4-methoxyphenyl)prop-2-en-1-one, C15H16N2O3
- Crystal structure of (Z)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-hydroxy-3-phenylprop-2-en-1-one, C14H14N2O2
- Crystal structure of (E)-2-(4-hydroxy-3-methoxybenzylidene)-6-methoxy-3,4-dihydronaphthalen-1(2H)-one, C19H18O4
- Crystal structure of (Z)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-(4-ethoxyphenyl)-3-hydroxyprop-2-en-1-one, C16H18N2O3
- Crystal structure of (Z)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-hydroxy-3-(p-toly)prop-2-en-1-one, C15H16N2O2
- Crystal structure of 1-acetyl-3-(3-chlorophenyl)-5-(4-isopropylphenyl)-4,5-dihydro-(1H)-pyrazole, C20H21ClN2O
- The crystal structure of 1-methyl-2,4-dinitro-5-iodoimidazole, C4H3IN4O4
- The crystal structure of 4-chloro-3,5-dinitroaniline, C6H4ClN3O4
- Crystal structure of N,N-dimethyl-N′-(2-methyl-4-oxo-5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidin-3(4H)-yl)formimidamide, C14H18N4OS
- Crystal structure of tetrakis(μ2-acetato-κ2O:O′)-bis[μ3-4-chloro-2,6-bis((methylimino)methyl)phenolato-κ2N,O:O,N′]-(μ4-oxido)tetracopper(II), C28H32Cl2Cu4N4O11
- Crystal structure of catena-poly[diaqua-bis(μ2-ethane-1,2-diyl-bis(pyridine-3-carboxylate-κ2N:N′))copper(II)] dinitrate, C28H28CuN6O16
- Synthesis and crystal structure of catena-poly[(μ2-nicotinato-κ2O,O′: κ1N)-(nitrato-κ1O)-(bis(2-benzimidazol-ylmethyl)amine-κ3N,N′,N′′)lead(II)], C22H18N7O5Pb
- The twinned crystal structure of (4SR)-7-benzyl-2,4,8,8-tetramethyl-7,8-dihydroimidazo[5,1-c][1,2,4]triazine-3,6(2H,4H)-dione, C16H20N4O2
- Crystal structure of (Z)-3-hydroxy-3-(4-methoxyphenyl)-1-(pyridin-2-yl)prop-2-en-1-one, C15H13NO3
- Crystal structure of 2-amino-4-(2,3-dichlorophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H12Cl2N2O2
- Crystal structure of catena-poly[(μ2-butane-1,4-diyl-bis(pyridine-3-carboxylato-κN))silver(I)] tetrafluoroborate, C16H16AgN2O4BF4
- Crystal structure of poly[diaqua-(1,10-phenanthroline-κ2N,N′)-(μ2-2,5-dihydroxytere-phthalato)-bis(μ4-2,5-dihydroxyterephthalato)dicerium(III)], C24H16CeN2O10
- Crystal structure of 5,7,4′-trihydroxy-3,8,3′-trymethoxyflavone, C18H16O8
- Crystal structure of N-(3,4-dichlorobenzylidene)-4-methylaniline, C14H11Cl2N
- Crystal structure of 4-(3-Methoxy-phenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylic acid ethyl ester, C22H27NO4
- Crystal structure of 2-amino-4-(3-fluorophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H13FN2O2
- Crystal structure of 1,1,(3,4-dihydroxythieno[2,3-b] thiophene-2,5-diyl)bis(2-bromoethanone), C10H6Br2O4S2
- The crystal structure of N,N′-(4,4′-oxydibenzyl)-bisisonicotinamide 3.5 hydrate, C24H24N4O6
- Crystal structure of catena-poly[hexakis(μ2-chlorido)-hexakis(4-(1H-pyrazol-5-yl)pyridine-κN)tricadmium(II)], Cd3C48H42Cl6N18
- Crystal structure of 2-(4-(dimethylamino)phenyl)-1,3-dimethyl-1H-perimidin-3-ium iodide, C21H22I1N3
- Crystal structure of 4-(1,3-dimethyl-2,3-dihydro-1H-perimidin-2-yl)benzonitrile, C20H17N3
- Crystal structure of tetrakis(μ2-acetato-κ2O:O′)-bis(2,2′-sulfonyldipyrazine-κ1N)dicopper(II), C24H24Cu2N8O12S2
- Crystal structure of 1-(4-chlorophenyl)-6,8-diphenyl-1H-pyrazolo[4,3-c]quinoline, C28H18ClN3
- Crystal structure of methyl 3-((1-(2-(methoxycarbonyl)benzyl)-1H-1,2,3-triazol-4-yl)methoxy)-2-naphthoate, C24H21N3O5
- Crystal structure of (tris(2-pyridylmethyl)amine-κ4N,N′,N′′,N′′′′)-chloranilato-κO,O′-zinc(II) – methanol (1/1), C25H22Cl2N4O5Zn
- Crystal structure of 1,1-dimethyl-3-(4-methoxyphenyl)urea, C10H14N2O2
- Crystal structure of 4a-Hydroxy-9-(2-nitro-phenyl)-3,4,4a,5,6,7,9,9a-octahydro-2H-xanthene-1,8-dione, C19H19NO6
- Crystal structure of chlorido-(η6–1-isopropyl-4-methyl benzene)-(1-(pyridin-2-yl)-N-(p-tolyl)methanimine-κ2N,N′)ruthenium(II) hexafluorophosphate(V), C23H26ClF6N2PRu
- Crystal structure of phenyl(2-phenyl-2,3-dihydro-1H-perimidin-2-yl)methanone, C24H18N2O
- Crystal structure of (E)-3-methyl-4-((3-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)methylene)-1-phenyl-1H-pyrazol-5(4H)-one, C29H23N7O
- Crystal structure of 2-(4-(2-butyl-1,3-dioxo-2,3-dihydro-1H-benzo[de]isoquinolin-6-yl)piperazin-1-yl)-2-oxoethyldiethylcarbamodithioate, C27H34N4O3S2
- Crystal structure of poly-[diaqua-bis(μ-4,4′-bipyridine-κ2N:N′)cobalt(II)] bis(4-chlorobenzenesulfonate) – 4,4′-bipyridine – water (1/1/2), C42H40Cl2CoN6O10S2
- Crystal structure of (η6-benzene)-(N-(2,6-dimethylphenyl)-1-(pyridin-2-yl)methanimine-κ2N,N′)ruthenium(II) perchlorate monohydrate, C20H20Cl2N2O5Ru
- Crystal structure of 4,10,16,22-tetrahydroxy-6,12,18,24-tetramethoxy-2,8,14,20-tetraethylphenylresorcin[4]arene – ethyl acetate (1/1), C68H72O10
- Crystal structure of chlorido-(N-(2,5-dichlorophenyl)-1-(pyridin-2-yl)methanimine-κ2N,N′)(η6-1-isopropyl-4-methyl benzene) ruthenium (II) tetrafluoroborate, C22H22Cl3N2BF4Ru
- Crystal structure of 3-(5-methyl-1-p-tolyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazole-4-carbaldehyde, a rare Z′ = 3 structure, C20H17N5O
- Crystal structure of 5-(5-(4-chlorophenyl)-1-phenyl-1H-pyrazol-3-yl)-N-phenyl-1,3,4-thiadiazol-2-amine, C23H16ClN5S
- Crystal structure of 7-hydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one-N,N-dimethylformamide (1/1), C18H17NO5
- Crystal structure of halogen-bonded 2-chloro-1,10-phenanthroline—1,4-diiodotetrafluorobenzene (2/1), C30H14Cl2F4I2N4
- Crystal structure of 1-(4,4-dimethyl-2,6-dithioxo-1,3,5-triazinan-1-yl)-3-(diethylaminocarbonyl)thiourea, C11H20N6OS3
- Crystal structure of methyl 1-(4-fluorobenzyl)-3-phenyl-1H-pyrazole-5-carboxylate, C18H15FN2O2
- Crystal structure of 1,1-dimethyl-3-(4-methylphenyl)urea, C10H14N2O
- Crystal structure of yttrium gallium antimonide, Y5Ga1.24Sb2.77
- Crystal structure of 2-(bis(4-methoxyphenyl)amino)-2-oxoacetic acid, C16H15NO5

