Abstract
A novel method for CO2 injection direct smelting vanadium steel (CIDSVS) is proposed. Achieving selective oxidation of phosphorus is essential for the applicability of the suggested process. Under the guidance of thermodynamics, the mechanisms of CO2 injection dephosphorization and vanadium retention were investigated with CO2 flow rate and dephosphorization slag composition as experimental variables. The results indicate that CO2 as an oxygen source can remove 73.8% of phosphorus, while the oxidation rate of vanadium is 17.5%. The dephosphorization process can be divided into two stages: FeO- and CO2-dominated experimental processes. In the initial stage of slag feeding, [V] and [P] undergo fast oxidation, and the oxidation amount is positively correlated with the initial FeO content. The high basicity (CaO/SiO2 ratio) reduces the activity of V2O3 in the slag and promotes the oxidation of [V]. Under the experimental conditions of 1,400°C, the optimal conditions were determined to be a CO2 flow rate of 1.5 mL·g−1·min−1, a FeO content of 40%, and a basicity B of 2.5. Following the CIDSVS steelmaking operation, 80% of the vanadium is retained, and the impurity elements fulfill the specifications for steel. This method enhances vanadium utilization and is environmentally friendly.
1 Introduction
Vanadium steels are widely used in high-rise buildings, oil and gas pipelines, and large-span structures. Worldwide, vanadium consumption in the steel-making industry accounts for 92.9% of its total production [1]. In recent decades, urban construction and manufacturing development have created a huge demand for vanadium steel. There is an immediate need to develop technology for the clean and efficient use of vanadium in the steelmaking process.
Vanadium-titanium magnetite is the main vanadium-bearing resource, contributing over 88% of vanadium production [2,3]. Generally, the content of vanadium in the ore is less than 1%. To recover as much vanadium as possible, the ore is usually used as the feed of a blast furnace, where vanadium is co-reduced to molten metal. Then, oxygen is injected into the converter to obtain vanadium slag and 80–90% of V will be enriched in the vanadium slag to meet the needs of the vanadium extraction plant [4,5,6,7].
Roasting of vanadium slag followed by leaching produces V2O5 products [8,9,10,11,12,13]. The technique has been extensively investigated and provides a high vanadium recovery rate (85–90%). However, the roasting process requires a great deal of energy or generates toxic gases such as Cl2, SO2, and HCl. Significant quantities of hazardous wastewater and residues are produced by the leaching process [14], including ammonia-containing wastewater, Cr-containing wastewater, and hard-to-recover tailings. According to incomplete statistics, each ton of V2O5 production will produce 30–50 tons of ammonia-containing wastewater (Na2SO3, (NH4)2SO4, and NH4Cl) and 2–7 tons of difficult-to-recycle residues [8,15]. Aluminum and iron particulates reduce V2O5 at 1,800°C in an arc furnace to produce the V–Fe alloy [16]. The procedure has a high energy consumption, and the waste residue contains 0.35% V2O5, which is a potentially dangerous solid waste.
When V–Fe is put into the refining furnace as an alloy additive, 10% or more of vanadium is lost in the form of oxidation or volatilization [17]. In recent years, severe rules on high-strength steel have boosted demand for vanadium as a steel addition. Vanadium usage surged by 67.8% from 2011 to 2021, according to the International Vanadium Technical Committee (Vanitec), and Lee expects consumption to reach 130.1 kilotons by 2024 [1]. The high demand for vanadium will further drive the vanadium-producing industry to pollute the environment.
After converter oxidation, vanadium in vanadium-containing molten iron is enriched in vanadium slag. V–Fe alloy is prepared by the reduction of V2O5 obtained by roasting, leaching, and deposition of vanadium slag. Finally, in the form of V–Fe, vanadium participates in the alloying of steel. The utilization rate of vanadium decreases by degrees with the increase in the vanadium steel production process. About 40–50% of vanadium resources in vanadium-bearing hot metal are lost in the residue and effluent [17]. In order to be environmentally friendly and improve the utilization efficiency of vanadium, the authors proposed a CO2 injection direct smelting vanadium steel (CIDSVS) method. The vanadium-bearing hot metal is not used as the raw material for vanadium slag extraction; instead, the weak oxidizing gas CO2 is used to carry out the impurity removal and steelmaking operations directly. The process flowchart is depicted in Figure 1. The CIDSVS method reduces vanadium extraction and subsequent production steps. The proposed approach can decrease the vanadium loss caused by frequent vanadium transfer during the manufacture of vanadium steel, reduce energy consumption and hazardous waste emissions, and reduce the environmental impact of vanadium steel manufacturing.
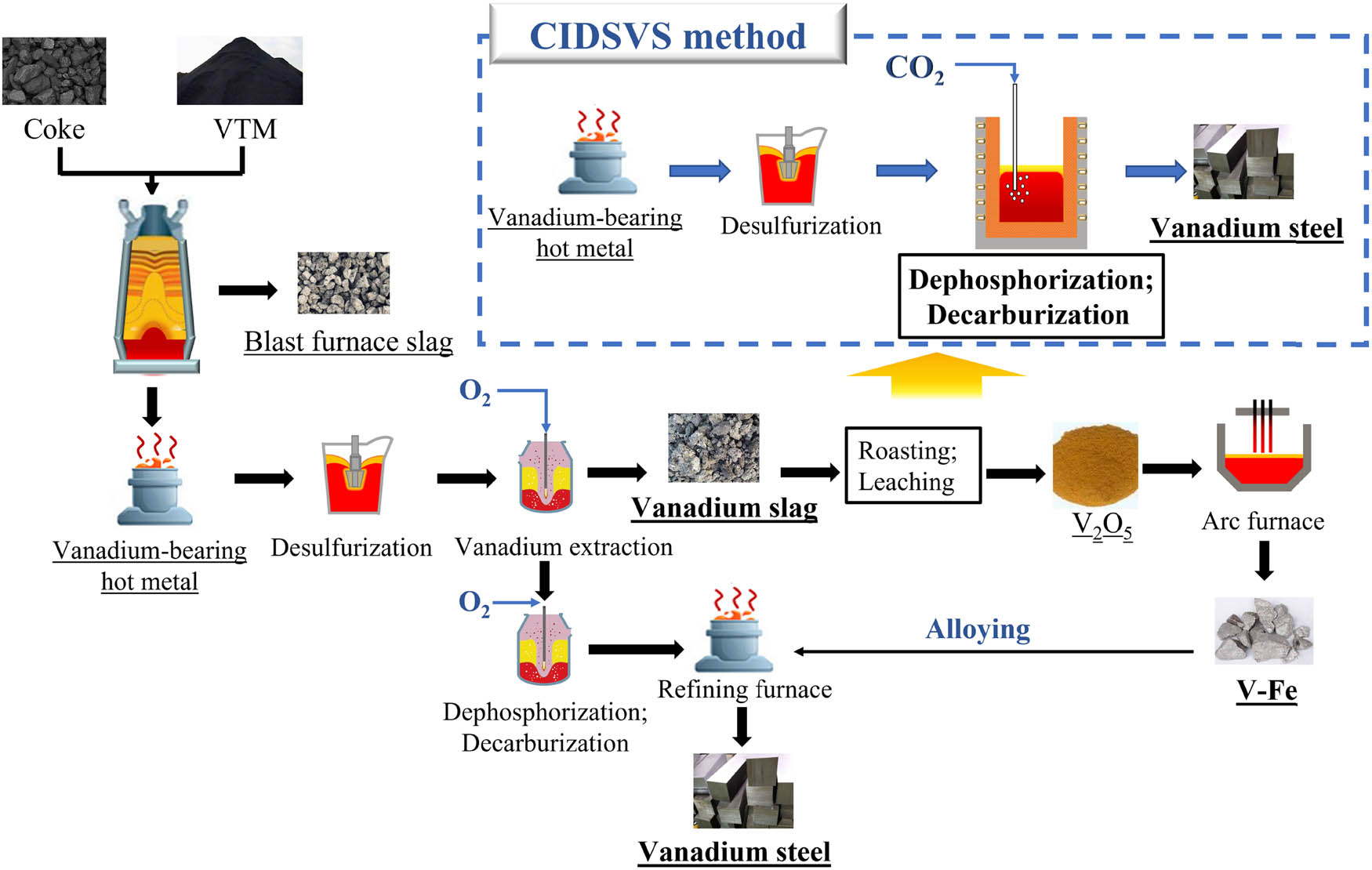
Traditional smelting process and the CIDSVS method flow chart of vanadium steel.
Phosphorus is the main harmful element in steel, which precipitates at the crystal boundary and reduces the impact resistance of steel. In general, [P] ≤ 0.045% is the limit for alloy steel (excluding low-phosphorus steel). Steel smelting has a long history, and the oxygen top-blown converter dephosphorization process is mature. The double-converter dephosphorization process can even produce [P] ≤ 0.01% of ultra-low phosphorus steel. However, selective oxidation of [V] and [P] cannot be achieved with traditional O2 steelmaking. CO2 is a weak oxidizing gas and is regarded as one of the culprits of the global greenhouse effect. As technology advances, CO2 is increasingly incorporated into the steelmaking process as an auxiliary gas [18,19]. The Boudouard reaction CO2(g) + [C] = 2CO(g) improves the gas stirring ability and has a good effect on suppressing the rapid increase in the furnace temperature [20,21,22]. Consequently, some investigations [23,24,25] have achieved effective phosphorus removal by injecting CO2–O2 mixed gas during dephosphorization.
The most important step in the CIDSVS technique is the selective oxidation of [V] and [P] in molten iron. This work focuses solely on the CO2 dephosphorization process in the CIDSVS method. This is a crucial prerequisite for applying the proposed method. Using the unique chemical properties of CO2, the material flow of vanadium and phosphorus is altered, leaving vanadium in the metal phase while meeting the steel’s impurity requirements. As the first investigation of short-process smelting of vanadium-containing hot metal, this study aims to employ CO2 and alter slag composition to dephosphorization and retain vanadium. At the same time, the effects of varying CO2 flux, slag FeO content, and basicity on the slag–metal distribution ratio of V and P were examined. According to the findings of the research, the process conditions for CO2 injection dephosphorization for the CIDSVS method were obtained.
2 Materials and methods
2.1 Materials
The pig iron used in the experiment is a pre-desulfurized hot metal produced by Panzhihua Iron and Steel Co., Ltd. Table 1 lists the principal components. Dephosphorization slag is a CaO–SiO2–FeO ternary slag system prepared by uniformly mixing chemical reagents according to the target components listed in Table 2. Ferrous oxalate (FeC2O4) was put into an Ar-filled resistance furnace at 800°C for 2 h to obtain FeO. The CO2 utilized in the experiment has a purity of 99.999%, and the injection rate is detailed in Table 2. The thermodynamic verification experiment findings in Section 3.1 show that the dephosphorization results of the single slag method with an 8% slag quantity cannot match the steel impurity standards; therefore, in the experiments, we adopt a slag–metal mass ratio of 12%. The dephosphorization slag basicity is calculated by binary basicity (B), as shown in the following equation:
Initial composition of hot metals (mass%)
| Element | [C] | [V] | [P] | [Si] | [Mn] | [Ti] | [S] | [Fe] |
|---|---|---|---|---|---|---|---|---|
| Mass% | 4.42 | 0.31 | 0.11 | 0.17 | 0.05 | 0.12 | 0.013 | 94.62 |
Dephosphorization slag composition (mass%) and varying CO2 injection rates
| No. | CaO | SiO2 | FeO | B | CO2 (mL·g−1·min−1) |
|---|---|---|---|---|---|
| #1 | 40 | 20 | 40 | 2.0 | 1.0 |
| #2 | 40 | 20 | 40 | 2.0 | 1.5 |
| #3 | 40 | 20 | 40 | 2.0 | 2.5 |
| #4 | 40 | 20 | 40 | 2.0 | 4.0 |
| #5 | 53.33 | 26.67 | 20 | 2.0 | 1.5 |
| #6 | 46.67 | 23.33 | 30 | 2.0 | 1.5 |
| #7 | 33.33 | 16.67 | 50 | 2.0 | 1.5 |
| #8 | 42.86 | 17.14 | 40 | 2.5 | 1.5 |
| #9 | 45 | 15 | 40 | 3.0 | 1.5 |
| #10 | 46.67 | 13.33 | 40 | 3.5 | 1.5 |
| #11 | 48 | 12 | 40 | 4.0 | 1.5 |
The removal ratio η i of element i in the hot metal is calculated by equation (2). [i]0 and [i]e represent the mass percent of element i in the hot metal at the initial point and the endpoint of dephosphorization stage, respectively:
2.2 Experimental apparatus and procedures
A graphite crucible containing a 200 g pig iron sample was placed in a vertical resistance furnace heated with the Mo–Si material. The experimental device is shown in Figure 2. The temperature was monitored by a B-type thermocouple, and the sample temperature was kept at 1,400°C for 30 min to guarantee sample consistency. Then, the uniformly mixed dephosphorization slag was put into the molten pool by a two-stage slag feeding method. In the first stage, 8% metal mass dephosphorization slag was fed into the molten pool, and, in the second stage, 4% metal mass dephosphorization slag was delivered. The reaction time of each stage was 15 min. The dephosphorization slag melts within 10–30 s, and then a corundum injection lance is inserted 10 mm below the liquid metal level to inject CO2 gas into the molten pool. To prevent unwanted oxidation, Ar gas (2 L·min−1) was supplied during heating and experimentation.

Diagram of the experimental apparatus.
2.3 Analysis methods
From the moment CO2 is injected into the molten bath, a sample (1–2 g) is taken out with a quartz tube (OD 6 mm, ID 4 mm) every 180 s and quenched with water. The samples were dried and acid-solubilized for chemical composition analysis and the quantitative determination of [V] and [P] in metals using inductively coupled plasma optical emission spectrometry (ICP-OES; Prodigy Plus, USA). At the end of the experiment, the crucible was removed and placed in an air-isolated container to cool at room temperature, and the final slag was taken for analysis. An X-ray fluorescence analyzer (XRF, ZSX Primus IV; Rigaku Corporation, Tokyo, Japan) was used to qualitatively analyze the sample’s chemical composition. The crystalline phase of samples was identified by an X-ray diffraction analyzer (XRD, D8 Advance, Bruker, Germany). XRD data were collected using a Cu-Kα radiation source in the range of 2θ = 10–90° with a scanning step of 0.04°·s−1. The obtained data were compared with the Joint Committee on Powder Diffraction Standards International Center for Diffraction Data (JXPDS-ICDD) database to determine the crystal composition. The microstructure and chemical composition of the dephosphorization slag were observed with a scanning electron microscope (SEM, TESCAN MIRA LMS, Czech) and energy dispersive spectrometer (EDS, Xplore 30, UK) after cold inlay polishing.
3 Results and discussion
3.1 The mechanism for CO2 dephosphorization
It is well known that dephosphorization requires low temperature, high oxidation, high basicity, and high fluidity. CO2 is a weak oxidant, and its effect on dephosphorization is rarely reported. The Gibbs free energy change ΔG θ of the reaction of CO2, FeO, and O2 with [V] and [P] was calculated with FactSage 8.1. For comparison purposes, 1 mol of O atoms is provided as a benchmark. The equation for the reaction is shown in Table 3, where ΔG θ is the standard Gibbs free energy change (J·mol−1), and T is the temperature (K).
Chemical reactions for the dephosphorization and vanadium oxidization and their corresponding Gibbs free energy calculated with FactSage 8.1 software
| No. | Reactions | ΔG θ |
|---|---|---|
| (3) | 2/5[P] + CO2(g) + 3/5CaO(s) = 1/5 (3CaO·P2O5)(s) + CO(g) | −148,406 + 1.67T |
| (4) | 2/5[P] + (FeO) + 3/5CaO(s) = 1/5 (3CaO·P2O5)(s) + Fe(l) | −184,101 + 35.05T |
| (5) | 2/5[P] + 1/2O2(g) + 3/5CaO(s) = 1/5 (3CaO·P2O5)(s) | −365,547 + 63.14T |
| (6) | 2/3[V] + CO2(g) = 1/3(V2O3) + CO(g) | −86445 − 21.12T |
| (7) | 2/3[V] + (FeO) = 1/3(V2O3) + Fe(l) | −122,140 + 12.27T |
| (8) | 2/3[V] + 1/2O2(g) = 1/3(V2O3) | −427,507 + 85.92T |
| (9) | 2/5[P] + 3/5(CaO)(s) + 1/3(V2O3) = 1/5(3CaO·P2O5)(s) + 2/3[V] | −61,961 + 22.79T |
As shown in Figure 3, ΔG θ values of dephosphorization and vanadium extraction reactions are negative in the temperature range of 1,523–1,973 K. The ΔG θ of the dephosphorization reaction is smaller than that of the vanadium oxidation reaction, which is independent of the oxygen source. The order of oxidizing power is O2 > CO2 > FeO. The dephosphorization and vanadium retention reaction in a CO2 atmosphere were obtained by combining reactions (3) and (6), as expressed in reaction (9).

ΔG θ –T diagrams for oxidation reactions under different oxygen sources.
The molten iron can be regarded as a dilute solution, and the hypothetical mass of 1% is selected as the standard state. The Gibbs free energy change (ΔG) in reaction (9) can be expressed as
where R is the gas constant (= 8.314 J·mol−1·K−1); f
V and f
P are the activity coefficients of V and P, respectively; [%V] and [%P] are the mass percentages of V and P, respectively, and
Furthermore, based on the Gibbs free energy minimization principle, the thermodynamic equilibrium phase concentration changes of 100 g vanadium-containing pig iron (Table 1) and 4.8 g CaO–2.4 g SiO2–0.8 g FeO–0.44 g CO2 at different temperatures at one atmospheric pressure were calculated, as shown in Figure 4.

The theoretical content of different components in an equilibrium system at different temperatures.
The dephosphorization process is highly dependent on low temperatures, and Figure 4 clearly shows that the equilibrium [P] content increases with temperature. The content of [V] in the hot metal remained relatively constant with the temperature change. Phosphorus was mainly fixed in the 2CaO·SiO2–3CaO·P2O5 (C2S-C3P) solid solution phase and reached a maximum content at 1,400°C. This is consistent with the conclusion of Sun et al.’s 180-ton industrial dephosphorization experiment [28], and the C2S-C3P enrichment is the largest at 1,400°C.’s The industrial experiment of Tian et al. involving the dephosphorization of a 210-ton converter demonstrates that the dephosphorization rate diminishes with increasing temperature between 1,327 and 1,427°C [29]. Zhou et al. found that with the increase in the dephosphorization temperature, dephosphorization increased first and then decreased; the optimum dephosphorization temperature was 1,400°C [30].
Further, to verify that CO2 does provide assistance in the dephosphorization process, slag + CO2, slag + N2, and slag-only experiments were carried out. As shown in Figure 5, [V] and [P] decrease first and then increase under the conditions of slag + N2 and slag only. This is due to the strong reducing ability of the [C]-rich liquid iron and, as the reaction proceeds, the FeO activity at the interface decreases continuously and V2O3 and P2O5 are reduced into the iron water by [C]. Under the condition of slag + CO2, no phosphorus reversion was observed, and [P] continued to decrease. Compared with the injection of N2, the dephosphorization rate increased from 6.7 to 36.7%. After proving that CO2 can carry out dephosphorization and vanadium retention operations, the author investigated the CO2 injection flow rate, slag FeO content, and basicity to expand the difference in oxidation behaviors of the two elements. The composition of the final slag and the contents of [V] and [P] in metals under different experimental conditions are shown in Table 4.
![Figure 5
The content of [V] and [P] under experimental conditions (200 g of pig iron, 7.68 g CaO–1.92 g SiO2–6.4 g FeO slag, CO2-4 mL·g−1·min−1 and N2-4 mL·g−1·min−1).](/document/doi/10.1515/htmp-2022-0281/asset/graphic/j_htmp-2022-0281_fig_005.jpg)
The content of [V] and [P] under experimental conditions (200 g of pig iron, 7.68 g CaO–1.92 g SiO2–6.4 g FeO slag, CO2-4 mL·g−1·min−1 and N2-4 mL·g−1·min−1).
Chemical compositions of slag and content of [V] and [P] in the metal at the end of the experiment
| No. | Chemical compositions of dephosphorization slags (mass%) | Hot metal (mass%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Al2O3 a) | SiO2 | P2O5 | CaO | TiO2 | V2O3 | MnO | SO2 | FeO | [V] | [P] | |
| #1 | 3.11 | 21.83 | 1.41 | 56.61 | 0.47 | 0.80 | 0.08 | 0.188 | 10.64 | 0.271 | 0.062 |
| #2 | 3.72 | 21.47 | 2.19 | 58.89 | 0.50 | 0.82 | 0.08 | 0.206 | 11.47 | 0.261 | 0.031 |
| #3 | 3.03 | 20.44 | 1.66 | 53.70 | 0.54 | 1.12 | 0.08 | 0.190 | 16.40 | 0.257 | 0.023 |
| #4 | 3.20 | 20.65 | 1.32 | 50.25 | 0.51 | 1.84 | 0.14 | 0.168 | 21.09 | 0.239 | 0.022 |
| #5 | 3.53 | 23.34 | 1.25 | 62.96 | 0.34 | 0.64 | 0.07 | 0.266 | 7.19 | 0.270 | 0.045 |
| #6 | 3.56 | 21.73 | 1.82 | 62.41 | 0.34 | 0.66 | 0.10 | 0.258 | 8.68 | 0.265 | 0.038 |
| #7 | 3.34 | 22.63 | 1.41 | 57.34 | 0.49 | 0.95 | 0.07 | 0.172 | 12.77 | 0.222 | 0.029 |
| #8 | 2.10 | 21.90 | 2.53 | 59.50 | 0.60 | 0.91 | 0.09 | 0.220 | 12.30 | 0.256 | 0.029 |
| #9 | 3.03 | 19.48 | 2.62 | 61.90 | 0.74 | 1.10 | 0.11 | 0.206 | 10.99 | 0.248 | 0.026 |
| #10 | 4.13 | 16.29 | 2.68 | 62.30 | 0.51 | 1.14 | 0.10 | 0.228 | 12.80 | 0.237 | 0.026 |
| #11 | 4.10 | 15.50 | 2.47 | 66.10 | 0.63 | 1.40 | 0.10 | 0.254 | 9.56 | 0.231 | 0.026 |
a)Al2O3 is sourced from the alumina lance.
3.2 Reaction behaviors of [V] and [P] for varying CO2 flow rates
Figure 6(a) depicts the effects of varying CO2 injection rates on [V] and [P]. The oxidation rate of [V] increased with the CO2 flow rate, and the oxidation amount of vanadium at the endpoint increased from 12.6 to 22.9%. When the CO2 flow rate exceeds 1.0 mL·g−1·min−1, the changing pattern of [P] tends to be the same, which indicates that the dephosphorization process is not controlled by the CO2 flow rate at this time.
![Figure 6
(a) The effect of varying CO2 injection rates on [V] and [P] and (b) removal rate of [P] and [V].](/document/doi/10.1515/htmp-2022-0281/asset/graphic/j_htmp-2022-0281_fig_006.jpg)
(a) The effect of varying CO2 injection rates on [V] and [P] and (b) removal rate of [P] and [V].
The thermodynamic feasibility of CO2 dephosphorization is proved in Section 3.1. However, the realistic course of the reaction is influenced by kinetic factors. At high temperatures, the mass transfer rate is lower than the chemical reaction rate [6]. The controlling step of the reaction process is the mass transfer of components (including the reactants and the oxidation products) in the hot metal and slag. At the interface of the blowing chamber, CO2 is adsorbed to the liquid metal surface and reacts. The total interfacial reaction can occur in the following steps:
Equation (14), the dissociation reaction of CO2, is considered to be the limiting step in the whole process. As illustrated in equation (17), Mannion and Fruehan apply the theories of absolute rate and microscopic reversibility to calculate the CO2 dissociation rate [31]:
where r is the rate of dissociation in mol·cm−2·s−1, n is the number of moles CO2 per unit area, K p represents the forward rate constant for the CO2 reaction, and θ i is the proportion of vacancy covered by species i. The experimental temperature is constant at 1,400°C, K p is a constant, and the liquid–metal composition is uniform, so the dissociation rate r of CO2 is a constant. However, the gas–liquid contact area increases with the CO2 flow, resulting in an increase in the [O] production per unit of time. This increases the equilibrium constant in equation (15). The oxidation amount of [V] increased.
Mukawa and Mizukami studied the effects of stirring energy and the oxygen supply rate on the dephosphorization rate of molten iron [32]. The results showed that the dephosphorization rate was always controlled by the slag–metal interfacial mass transfer process in both molten iron and slag stages. In the present study, the lower CO2 flow rate (1.0 mL·g−1·min−1) provides limited [O] for the molten pool and cannot further promote the dephosphorization reaction. Moreover, the low-speed gas flow fails to provide good mass transfer conditions for the molten pool, so the dephosphorization rate is low. The experimental results show that the CO2 flow rate at 1.5 and 2.5 mL·g−1·min−1 provides better dephosphorization ability and kinetic conditions, and the dephosphorization rate can reach 71.8 and 79.1% (Figure 6(b)).
3.3 Effect of FeO content in slag on [V] and [P] in the hot metal
In order to more intuitively see the oxidation law of V and P by the change in FeO content in the initial slag, the [V] and [P] contents and time were plotted. As seen in Figure 7(a), the contents of [V] and [P] in the molten metal decreased rapidly within the initial 6 min following the addition of the dephosphorization slag, and the transition value increased with the increase of FeO content in the slag. With the increase of FeO content in the slag from 20 to 50%, the endpoint [P] decreased from 0.045 to 0.029% and the dephosphorization rate increased by 24.5%. The oxidation rate of [V] is the most prominent at 50% FeO, which is 28.4%. This demonstrates that an excessive increase in FeO promotes [V] oxidation, which is detrimental to vanadium retention operations.
![Figure 7
(a) Variation of [V] and [P] with time under different FeO contents and (b) viscosity and [V] and [P] content transition values.](/document/doi/10.1515/htmp-2022-0281/asset/graphic/j_htmp-2022-0281_fig_007.jpg)
(a) Variation of [V] and [P] with time under different FeO contents and (b) viscosity and [V] and [P] content transition values.
Figure 7(b) shows the theoretical viscosity of the slag under different FeO contents calculated using FactSage 8.1 and the content transition value of the target element in the metal for 0–6 min. With the increase of FeO, the viscosity of the initial slag decreased from 1.55 to 0.26 Pa·s, the Δ[V] increased from 0.017 to 0.039%, and the Δ[P] increased from 0.032 to 0.0499%. The FeO in slag shoulders the role of reducing the melting point, improving fluidity, and oxidizing impurities in the metal. The viscosity of the slag is the key characteristic that controls the molten slag’s mass transfer and crystallization behavior. Considerable research has shown that FeO can depolymerize the Si–O tetrahedral network to reduce the degree of polymerization of the slag, resulting in lower viscosity of the CaO–SiO2–FeO–P2O5 melt and improving the dephosphorization efficiency [33]. But, excess FeO at the slag–metal interface causes reaction (7) to proceed positively, promoting [V] oxidation. Along the FeO consumed by [V], [P], and [C], the activity of FeO in the slag tends to balance and the oxidation process of the molten pool is controlled by CO2.
3.4 Effect of basicity on the dephosphorization and vanadium retention operation
Figure 8 shows the experimental results at different basicity levels. The dephosphorization rate increased from 71.8 to 76.1% with basicity, [V] showed a decreasing trend, and the oxidation rate increased from 15.9 to 25.6%.
![Figure 8
Removal rate and contents of [V] and [P] under different basicity levels.](/document/doi/10.1515/htmp-2022-0281/asset/graphic/j_htmp-2022-0281_fig_008.jpg)
Removal rate and contents of [V] and [P] under different basicity levels.
Studies have shown that the dephosphorization slag is a multiphase slag composed of a homogeneous glass phase (2CaO·SiO2), a phosphorus-rich phase (C2S-C3P), and an iron-rich phase [34,35]. At steelmaking temperature, C2S and C3P can form a solid solution in a wide composition range. As a result, the activity of P2O5 in the slag decreases, which increases the driving force for the transformation of [P] to P2O5. As shown in Figure 8, the dephosphorization rate increases with basicity.
In the process of vanadium extraction, vanadium is enriched in the converter vanadium slag. The CaO content of the vanadium slag is generally less than 2.5%, and V2O3 is mainly in the spinel phase. However, the content of CaO in the dephosphorization slag is more than 50%, so it is necessary to characterize the final slag by XRD and SEM. The XRD of the final slag is shown in Figure 9(a). Phosphorus and vanadium are mainly enriched in C3P, C2S-C3P, and CaV2O4 phases. (V2O3) is a neutral oxide, acidic in a basic slag system. The increasing alkalinity promotes the combination of V2O3 and CaO to form CaV2O4, which further reduces the activity of V2O3 in the slag and expands the equilibrium constant of reaction (6), causing a decrease in [V]. Figure 9(b) shows the SEM image of the final slag with B = 4. By EDS surface scanning analysis, it is shown that the slag is mainly composed of CaO, phosphorus-rich phase, and iron-rich phase. The vanadium and phosphorus in the slag are evenly distributed in the calcium-containing mineral phase and no vanadium-containing spinel phase is found.

(a) XRD results of the dephosphorization slag in the basicity range of 2–4 and (b) SEM images of the dephosphorization slag at B = 4.
3.5 Effect of dephosphorization conditions on [V] and [P] slag–metal distribution ratio
From the above discussion, CO2 gas not only participates in the dephosphorization reaction but also has a low oxidation rate of vanadium, which can be used as a dephosphorization oxygen source for smelting vanadium steel in the CIDSVS method. Further, in order to analyze the influence of different factors on dephosphorization and vanadium retention, based on the experimental data in Table 4, the slag-to-metal distribution ratios L V and L P of vanadium and phosphorus were calculated as follows:
The CIDSVS method pursues high L P and low L V, defines L P/L V to find the best process conditions in the feasible region, and high L P/L V represents a high dephosphorization and vanadium retention effect. Figure 10 shows the correlation obtained between L V/L P and experimental factors.

Correlation between L P/L V and experimental factors: (a) CO2 flow rate, (b) FeO content, (c) basicity, and (d) correlation between L P and L V.
Figure 10(a) shows the relationship between the CO2 flow and L P/L V, where L P/L V shows a maximum at 1.5 mL·g−1·min−1. However, the correlation between the CO2 flow and L P/L V was unsatisfactory. The curve for the current studied data shows a coefficient of determination (R 2) of 0.6159. In Figure 10(b), L P/L V shows a trend of increase to decrease with the increase of FeO. As discussed in Section 3.3, the low FeO content slag has poor fluidity and low dephosphorization efficiency. A high FeO content leads to a large amount of vanadium oxidation at the slag–metal interface, and a large amount of V2O3 enters the slag phase. The results in Figure 10(c) show that the effect of dephosphorization and vanadium retention is better under low basicity operation. Figure 10(d) shows the correlation between L P and L V. L P increases with L V, indicating that a small amount of V2O3 in the slag can increase dephosphorization efficiency.
In summary, the researchers concluded that a CO2 flow rate of 1.5 mL·g−1·min−1 can meet the dissolved oxygen required for dephosphorization and control the redox reaction of vanadium at equilibrium. A 40% FeO content in the initial slag and a basicity of 2.5 provide better kinetic conditions for the dephosphorization operation. Under the above experimental conditions, the vanadium retention rate was 82.5%, and the dephosphorization rate was 73.8%. The main composition of the dephosphorization iron is shown in Table 5. The [C] content is elevated. The graphite crucible, which is selected to ensure the stability of the dephosphorization slag composition, has a certain influence on the experimental results. Due to the dissolution of graphite crucibles, C → [C], [C] in molten iron has been at a high content. The reactions [C] + (FeO) = Fe + CO and 5[C] + (P2O5) = 5CO + 2[P] diminished the dephosphorization capacity of THE dephosphorization slag. The reducibility of molten iron in an industrial production environment will be lower than that in laboratory research, and the quantity of slag and FeO content required for dephosphorization can be reduced further.
Main components of hot metals at different stages and specific vanadium steel grade standard (mass%)
| Element | V | P | C | Si | Mn | S | Cr | Mo | W |
|---|---|---|---|---|---|---|---|---|---|
| After De-P | 0.256 | 0.029 | 4.84 | 0.018 | <0.01 | 0.011 | / | / | / |
| After De-C | 0.248 | 0.028 | 0.43 | <0.01 | <0.01 | 0.008 | / | / | / |
| 3Cr2W8V | 0.20–0.50 | ≤0.03 | 0.30–0.40 | ≤0.40 | ≤0.40 | ≤0.03 | 2.20–2.70 | / | 7.5–9.0 |
| Cr12MoV | 0.15–0.30 | ≤0.03 | 1.45–1.70 | ≤0.40 | ≤0.40 | ≤0.03 | 11.0–12.5 | 0.4–0.6 | / |
Further decarburization of steelmaking is needed after the dephosphorization of molten iron. According to previous studies [36], the oxidation order of carbon and vanadium reverses at 1,357°C, and carbon preferentially oxidizes vanadium when the smelting temperature is higher than 1,357°C; a decarburization rate of 95.6% was achieved at 1,550°C. Vanadium-bearing steel was obtained by decarburization of dephosphorization pig iron at 1,550°C, a CO2 flow rate of 3 mL·g−1·min−1, and an injection time of 80 min. The main components are shown in Table 5. At this time, the impurity content satisfies the specifications for a particular vanadium steel, and after alloying, the desirable steel can be produced.
In comparison to the current process’s yield of 50–60%, the CIDSVS method can retain 80% of the vanadium in steel. The resource utilization efficiency of vanadium has improved. According to the production route of vanadium extraction, the cost of processing per kilogram of vanadium slag is 75.23 RMB and does not include the treatment of wastewater and tailings [37]. The CIDSVS process does not generate hazardous waste and can lower manufacturing costs by 4.8 kg of 50V–Fe per ton (about 55 kg of vanadium slag) of vanadium steel. Considering the low calorific value and long impurity removal time generated by CO2 participating in the oxidation reaction, it is necessary to use electric energy to heat the system during the steelmaking process. The CIDSVS process has a good application prospect in the electric furnace steelmaking process [38] and has positive economic and environmental effects.
4 Conclusions
According to thermodynamic calculations, the oxidation potency of the oxygen source for dephosphorization is as follows: O2 > CO2 > FeO. The dephosphorization reaction occurs before the vanadium oxidation reaction, and low temperatures are advantageous to the dephosphorization process.
The experimental procedure consists of two distinct phases: FeO- and CO2-dominated. The oxidation reactions of [V] and [P] were intense in the FeO-dominant stage, and the oxidation amount was positively correlated with the FeO content. The higher CO2 flow rate increases the gas–liquid reaction area, resulting in an increase in the formation of dissolved oxygen per unit of time and an increase in [V] oxidation.
Excessive FeO content in the initial slag enhanced the oxidation at the slag–metal interface, and a high CaO/SiO2 ratio reduced the activity of V2O3 in the slag. An appropriate FeO content and low CaO/SiO2 are the keys to dephosphorization and vanadium retention.
The L P/L V parameters were defined and fitted with experimental factors. It was found that the L P/L V was well correlated with the FeO content and basicity. The optimum conditions in the feasible region are a CO2 flow rate of 1.5 mL·g−1·min−1, FeO content of 40%, and basicity B of 2.5. Under the above conditions, the retention rate of vanadium is 82.5%, and the removal rate of phosphorus is 73.8%. After decarbonization, the [V], [P], and [C] percentages in the molten steel are, respectively, 0.248, 0.028, and 0.43%, which meet the requirements for special vanadium steels. This investigation provides the groundwork for the dephosphorization process of the CIDSVS method.
Acknowledgement
The authors deeply appreciate the financial support received from the National Natural Science Foundation of China (51874094, U1908225).
-
Funding information: This research was funded by National Natural Science Foundation of China (51874094, U1908225).
-
Author contributions: Han Yang: writing – original draft, writing – review & editing; Yan Liu: writing – review & editing; Kun Wang: formal analysis; Ting-an Zhang: methodology, project administration; and Shengnan Lin: resources.
-
Conflict of interest: The authors state that there was no conflict of interest.
References
[1] Lee, J. C., K. Kurniawan, E. Y. Kim, K. W. Chung, R. Kim, and H. S. Jeon. A review on the metallurgical recycling of vanadium from slags: towards a sustainable vanadium production. Journal of Materials Research and Technology, Vol. 12, 2021, pp. 343–364.10.1016/j.jmrt.2021.02.065Search in Google Scholar
[2] Moskalyk, R. R. and A. M. Alfantazi. Processing of vanadium: a review. Minerals Engineering, Vol. 16, No. 9, 2003, pp. 793–805.10.1016/S0892-6875(03)00213-9Search in Google Scholar
[3] Gao, M. L., X. X. Xue, L. J. Li, H. Yang, and D. H. Cheng. Leaching behavior and kinetics of vanadium extraction from vanadium-bearing steel slag. Metallurgical Research & Technology, Vol. 116, No. 4, 2019, id. 407.10.1051/metal/2018129Search in Google Scholar
[4] Ji, Y. L., S. B. Shen, J. H. Liu, S. Y. Yan, and Z. T. Zhang. Green and efficient process for extracting chromium from vanadium slag by an innovative three-phase roasting reaction. ACS Sustainable Chemistry & Engineering, Vol. 5, No. 7, 2017, pp. 6008–6015.10.1021/acssuschemeng.7b00836Search in Google Scholar
[5] Lindvall, M., J. Tikka, M. Berg, G. Ye, and D. Sichen. Vanadium extraction from a Fe–V (2.0 Mass%)–P (0.1 Mass%) melt and investigation of the phase relations in the formed FeO–SiO2-based slag with 20 Mass% V. Journal of Sustainable Metallurgy, Vol. 3, 2017, pp. 808–822.10.1007/s40831-017-0147-zSearch in Google Scholar
[6] Huang, W. J., S. Yu, X. Shen, L. Xu, N. Wang, and M. Chen. Kinetic study on the oxidation of elements in hot metal during vanadium-extraction process. Steel Research International, Vol. 87, No. 9, 2016, pp. 1228–1237.10.1002/srin.201500333Search in Google Scholar
[7] Li, H. Y., C. J. Wang, M. M. Lin, Y. Guo, and B. Xie. Green one-step roasting method for efficient extraction of vanadium and chromium from vanadium-chromium slag. Powder Technology, Vol. 360, 2020, pp. 503–508.10.1016/j.powtec.2019.10.074Search in Google Scholar
[8] Li, H. Y., C. J. Wang, M. M. Lin, Y. Guo, J. Diao, and B. Xie. Magnetisation roasting-acid leaching: A zero-discharge method for vanadium extraction from vanadium slag. Journal of Cleaner Production, Vol. 260, 2020, id. 121091.10.1016/j.jclepro.2020.121091Search in Google Scholar
[9] Fan, H. L., H. M. Duan, W. J. He, D. F. Chen, T. Liu, M. J. Long, et al. Sequential extraction of vanadium and chromium from chromium-bearing vanadium slag through two-stage soda roasting-water leaching. Metallurgical Research & Technology, Vol. 115, No. 6, 2018, id. 607.10.1051/metal/2018093Search in Google Scholar
[10] Wang, Z. H., S. L. Zheng, S. N. Wang, B. Liu, D. W. Wang, H. Du, et al. Research and prospect on extraction of vanadium from vanadium slag by liquid oxidation technologies. Transactions of Nonferrous Metals Society of China, Vol. 24, No. 5, 2014, pp. 1273–1288.10.1016/S1003-6326(14)63189-7Search in Google Scholar
[11] Li, M., H. Du, S. L. Zheng, S. N. Wang, Y. Zhang, B. Liu, et al. Extraction of vanadium from vanadium slag via non-salt roasting and ammonium oxalate leaching. JOM, Vol. 69, 2017, pp. 1970–1975.10.1007/s11837-017-2494-4Search in Google Scholar
[12] Diao, J., W. Zhou, P. Gu, Z. Q. Ke, Y. Qiao, and B. Xie. Competitive growth of crystals in vanadium–chromium slag. CrystEngComm, Vol. 18, No. 33, 2016, pp. 6272–6281.10.1039/C6CE01087CSearch in Google Scholar
[13] Ji, Y. L., S. B. Shen, J. H. Liu, and Y. Xue. Cleaner and effective process for extracting vanadium from vanadium slag by using an innovative three-phase roasting reaction. Journal of Cleaner Production, Vol. 149, 2017, pp. 1068–1078.10.1016/j.jclepro.2017.02.177Search in Google Scholar
[14] Peng, H. A literature review on leaching and recovery of vanadium. Journal of Cleaner Production, Vol. 7, No. 5, 2019, id. 103313.10.1016/j.jece.2019.103313Search in Google Scholar
[15] Li, M., B. Liu, S. L. Zheng, S. N. Wang, H. Du, D. B. Dreisinger, et al. A cleaner vanadium extraction method featuring non-salt roasting and ammonium bicarbonate leaching. Journal of Cleaner Production, Vol. 149, 2017, pp. 206–217.10.1016/j.jclepro.2017.02.093Search in Google Scholar
[16] Yuan, R., S. L. Li, Y. S. Che, J. L. He, J. X. Song, and B. Yang. A critical review on extraction and refining of vanadium metal. International Journal of Refractory Metals and Hard Materials, Vol. 101, 2021, id. 105696.10.1016/j.ijrmhm.2021.105696Search in Google Scholar
[17] Smirnov, L. A., V. I. Zhuchkov, O. V. Zayakin, and L. Y. Mikhailova. Complex vanadium-containing ferroalloys. Metallurgist, Vol. 64, 2021, pp. 1249–1255.10.1007/s11015-021-01112-1Search in Google Scholar
[18] Dong, K. and X. Wang. CO2 utilization in the ironmaking and steelmaking process. Metals, Vol. 9, No. 3, 2019, id. 273.10.3390/met9030273Search in Google Scholar
[19] Chung, W., K. Roh, and J. H. Lee. Design and evaluation of CO2 capture plants for the steelmaking industry by means of amine scrubbing and membrane separation. International Journal of Greenhouse Gas Control, Vol. 74, 2018, pp. 259–270.10.1016/j.ijggc.2018.05.009Search in Google Scholar
[20] Song, H. L., J. P. Zhang, G. J. Cheng, S. T. Yang, and X. X. Xue. CO2 injection improves the high-temperature performances of Cr-bearing vanadia-titania magnetite smelting in blast furnace. Journal of CO2 Utilization, Vol. 43, 2021, id. 101363.10.1016/j.jcou.2020.101363Search in Google Scholar
[21] Han, B. C., G. S. Wei, R. Zhu, W. H. Wu, J. J. Jiang, C. Feng, et al. Utilization of carbon dioxide injection in BOF–RH steelmaking process. Journal of CO2 Utilization, Vol. 34, 2019, pp. 53–62.10.1016/j.jcou.2019.05.038Search in Google Scholar
[22] Dong, W., A. Xu, H. Li, S. Guan, C. Ji, N. Hao, et al. Study on the Reaction Behavior of CO2 With Molten Steel in Converter Process by Top Blowing Mixed CO2–O2 Gas. Metallurgical and Materials Transactions B: Process Metallurgy and Materials Processing Science, Vol. 53, 2022, pp. 3575–3584.10.1007/s11663-022-02621-3Search in Google Scholar
[23] Lv, M., R. Zhu, and L. Z. Yang. High efficiency dephosphorization by mixed injection during steelmaking process. Steel Research International, Vol. 90, No. 3, 2019, id. 1800454.10.1002/srin.201800454Search in Google Scholar
[24] Wei, G., C. Zhou, S. Hu, J. Tian, R. Zhu, D. Wang, et al. Research on the jet characteristics and dephosphorization efficiency of converter oxygen lance blowing CO2–O2 mixed gas. Metals, Vol. 12, No. 9, 2022, id. 1457.10.3390/met12091457Search in Google Scholar
[25] Chao, F., R. Zhu, R. Liu, K. Dong, and G. Wei. Industrial application of bottom–blown CO2 in basic oxygen furnace steelmaking process. Steel Research International, Vol. 92, No. 10, 2021, id. 2000704.10.1002/srin.202000704Search in Google Scholar
[26] Wagner, C. Theorie der alterung von niederschlägen durch umlösen (Ostwald-Reifung). Zeitschrift für Elektrochemie, Berichte der Bunsengesellschaft für physikalische Chemie, Vol. 65, 1961, pp. 581–591.10.1002/bbpc.19610650704Search in Google Scholar
[27] Sigworth, G. K. and J. F. Elliott. The thermodynamics of liquid dilute iron alloys. Metal Science, Vol. 8, No. 1, 1974, pp. 298–310.10.1179/msc.1974.8.1.298Search in Google Scholar
[28] Sun, H., J. Yang, R. H. Zhang, and W. K. Yang. Influence of temperature on dephosphorization at lower basicity and lower temperature based on industrial experiments and IMCT. ISIJ International, Vol. 62, No. 6, 2022, pp. 1078–1090.10.2355/isijinternational.ISIJINT-2021-529Search in Google Scholar
[29] Tian, Z. H., B. H. Li, X. M. Zhang, and Z. H. Jiang. Double slag operation dephosphorization in BOF for producing low phosphorus steel. Journal of Iron and Steel Research, International, Vol. 16, 2009, pp. 6–14.10.1016/S1006-706X(09)60036-4Search in Google Scholar
[30] Zhou, C. G., J. Li, C. B. Shi, W. T. Yu, Z. M. Zhang, Z. M. Liu, et al. Dependence of temperature and slag composition on dephosphorization at the first deslagging in BOF steelmaking process. High Temperature Materials and Processes, Vol. 35, No. 4, 2016, pp. 433–440.10.1515/htmp-2014-0187Search in Google Scholar
[31] Mannion, F. J. and R. J. Fruehan. Decarburization kinetics of liquid Fe−Csat alloys by CO2. Metallurgical and Materials Transactions B: Process Metallurgy and Materials Processing Science, Vol. 20, 1989, pp. 853–861.10.1007/BF02670190Search in Google Scholar
[32] Mukawa, S. and Y. Mizukami. Effect of stirring energy and rate of oxygen supply on the rate of hot metal dephosphorization. ISIJ International, Vol. 35, No. 11, 1995, pp. 1374–1380.10.2355/isijinternational.35.1374Search in Google Scholar
[33] Wang, Z. J., Q. F. Shu, S. Sridhar, M. Zhang, M. Guo, and Z. T. Zhang. Effect of P2O5 and FetO on the viscosity and slag structure in steelmaking slags. Metallurgical and Materials Transactions B: Process Metallurgy and Materials Processing Science, Vol. 46, 2015, pp. 758–765.10.1007/s11663-014-0270-1Search in Google Scholar
[34] Lin, W. H., S. Q. Jiao, K. X. Zhou, J. K. Sun, X. M. Feng, and Q. Liu. A review of multi-phase slag refining for dephosphorization in the steelmaking process. Frontiers in Materials, Vol. 7, 2020, id. 602522.10.3389/fmats.2020.602522Search in Google Scholar
[35] Ye, G. F., J. Yang, R. H. Zhang, W. K. Yang, and H. Sun. Behavior of phosphorus enrichment in dephosphorization slag at low temperature and low basicity. International Journal of Minerals, Metallurgy, and Materials, Vol. 28, 2021, pp. 66–75.10.1007/s12613-020-2036-xSearch in Google Scholar
[36] Luo, Y., T. A. Zhang, L. P. Niu, Z. L. Liu, and B. J. Zhang. Research on directly smelting semi-steel by blowing CO2 into blast furnace vanadium-bearing molten iron. Iron Steel Vanadium Titanium, Vol. 39, No. 5, 2018, pp. 116–121.Search in Google Scholar
[37] Liu, S., S. Li, S. Wu, L. Wang, and K. Chou. A novel method for vanadium slag comprehensive utilization to synthesize Zn-Mn ferrite and Fe-V-Cr alloy. Journal of Hazardous Materials, Vol. 354, 2018, pp. 99–106.10.1016/j.jhazmat.2018.04.061Search in Google Scholar PubMed
[38] Toshihiko, E. M. I. Steelmaking technology for the last 100 years: Toward highly efficient mass production systems for high quality steels. ISIJ International, Vol. 55, No. 1, 2015, pp. 36–66.10.2355/isijinternational.55.36Search in Google Scholar
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- First-principles investigation of phase stability and elastic properties of Laves phase TaCr2 by ruthenium alloying
- Improvement and prediction on high temperature melting characteristics of coal ash
- First-principles calculations to investigate the thermal response of the ZrC(1−x)Nx ceramics at extreme conditions
- Study on the cladding path during the solidification process of multi-layer cladding of large steel ingots
- Thermodynamic analysis of vanadium distribution behavior in blast furnaces and basic oxygen furnaces
- Comparison of data-driven prediction methods for comprehensive coke ratio of blast furnace
- Effect of different isothermal times on the microstructure and mechanical properties of high-strength rebar
- Analysis of the evolution law of oxide inclusions in U75V heavy rail steel during the LF–RH refining process
- Simultaneous extraction of uranium and niobium from a low-grade natural betafite ore
- Transfer and transformation mechanism of chromium in stainless steel slag in pedosphere
- Effect of tool traverse speed on joint line remnant and mechanical properties of friction stir welded 2195-T8 Al–Li alloy joints
- Technology and analysis of 08Cr9W3Co3VNbCuBN steel large diameter thick wall pipe welding process
- Influence of shielding gas on machining and wear aspects of AISI 310–AISI 2205 dissimilar stainless steel joints
- Effect of post-weld heat treatment on 6156 aluminum alloy joint formed by electron beam welding
- Ash melting behavior and mechanism of high-calcium bituminous coal in the process of blast furnace pulverized coal injection
- Effect of high temperature tempering on the phase composition and structure of steelmaking slag
- Numerical simulation of shrinkage porosity defect in billet continuous casting
- Influence of submerged entry nozzle on funnel mold surface velocity
- Effect of cold-rolling deformation and rare earth yttrium on microstructure and texture of oriented silicon steel
- Investigation of microstructure, machinability, and mechanical properties of new-generation hybrid lead-free brass alloys
- Soft sensor method of multimode BOF steelmaking endpoint carbon content and temperature based on vMF-WSAE dynamic deep learning
- Mechanical properties and nugget evolution in resistance spot welding of Zn–Al–Mg galvanized DC51D steel
- Research on the behaviour and mechanism of void welding based on multiple scales
- Preparation of CaO–SiO2–Al2O3 inorganic fibers from melting-separated red mud
- Study on diffusion kinetics of chromium and nickel electrochemical co-deposition in a NaCl–KCl–NaF–Cr2O3–NiO molten salt
- Enhancing the efficiency of polytetrafluoroethylene-modified silica hydrosols coated solar panels by using artificial neural network and response surface methodology
- High-temperature corrosion behaviours of nickel–iron-based alloys with different molybdenum and tungsten contents in a coal ash/flue gas environment
- Characteristics and purification of Himalayan salt by high temperature melting
- Temperature uniformity optimization with power-frequency coordinated variation in multi-source microwave based on sequential quadratic programming
- A novel method for CO2 injection direct smelting vanadium steel: Dephosphorization and vanadium retention
- A study of the void surface healing mechanism in 316LN steel
- Effect of chemical composition and heat treatment on intergranular corrosion and strength of AlMgSiCu alloys
- Soft sensor method for endpoint carbon content and temperature of BOF based on multi-cluster dynamic adaptive selection ensemble learning
- Evaluating thermal properties and activation energy of phthalonitrile using sulfur-containing curing agents
- Investigation of the liquidus temperature calculation method for medium manganese steel
- High-temperature corrosion model of Incoloy 800H alloy connected with Ni-201 in MgCl2–KCl heat transfer fluid
- Investigation of the microstructure and mechanical properties of Mg–Al–Zn alloy joints formed by different laser welding processes
- Effect of refining slag compositions on its melting property and desulphurization
- Effect of P and Ti on the agglomeration behavior of Al2O3 inclusions in Fe–P–Ti alloys
- Cation-doping effects on the conductivities of the mayenite Ca12Al14O33
- Modification of Al2O3 inclusions in SWRH82B steel by La/Y rare-earth element treatment
- Possibility of metallic cobalt formation in the oxide scale during high-temperature oxidation of Co-27Cr-6Mo alloy in air
- Multi-source microwave heating temperature uniformity study based on adaptive dynamic programming
- Round-robin measurement of surface tension of high-temperature liquid platinum free of oxygen adsorption by oscillating droplet method using levitation techniques
- High-temperature production of AlN in Mg alloys with ammonia gas
- Review Article
- Advances in ultrasonic welding of lightweight alloys: A review
- Topical Issue on High-temperature Phase Change Materials for Energy Storage
- Compositional and thermophysical study of Al–Si- and Zn–Al–Mg-based eutectic alloys for latent heat storage
- Corrosion behavior of a Co−Cr−Mo−Si alloy in pure Al and Al−Si melt
- Al–Si–Fe alloy-based phase change material for high-temperature thermal energy storage
- Density and surface tension measurements of molten Al–Si based alloys
- Graphite crucible interaction with Fe–Si–B phase change material in pilot-scale experiments
- Topical Issue on Nuclear Energy Application Materials
- Dry synthesis of brannerite (UTi2O6) by mechanochemical treatment
- Special Issue on Polymer and Composite Materials (PCM) and Graphene and Novel Nanomaterials - Part I
- Heat management of LED-based Cu2O deposits on the optimal structure of heat sink
- Special Issue on Recent Developments in 3D Printed Carbon Materials - Part I
- Porous metal foam flow field and heat evaluation in PEMFC: A review
- Special Issue on Advancements in Solar Energy Technologies and Systems
- Research on electric energy measurement system based on intelligent sensor data in artificial intelligence environment
- Study of photovoltaic integrated prefabricated components for assembled buildings based on sensing technology supported by solar energy
- Topical Issue on Focus of Hot Deformation of Metaland High Entropy Alloys - Part I
- Performance optimization and investigation of metal-cored filler wires for high-strength steel during gas metal arc welding
- Three-dimensional transient heat transfer analysis of micro-plasma arc welding process using volumetric heat source models
Articles in the same Issue
- Research Articles
- First-principles investigation of phase stability and elastic properties of Laves phase TaCr2 by ruthenium alloying
- Improvement and prediction on high temperature melting characteristics of coal ash
- First-principles calculations to investigate the thermal response of the ZrC(1−x)Nx ceramics at extreme conditions
- Study on the cladding path during the solidification process of multi-layer cladding of large steel ingots
- Thermodynamic analysis of vanadium distribution behavior in blast furnaces and basic oxygen furnaces
- Comparison of data-driven prediction methods for comprehensive coke ratio of blast furnace
- Effect of different isothermal times on the microstructure and mechanical properties of high-strength rebar
- Analysis of the evolution law of oxide inclusions in U75V heavy rail steel during the LF–RH refining process
- Simultaneous extraction of uranium and niobium from a low-grade natural betafite ore
- Transfer and transformation mechanism of chromium in stainless steel slag in pedosphere
- Effect of tool traverse speed on joint line remnant and mechanical properties of friction stir welded 2195-T8 Al–Li alloy joints
- Technology and analysis of 08Cr9W3Co3VNbCuBN steel large diameter thick wall pipe welding process
- Influence of shielding gas on machining and wear aspects of AISI 310–AISI 2205 dissimilar stainless steel joints
- Effect of post-weld heat treatment on 6156 aluminum alloy joint formed by electron beam welding
- Ash melting behavior and mechanism of high-calcium bituminous coal in the process of blast furnace pulverized coal injection
- Effect of high temperature tempering on the phase composition and structure of steelmaking slag
- Numerical simulation of shrinkage porosity defect in billet continuous casting
- Influence of submerged entry nozzle on funnel mold surface velocity
- Effect of cold-rolling deformation and rare earth yttrium on microstructure and texture of oriented silicon steel
- Investigation of microstructure, machinability, and mechanical properties of new-generation hybrid lead-free brass alloys
- Soft sensor method of multimode BOF steelmaking endpoint carbon content and temperature based on vMF-WSAE dynamic deep learning
- Mechanical properties and nugget evolution in resistance spot welding of Zn–Al–Mg galvanized DC51D steel
- Research on the behaviour and mechanism of void welding based on multiple scales
- Preparation of CaO–SiO2–Al2O3 inorganic fibers from melting-separated red mud
- Study on diffusion kinetics of chromium and nickel electrochemical co-deposition in a NaCl–KCl–NaF–Cr2O3–NiO molten salt
- Enhancing the efficiency of polytetrafluoroethylene-modified silica hydrosols coated solar panels by using artificial neural network and response surface methodology
- High-temperature corrosion behaviours of nickel–iron-based alloys with different molybdenum and tungsten contents in a coal ash/flue gas environment
- Characteristics and purification of Himalayan salt by high temperature melting
- Temperature uniformity optimization with power-frequency coordinated variation in multi-source microwave based on sequential quadratic programming
- A novel method for CO2 injection direct smelting vanadium steel: Dephosphorization and vanadium retention
- A study of the void surface healing mechanism in 316LN steel
- Effect of chemical composition and heat treatment on intergranular corrosion and strength of AlMgSiCu alloys
- Soft sensor method for endpoint carbon content and temperature of BOF based on multi-cluster dynamic adaptive selection ensemble learning
- Evaluating thermal properties and activation energy of phthalonitrile using sulfur-containing curing agents
- Investigation of the liquidus temperature calculation method for medium manganese steel
- High-temperature corrosion model of Incoloy 800H alloy connected with Ni-201 in MgCl2–KCl heat transfer fluid
- Investigation of the microstructure and mechanical properties of Mg–Al–Zn alloy joints formed by different laser welding processes
- Effect of refining slag compositions on its melting property and desulphurization
- Effect of P and Ti on the agglomeration behavior of Al2O3 inclusions in Fe–P–Ti alloys
- Cation-doping effects on the conductivities of the mayenite Ca12Al14O33
- Modification of Al2O3 inclusions in SWRH82B steel by La/Y rare-earth element treatment
- Possibility of metallic cobalt formation in the oxide scale during high-temperature oxidation of Co-27Cr-6Mo alloy in air
- Multi-source microwave heating temperature uniformity study based on adaptive dynamic programming
- Round-robin measurement of surface tension of high-temperature liquid platinum free of oxygen adsorption by oscillating droplet method using levitation techniques
- High-temperature production of AlN in Mg alloys with ammonia gas
- Review Article
- Advances in ultrasonic welding of lightweight alloys: A review
- Topical Issue on High-temperature Phase Change Materials for Energy Storage
- Compositional and thermophysical study of Al–Si- and Zn–Al–Mg-based eutectic alloys for latent heat storage
- Corrosion behavior of a Co−Cr−Mo−Si alloy in pure Al and Al−Si melt
- Al–Si–Fe alloy-based phase change material for high-temperature thermal energy storage
- Density and surface tension measurements of molten Al–Si based alloys
- Graphite crucible interaction with Fe–Si–B phase change material in pilot-scale experiments
- Topical Issue on Nuclear Energy Application Materials
- Dry synthesis of brannerite (UTi2O6) by mechanochemical treatment
- Special Issue on Polymer and Composite Materials (PCM) and Graphene and Novel Nanomaterials - Part I
- Heat management of LED-based Cu2O deposits on the optimal structure of heat sink
- Special Issue on Recent Developments in 3D Printed Carbon Materials - Part I
- Porous metal foam flow field and heat evaluation in PEMFC: A review
- Special Issue on Advancements in Solar Energy Technologies and Systems
- Research on electric energy measurement system based on intelligent sensor data in artificial intelligence environment
- Study of photovoltaic integrated prefabricated components for assembled buildings based on sensing technology supported by solar energy
- Topical Issue on Focus of Hot Deformation of Metaland High Entropy Alloys - Part I
- Performance optimization and investigation of metal-cored filler wires for high-strength steel during gas metal arc welding
- Three-dimensional transient heat transfer analysis of micro-plasma arc welding process using volumetric heat source models

