High-temperature corrosion behaviours of nickel–iron-based alloys with different molybdenum and tungsten contents in a coal ash/flue gas environment
Abstract
High-temperature coal ash/flue gas corrosion behaviours of three nickel–iron based superalloys for boiler tubes with different Mo and W contents (1.8% Mo + 1.2% W for S1 alloy, 0.8% Mo + 0.2% W for S2 alloy, and 0.5% Mo + 2.2% W for S3 alloy) at 650 and 700°C were studied. The microstructure, phase compositions, and morphologies together with element distribution for corrosion products were investigated with a metallographic microscope, a scanning electron microscope equipped with an energy-dispersive spectroscope, and X-ray diffractometer. The results show that the corrosion resistance of the Ni–Fe-based alloy decreases with the increasing total content of Mo and W in the alloy at both 650 and 700°C; the outer layer of the corrosion products was mainly composed of FeCr2O4 while the inner layer was mainly composed of Cr2O3. Peeling of the oxide films formed on the surfaces of S1 and S3 alloys was observed but no obvious spalling was observed for the S2 alloy. The reactions among Mo, W, and S in the coal ash/flue gas increased the internal stress in the oxide scale, which would cause the failure of the oxide scale.
Graphical Abstract
High-temperature corrosion behaviours of three nickel–iron-based superalloys for boiler tubes with different Mo and W contents in simulated coal ash/flue gas at 650 and 700°C were studied. The corrosion resistance of the Ni–Fe-based alloy decreases with the increasing total contents of Mo and W in the alloy at both 650 and 700°C; peeling of the oxide films formed on the surfaces of S1 and S3 alloys was observed but no obvious spalling was observed for the S2 alloy. Reactions between Mo, W, and S in the coal ash/flue gas increased the internal stress in the oxide scale, which would cause the failure of the oxide scale.
1 Introduction
Increasing demand for more electricity, reduced plant emissions, and greater efficiency is forcing power plants to increase the steam temperature and pressure of pulverized coal-fired boilers. Coal-fired steam generation plants designed based on supercritical and ultra-supercritical steam conditions have been adopted to increase the efficiency of boilers to about over 50%. The ultimate goal is to change the steam pressure and temperature to 34.5 MPa and 700°C, respectively [1], which will therefore require component materials performing a high creep rupture strength at temperatures of about 750°C (100 MPa), together with good corrosion resistance (= 2 mm cross-sectional loss in 2 × 105 h) [2]. The use of high-parameter ultra-supercritical thermal power units is one of the main development directions for coal-fired power generation. Several nickel-based alloys with high strength and good properties of corrosion resistance have been developed to meet the requirement of high temperature and pressure (700°C per 35 MPa) of an advanced ultra-supercritical coal-fired boiler [2,3]. The corrosion behaviours of nickel-based alloys in the presence of mixtures of synthetic coal ash, flue gas, alkali sulphates, and alkali chlorides have been widely studied [2,3,4,5,6]. The corrosion mechanism of nickel-based alloys in the above environments is also discussed in these studies.
In general, nickel-based alloys exert acceptable corrosion resistance in a coal-fired environment at the temperature range of an ultra-supercritical coal-fired boiler. However, the highly expensive and hard processing of the nickel-based alloy improved the building cost of the coal-fired boiler. Recently, a novel precipitation-hardened nickel–iron-based superalloy HT700T, which exhibits good high-temperature mechanical properties, high microstructural stability, and good environmental resistance, has been developed and evaluated as a candidate material for 700°C ultra-supercritical technology [7,8,9,10,11]. It is reported that HT700T shows good oxidation resistance in pure steam [12]. However, alloys should endure much more severe corrosion in a fireside environment because of the presence of impurities such as sodium, potassium, sulphur, and chlorine in the coal feedstock sulphate. The corrosion behaviours of HT700T in simulated fireside environments have also been studied [13,14,15].
In the present work, corrosion behaviours of three nickel–iron-based superalloys in simulation coal ash/flue gas environments were studied to understand the roles of alloy elements Mo and W, both added to improve the mechanical properties at high temperatures, in the corrosion resistance of nickel–iron-based superalloys.
2 Experimental procedures
2.1 Preparation of samples
The nickel–iron-based superalloys used in the present work are fabricated in a frequency crucible induction melting furnace. The high-temperature strength of the Fe–Ni alloy can be found in the study of Yuan et al. [16]. S1, S2, and S3 stand for the alloys with different compositions (Table 1). All three types of alloys are austenitic alloys (Figure 1). The grain sizes of S1, S2, and S3 alloys are measured to be 7, 136, and 102 nm, respectively. The cast alloys were cut into pieces with dimensions of 15 mm × 10 mm × 2 mm and a hole with a diameter of 0.8 mm on the middle top of each sample. The samples were ground with 800# and 1200# sandpaper, and then ultrasonic degreased with acetone, thoroughly rinsed with distilled water, and dried by warm air before the corrosion tests.
Chemical composition of the experimental alloy (wt%)
| Cr | Fe | Mo | W | Ti | Al | Ni | |
|---|---|---|---|---|---|---|---|
| S1 | 16 | 38 | 1.8 | 1.2 | 2.1 | 1.4 | Bal. |
| S2 | 16 | 42 | 0.8 | 0.2 | 2.1 | 1.4 | Bal. |
| S3 | 16 | 47 | 0.5 | 2.2 | 2.1 | 1.4 | Bal. |

Photomicrographs of the nickel–iron-based alloys: (a) S1, (b) S2, and (c) S3.
2.2 Corrosion tests
The corrosion tests were carried out in simulated coal ash/flue gas environments with high sulphur contents at 650 and 700°C (Figure 2). A mixture of 6% Fe2O3 + 2% Na2SO4 + 29% K2SO4 + 29% CaSO4 + 39% SiO2 + 22% Al2O3 (weight percentage) was milled and then suspended in acetone to make a suspension liquid. Then, the samples were put on a hot steel plate, and the suspension liquid was scrubbed onto the surface of the samples uniformly to yield slightly compacted coal ash layers with a density of about 45 mg·cm−2. The furnace was heated up to the desired temperature at a rate of 40°C·min−1 after the coated samples were placed in the furnace; at the same time, simulated flue gas with a composition of 0.3% SO2 + 81.2% N2 + 10% CO2 + 3.5% O2 + 5% H2O (volume percentage) was introduced into the furnace with a flow rate of 100 mL·min−1 controlled by an electronic mass flow meter. To keep the balance of the reaction 2SO2 + O2 → 2SO3, a platinum wire was used as a catalyst. An analytical balance with a sensitivity of 10−5 g was used to measure the weight of the specimens at intervals of 50, 100, 200, 500, and 1,000 h during the corrosion tests; three duplications for every alloy were conducted under each condition. After weighing, the specimens were re-coated with simulated coal ash and put into the furnace again for corrosion testing. The duration of the whole corrosion test was 1,000 h. The exhausted gas was treated with saturated NaOH solution before discharging into the air.

Schematic diagram of the corrosion experiment setup in a simulative coal ash/flue gas environment.
2.3 Characterization of the corrosion products
X-ray diffraction analysis (Bruker D8 Discover) was used to identify the crystal structure of the corrosion products after corrosion tests. The scanning range was 20–90°, and the scanning rate was 4°·min−1. A scanning electron microscope (Hitachi-S4800 FESEM) equipped with an energy-dispersive spectroscope was used to observe the surface and cross-sectional morphology of the corroded sample, and the surface distribution of the main elements in the corroded layer was determined.
3 Results
3.1 Corrosion kinetics of nickel–iron-based alloys in simulated environments
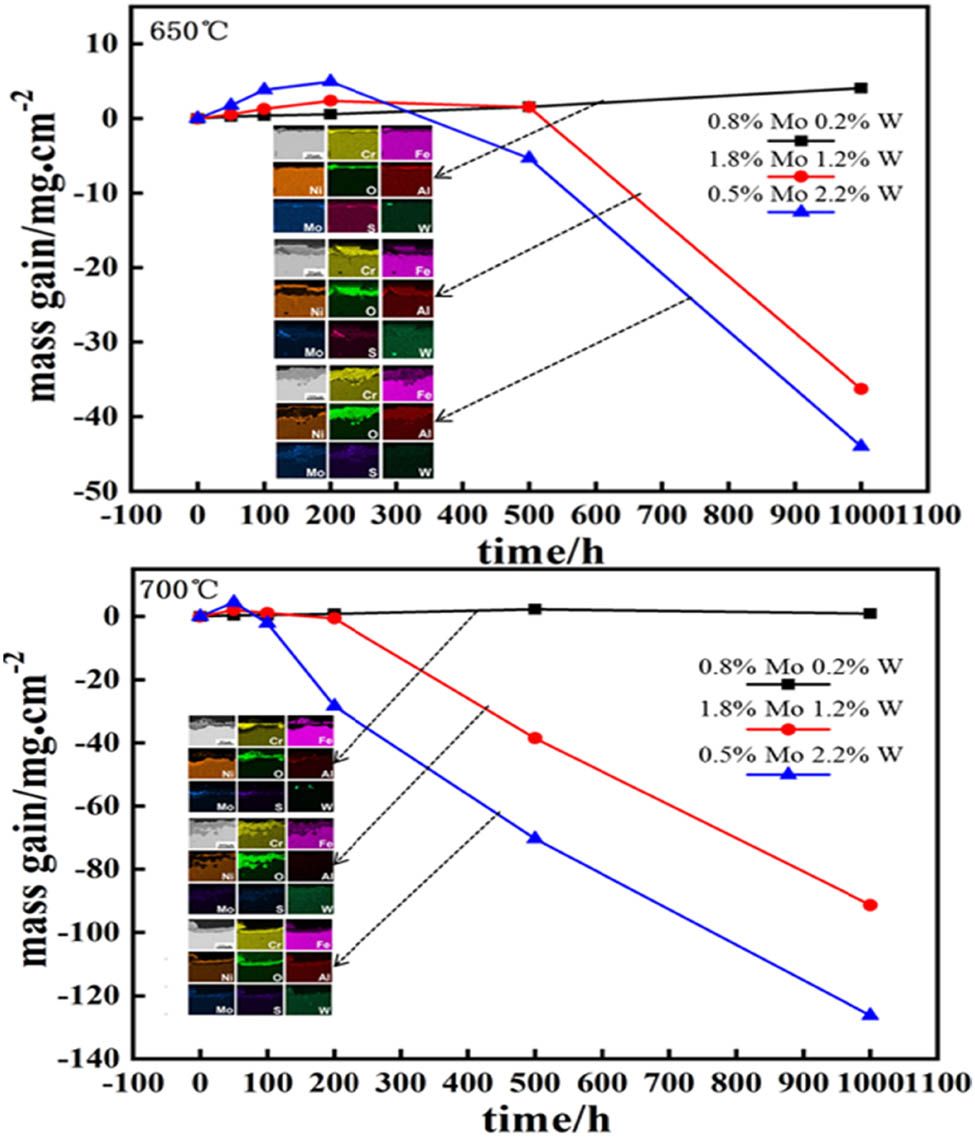
The mass of all three alloys with different compositions increases and then decreases with the corrosion time at corrosion temperatures of 650 and 700°C, but the mass loss of the S2 alloy is not obvious at 650°C, as shown in Figure 3(a). The enlarged images of the first 200 h of the corrosion process are also shown as an inset. S2 is more corrosion-resistant than S1 and S3, and the mass gains of S1 and S3 alloys are 4 and 9 times that of S2 alloy at 650°C at the mass-increasing stage of the corrosion process, respectively, while the data are 5 and 12 times at 700°C, respectively, as shown in Figure 3(b). The mass loss for the S3 alloy started early than that for the S1 alloy during the corrosion process at 650 and 700°C. It can be concluded that the corrosion resistance of the three tested alloys in the simulated coal ash/flue gas at 650 and 700°C was as follows: S2 > S1 > S3.

Corrosion kinetics of alloys corrosion in simulated coal ash/flu gas at (a) 650 and (b) 700°C.
3.2 Morphologies of the corrosion products
From the surface morphologies of corrosion products formed on the surfaces of S1, S2, and S3 alloys after corrosion for 1,000 h, it can be observed that the amount of “tubercular” raised increased corrosion at 700°C is significantly higher than that at 650°C (Figures 4 and 5). Besides, corrosion layers of S1 (Figures 4(a and b) and 5(a and b)) and S3 (Figures 4(e and f) and 5(e and f)) show severe lamellar peeling and rupture, whereas the corrosion layer of S2 (Figures 4(c and d) and 5(c and d)) does not crack or peel. It can be seen from the partial magnification at 650°C that S1 corrosion products are looser than S3, and “tubercular” raised corrosion products have grown on some areas of the S2 surface. At 700°C, the S3 surface corrosion products are looser, whereas the S1 surface corrosion products are regular, tight lumps. In addition, the S2 surface “tubercular” raised products are more and larger. The relative contents of the main elements in the corrosion products after corrosion at 650 and 700°C for 1,000 h are shown in Tables 2 and 3; the sulphur contents of S3 and S1 are much higher than that of S1, whereas the tungsten contents of S3 and S1 are lower than that of S1.

Surface morphologies of the corrosion products formed on the nickel–iron-based alloys at 650°C, (a and b) S1, (c and d) S2, (e and f) S3.

Surface morphologies of the corrosion products formed on the nickel–iron-based alloys at 700°C, (a and b) S1, (c and d) S2, (e and f) S3.
Percentage of surface atoms of alloys corroded at 650°C for 1,000 h
| Alloy | O | S | Cr | Fe | Ni | W |
|---|---|---|---|---|---|---|
| S1(A) | 57.62 | 8.27 | 15.29 | 5.80 | 12.77 | 0.26 |
| S1(B) | 3.17 | 3.91 | 59.82 | 16.17 | 18.84 | 0.08 |
| S2(A) | 26.36 | 0.86 | 9.87 | 56.54 | 3.96 | 2.42 |
| S2(B) | 63.38 | 1.17 | 8.78 | 21.66 | 2.98 | 2.04 |
| S3(A) | 38.49 | 1.62 | 12.76 | 22.93 | 23.59 | 0.62 |
| S3(B) | 58.03 | 0.46 | 15.01 | 19.27 | 4.80 | 2.32 |
Percentage of surface atoms of alloys corroded at 700°C for 1,000 h
| Alloy | O | S | Cr | Fe | Ni | W |
|---|---|---|---|---|---|---|
| S1(A) | 56.81 | 0.65 | 16.13 | 13.24 | 12.68 | 0.49 |
| S1(B) | 8.89 | 0.27 | 40.17 | 29.75 | 20.86 | 0.06 |
| S2(A) | 2.82 | 0.07 | 37.08 | 51.70 | 8.30 | 0.02 |
| S2(B) | 57.58 | 0.40 | 19.56 | 17.16 | 5.13 | 0.16 |
| S3(A) | 64.96 | 0.70 | 1.00 | 27.93 | 5.38 | 0.04 |
| S3(B) | 54.76 | 0.58 | 22.24 | 19.19 | 2.90 | 0.34 |
The cross-sectional morphologies of the corrosion products formed on the surfaces of S1, S2, and S3 alloys after corrosion at 650 and 700°C for 1,000 h are different, as shown in Figure 6. The corrosion layers formed on S1 (Figure 6(a and d)) and S3 (Figure 6(c and f)) are uneven, which is attributed to the loss of the oxide film on the surface after corrosion. Cracks were observed on the corrosion layers formed on S1 and S3 alloys, which did not occur for the S2 alloy. The thicknesses of the corrosion layers formed on S1, S2, and S3 alloys at 650°C are 53.6, 6, and 76.8 μm, respectively, and the values at 700°C are 71.4, 27, and 40.17 μm, respectively. Additionally, the corrosion layers formed on the S2 alloy (Figure 6(b and e)) are much thinner than those formed on S1 (Figure 6(a and d) and S3 (Figure 6(c and f)) alloys. The thickness of the corrosion layer formed on the S3 alloy should be thicker than that formed on the S1 alloy according to the weight changes shown in Figure 3; however, the accurate thickness of the corrosion layers formed on S3 is thinner than those formed on the S1 alloy, indicating the peeling of the corrosion layers of the S3 alloy.

Cross-sectional morphologies of the alloys after corrosion tests at 650 and 700°C. (a) S1 650°C, (b) S1 700°C, (c) S2 650°C, (d) S2 700°C, (e) S3 650°C, and (f) S3 700°C.
The corrosion products formed on the surface of the three Ni–Fe-based alloys after corrosion at 650 and 700°C for 1,000 h contained Ni, Fe, and Cr, as shown in Figure 7. Enrichments of Al beneath the oxide scale were observed; however, the Al-enriched layer formed on S1 and S3 alloys is not continuous and dense. Molybdenum and sulphur were distributed in the total corrosion layer of S1 and S3 alloys after corrosion at 650 and 700°C but they were only enriched at the oxide scale/substrate interface for the S2 alloy.

Element mapping of the corrosion scale of the corrosion of three alloys at 650 and 700°C. (a) S1650°C, (b) S1700°C, (c) S2650°C, (d) S2700°C, (e) S3650°C, and (f) S3700°C.
3.3 X-ray diffraction patterns of the corrosion products
The main corrosion products of S1, S2, and S3 are mainly oxides of chromium and iron (Figures 8 and 9). The main phases of the corrosion products formed on the surfaces of the three alloys are Cr2O3 and FeCr2O4, which is consistent with the results shown in Figure 7; the peaks corresponding to substrates were also detected. Although the enrichment of Mo, W, and S was found (Figure 7), their compounds were not detected by XRD due to their relatively low contents compared to others. Similarly, it cannot be ruled out that the sensitivity of the XRD analyser was too low to detect.

X-ray diffraction patterns of the corrosion of three alloys at 650°C.

X-ray diffraction patterns of the corrosion of three alloys at 700°C.
4 Discussion
The three alloys selected for the experiments were all austenitic heat-resistant steels, which differed significantly in their resistance to coal ash/flue gas corrosion due to the differences in alloying elements. The corrosion resistance of the three alloys was similar at 650 and 700°C, with the corrosion resistance S2 > S1 > S3. For the same alloy, the higher the temperature, the worse the corrosion resistance. In addition, both S1 and S3 present significant weight loss at 650°C, with rupture and peeling of the oxide film, and an early appearance at 700°C. This might be because the thermal expansion coefficients of the oxide film and the substrate were different; the thicker the oxide film, the greater the internal stress, which led to oxide film rupture and peeling [17,18]. In addition, the volume fraction of sulphate on the side of the substrate near the corrosion layer was larger, and the sulphate entrained in the oxide layer increased the internal stress in the film [19], which resulted in a direct peeling of the oxide film where the strength of the substrate bond was greatly reduced. With the progress of corrosion, the corrosion products completely covered the alloy substrate, while the molten salts in the coated coal ash formed and adhered to the surface of the damaged film layer, which also contributed to the rupture. When the temperature increased to 700°C, the number and size of “tubercular” raised products increased and accelerated the change in the low melting point sulphate to a molten state and the dissolution reaction with the film, thereby causing serious damage to the oxide film. During the high-temperature corrosion test, there was no significant change in the microstructure of the alloy.
As shown by the experimental results, the elements playing a major role in the degree of corrosion were iron, chromium, oxygen, and sulphur. Due to the low chromium content, a continuous film of Cr2O3 could not be grown at the beginning of corrosion. As the alloying element iron was also involved in the oxidation and the diffusion rate of iron was higher than that of chromium, the alloy surface preferentially formed a large amount of oxidation products of iron, whose loose structure was not protective to the substrate and inhibited the continuous formation of Cr2O3. At the same time, Cr diffused outwards and reacted with iron-containing oxides to form FeCr2O4 spinel. In addition, the coal ash/flue gas contained a large number of sulphate substances in the initial stage of corrosion. Apart from the generation of the oxide film, the low melting point sulphate was in a molten state and adhered to the surface of the oxide film. The reaction occurred to yield Na3Fe(SO4)3 (melting point of 624°C) and K3Fe(SO4)3 (melting point of 618°C), as well as a small amount of sulphate, which accelerated the corrosion process [20,21]. Furthermore, the alloying elements molybdenum and tungsten easily reacted with oxygen to generate MoO3 and WO3 [22]; MoO3 preferentially reacted with Na2SO4: MoO3 + Na2SO4 = Na2MoO4 + SO3. Therefore, the partial pressure of SO3 increased, which accelerated the pitting within the micro-zone and reduced the bond between the film layer and the substrate. In this way, micro-cracks and holes appeared in the film layer. At the same time, the oxygen ion activity was relatively reduced, and the molten eutectic salt became acidic, promoting the decomposition of Cr2O3, NiO, and other products. The cations formed in the outer layer through decomposition combined with oxygen to form a loose unprotected oxide film layer, which triggered rapid penetration of the corrosive medium oxygen/sulphur into the interior of the film layer and further induced corrosion of alloy elements. Thus, as the iron content of S1 was lower than that of S2, the influence of molybdenum and tungsten on the degree of corrosion of the alloy may be one of the reasons for the corrosion resistance of S2 being greater than that of S1.
Due to the catalytic effect of the platinum wire, SO2 reacted with O2 at high temperatures to form SO3, which induced low-temperature corrosion. Owing to the presence of tungsten in the alloy, which has a high affinity to oxygen, the reaction with oxygen easily forms WO3, which may further react with the oxide of chromium to form the molten tungstate. It leaves empty spaces in the oxide film, reduces the bond between the film and the substrate, and promotes the corrosion of the alloy [23]. Furthermore, the thermal stability of tungstate was weaker than that of molybdate, and the total contents of iron, molybdenum, and tungsten in S3 were higher than those of S2. Thus, S2 was more resistant to corrosion than S3. As shown by the element mapping, the formation of aluminium oxide film on the surface of S2 could hinder the rapid penetration of oxygen/sulphur and reduce the corrosion rate. In summary, S2 has a stronger corrosion resistance than S1, while S1 has a stronger corrosion resistance than S3.
5 Conclusions
At 650 and 700°C, all three alloys exhibited significant high-temperature corrosion in coal ash/flue gas media. After corrosion for 1,000 h, S2 was more corrosion resistant than S1, and S1 was more corrosion resistant than S3.
After 1,000 h of the corrosion experiment, the S1 and S3 outer oxide film formed Fe2O3, the inner oxide film formed Cr2O3, the multicomponent oxide film formed layered growth, and interlayer defects reduced the bonding force of the oxide film. As the corrosion time increased, the oxide film/substrate interface was enriched with a large amount of sulphide and the internal stress increased, resulting in the peeling of large areas of the oxide film. The outer oxide film of S2 is Fe2O3 and the inner layer is Cr2O3 and sulphide, with no obvious peeling seen.
The elements molybdenum and tungsten increased the degree of corrosion of nickel–iron-based superalloys; the higher the content, the more likely catastrophic corrosion occurred.
Acknowledgments
This work was supported by the Youth Special Support Program of Shaanxi Province under Grant No. ZD18SST05; National Natural Science Foundation of China under Grant No. 51901178; and Science and Technology Project of China Huaneng Group under Grant No. HNKJ40-H43.
-
Author contributions: Ming Zhu and Jintao Lu contributed to the concept of the study; Xin Zhang and Chunlin Huang performed the experiment; Huihui Zhang and Yimeng Ma contributed significantly to the analysis and manuscript preparation; Ming Zhu and Xin Zhang performed the data analyses and wrote the manuscript; and Mingjing Wang helped in the analysis with constructive discussions.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
[1] Natesan, K. and J. H. Park. Fireside and steamside corrosion of alloys for USC plants. International Journal of Hydrogen Energy, Vol. 32, 2007, pp. 3689–3697.10.1016/j.ijhydene.2006.08.038Suche in Google Scholar
[2] Zhao, S., X. Xie, G. D. Smith, and S. J. Patel. The corrosion of INCONEL alloy 740 in simulated environments for pulverized coal-fired boiler. Materials Chemistry and Physics, Vol. 90, 2005, pp. 275–281.10.1016/j.matchemphys.2004.04.006Suche in Google Scholar
[3] Liu, D., L. Yan, and S. Hou. The evaluation of high temperature corrosion of the Inconel 740H in simulated coal-fired environments. Corrosion Science, Vol. 161, 2019, id. 108150.10.1016/j.corsci.2019.108150Suche in Google Scholar
[4] Stein-Brzozowska, G., D. M. Flórez, J. Maier, and G. Scheffknecht. Nickel-base superalloys for ultra-supercritical coal-fired power plants: Fireside corrosion. Laboratory studies and power plant exposures. Fuel, Vol. 108, 2013, pp. 521–533.10.1016/j.fuel.2012.11.081Suche in Google Scholar
[5] Han, T., C. Wang, R. Sun, C. Zhu, Y. Liu, and D. Che. Experimental study on ash deposition of Zhundong coal in oxy-fuel combustion. Journal of the Energy Institute, Vol. 92, 2019, pp. 1697–1709.10.1016/j.joei.2019.01.004Suche in Google Scholar
[6] Aung, N. N. and X. Liu. High temperature electrochemical sensor for in situ monitoring of hot corrosion. Corrosion Science, Vol. 65, 2012, pp. 1–4.10.1016/j.corsci.2012.08.010Suche in Google Scholar
[7] Zhang, P., Y. Yuan, Y. F. Gu, Y. Y. Dang, J. B. Yan, J. T. Lu, et al. Creep deformation behavior of a novel precipitate-hardened Ni-Fe-base superalloy at 750° C. Metallurgical and Materials Transactions A, Vol. 51, 2020, pp. 1062–1066.10.1007/s11661-019-05611-4Suche in Google Scholar
[8] Zhang, P., Y. Yuan, Y. F. Gu, J. B. Yan, Y. Y. Dang, J. T. Lu, et al. Investigation on the tensile deformation mechanisms in a new Ni–Fe-base superalloy HT700T at 750° C. Journal of Alloys and Compounds, Vol. 825, 2020, id. 154012.10.1016/j.jallcom.2020.154012Suche in Google Scholar
[9] Yan, J., Y. Gu, and J. Lu. On precipitates in Fe–Ni base alloys used for USC boilers. Materials Science and Technology, Vol. 31, 2015, pp. 389–399.10.1179/1743284714Y.0000000620Suche in Google Scholar
[10] Yan, J., Y. Gu, H. Li, F. Yang, Y. Yuan, P. Zhang, et al. Impact of aging temperature on the performance of a nickel–iron-based superalloy. Metallurgical and Materials Transactions A, Vol. 49, 2018, pp. 1561–1570.10.1007/s11661-018-4514-6Suche in Google Scholar
[11] Zhao, X., Y. Dang, H. Yin, Y. Yuan, J. Lu, Z. Yang, et al. Microstructural stability of long-term aged wrought Ni–Fe-based superalloys. Materials Science and Technology, Vol. 33, 2017, pp. 1252–1257.10.1080/02670836.2017.1282668Suche in Google Scholar
[12] Yang, Z., J. Lu, Z. Yang, Y. Li, Y. Yuan, and Y. Gu. Oxidation behavior of a new wrought Ni-30Fe-20Cr based alloy at 750°C in pure steam and the effects of alloyed yttrium. Corrosion Science, Vol. 125, 2017, pp. 106–113.10.1016/j.corsci.2017.06.009Suche in Google Scholar
[13] Lu, J., J. Huang, Z. Yang, Y. Zhou, Y. Dang, Y. Yuan, et al. Simulated fireside corrosion behavior of a wrought Ni-Fe-based superalloy for 700°C-class ultra-supercritical power plant applications. Journal of Materials Engineering and Performance, Vol. 28, 2019, pp. 7390–7397.10.1007/s11665-019-04515-zSuche in Google Scholar
[14] Lu, J. T., J. Y. Huang, Y. L. Zhou, Y. Y. Dang, Y. Yuan, and Y. F. Gu. Effect of cobalt content on the oxidation and corrosion behavior of Ni–Fe-based superalloy for ultra-supercritical boiler applications. Oxidation of Metals, Vol. 89, 2017, pp. 197–209.10.1007/s11085-017-9783-8Suche in Google Scholar
[15] Lu, J. Y., Z. Yang, J. Y. Huang, X. B. Zhao, and Y. Yuan. Effect of alloying chemistry on fireside corrosion behavior of Ni–Fe-based superalloy for ultra-supercritical boiler applications. Oxidation of Metals, Vol. 89, 2017, pp. 609–621.10.1007/s11085-017-9804-7Suche in Google Scholar
[16] Yuan, Y., Y. Dang, Z. Yang, P. Zhang, H. Yin, J. Huang, et al. Microstructure and properties of Ni-Fe-base superalloy for 700°C advanced Ultra Supercritical Unit Final Superheater. Materials for Mechanical Engineering, Vol. 44, 2020, pp. 44–50.Suche in Google Scholar
[17] Yang, Z., J. T. Lu, Y. Yuan, X. B. Zhao, Y. F. Gu, H. F. Yin, et al. Study on the oxidation behavior of high temperature alloy GH2984 in water vapor at 750°C. Rare Metal Materials and Engineering, Vol. 46, 2017, pp. 1013–1019.Suche in Google Scholar
[18] Dudziak, T., T. Hussain, D. Orlicka, A. Pokrywa, and N. J. Simms. Fireside corrosion degradation of 15Mo3, T22, T23 & T91 in simulated coal-biomass co-fired environment. Materials and Corrosion, Vol. 66, 2015, pp. 839–850.10.1002/maco.201407886Suche in Google Scholar
[19] Lu, J. T., Y. F. Gu, and Z. Yang. Corrosion behavior of coal ash from three alternative high temperature alloys for 700°C class ultra-supercritical coal-fired boilers. Corrosion Science and Protection Technology, Vol. 26, 2014, pp. 205–210.Suche in Google Scholar
[20] Xiong, X., Z. Lv, H. Tan, and B. Wei. A typical super-heater tube leakage and high temperature corrosion mechanism investigation in a 260 t/h circulated fluidized boiler. Engineering Failure Analysis, Vol. 109, 2020, id. 104255.10.1016/j.engfailanal.2019.104255Suche in Google Scholar
[21] Castello, P., V. Guttmann, N. Farr, and G. Smith. Laboratory-simulated fuel-ash corrosion of superheater tubes in coal-fired ultra-supercritical-boilers. Materials and Corrosion, Vol. 51, 2000, pp. 786–790.10.1002/1521-4176(200011)51:11<786::AID-MACO786>3.0.CO;2-MSuche in Google Scholar
[22] Lloyd, A. C., J. J. Noël, S. McIntyre, and D. W. Shoesmith. Cr, Mo and W alloying additions in Ni and their effect on passivity. Electrochimica Acta, Vol. 49, 2004, pp. 3015–3027.10.1016/j.electacta.2004.01.061Suche in Google Scholar
[23] Li, M. S. High temperature corrosion of metals, Metallurgical Industry Press, Beijing, 2001.Suche in Google Scholar
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- First-principles investigation of phase stability and elastic properties of Laves phase TaCr2 by ruthenium alloying
- Improvement and prediction on high temperature melting characteristics of coal ash
- First-principles calculations to investigate the thermal response of the ZrC(1−x)Nx ceramics at extreme conditions
- Study on the cladding path during the solidification process of multi-layer cladding of large steel ingots
- Thermodynamic analysis of vanadium distribution behavior in blast furnaces and basic oxygen furnaces
- Comparison of data-driven prediction methods for comprehensive coke ratio of blast furnace
- Effect of different isothermal times on the microstructure and mechanical properties of high-strength rebar
- Analysis of the evolution law of oxide inclusions in U75V heavy rail steel during the LF–RH refining process
- Simultaneous extraction of uranium and niobium from a low-grade natural betafite ore
- Transfer and transformation mechanism of chromium in stainless steel slag in pedosphere
- Effect of tool traverse speed on joint line remnant and mechanical properties of friction stir welded 2195-T8 Al–Li alloy joints
- Technology and analysis of 08Cr9W3Co3VNbCuBN steel large diameter thick wall pipe welding process
- Influence of shielding gas on machining and wear aspects of AISI 310–AISI 2205 dissimilar stainless steel joints
- Effect of post-weld heat treatment on 6156 aluminum alloy joint formed by electron beam welding
- Ash melting behavior and mechanism of high-calcium bituminous coal in the process of blast furnace pulverized coal injection
- Effect of high temperature tempering on the phase composition and structure of steelmaking slag
- Numerical simulation of shrinkage porosity defect in billet continuous casting
- Influence of submerged entry nozzle on funnel mold surface velocity
- Effect of cold-rolling deformation and rare earth yttrium on microstructure and texture of oriented silicon steel
- Investigation of microstructure, machinability, and mechanical properties of new-generation hybrid lead-free brass alloys
- Soft sensor method of multimode BOF steelmaking endpoint carbon content and temperature based on vMF-WSAE dynamic deep learning
- Mechanical properties and nugget evolution in resistance spot welding of Zn–Al–Mg galvanized DC51D steel
- Research on the behaviour and mechanism of void welding based on multiple scales
- Preparation of CaO–SiO2–Al2O3 inorganic fibers from melting-separated red mud
- Study on diffusion kinetics of chromium and nickel electrochemical co-deposition in a NaCl–KCl–NaF–Cr2O3–NiO molten salt
- Enhancing the efficiency of polytetrafluoroethylene-modified silica hydrosols coated solar panels by using artificial neural network and response surface methodology
- High-temperature corrosion behaviours of nickel–iron-based alloys with different molybdenum and tungsten contents in a coal ash/flue gas environment
- Characteristics and purification of Himalayan salt by high temperature melting
- Temperature uniformity optimization with power-frequency coordinated variation in multi-source microwave based on sequential quadratic programming
- A novel method for CO2 injection direct smelting vanadium steel: Dephosphorization and vanadium retention
- A study of the void surface healing mechanism in 316LN steel
- Effect of chemical composition and heat treatment on intergranular corrosion and strength of AlMgSiCu alloys
- Soft sensor method for endpoint carbon content and temperature of BOF based on multi-cluster dynamic adaptive selection ensemble learning
- Evaluating thermal properties and activation energy of phthalonitrile using sulfur-containing curing agents
- Investigation of the liquidus temperature calculation method for medium manganese steel
- High-temperature corrosion model of Incoloy 800H alloy connected with Ni-201 in MgCl2–KCl heat transfer fluid
- Investigation of the microstructure and mechanical properties of Mg–Al–Zn alloy joints formed by different laser welding processes
- Effect of refining slag compositions on its melting property and desulphurization
- Effect of P and Ti on the agglomeration behavior of Al2O3 inclusions in Fe–P–Ti alloys
- Cation-doping effects on the conductivities of the mayenite Ca12Al14O33
- Modification of Al2O3 inclusions in SWRH82B steel by La/Y rare-earth element treatment
- Possibility of metallic cobalt formation in the oxide scale during high-temperature oxidation of Co-27Cr-6Mo alloy in air
- Multi-source microwave heating temperature uniformity study based on adaptive dynamic programming
- Round-robin measurement of surface tension of high-temperature liquid platinum free of oxygen adsorption by oscillating droplet method using levitation techniques
- High-temperature production of AlN in Mg alloys with ammonia gas
- Review Article
- Advances in ultrasonic welding of lightweight alloys: A review
- Topical Issue on High-temperature Phase Change Materials for Energy Storage
- Compositional and thermophysical study of Al–Si- and Zn–Al–Mg-based eutectic alloys for latent heat storage
- Corrosion behavior of a Co−Cr−Mo−Si alloy in pure Al and Al−Si melt
- Al–Si–Fe alloy-based phase change material for high-temperature thermal energy storage
- Density and surface tension measurements of molten Al–Si based alloys
- Graphite crucible interaction with Fe–Si–B phase change material in pilot-scale experiments
- Topical Issue on Nuclear Energy Application Materials
- Dry synthesis of brannerite (UTi2O6) by mechanochemical treatment
- Special Issue on Polymer and Composite Materials (PCM) and Graphene and Novel Nanomaterials - Part I
- Heat management of LED-based Cu2O deposits on the optimal structure of heat sink
- Special Issue on Recent Developments in 3D Printed Carbon Materials - Part I
- Porous metal foam flow field and heat evaluation in PEMFC: A review
- Special Issue on Advancements in Solar Energy Technologies and Systems
- Research on electric energy measurement system based on intelligent sensor data in artificial intelligence environment
- Study of photovoltaic integrated prefabricated components for assembled buildings based on sensing technology supported by solar energy
- Topical Issue on Focus of Hot Deformation of Metaland High Entropy Alloys - Part I
- Performance optimization and investigation of metal-cored filler wires for high-strength steel during gas metal arc welding
- Three-dimensional transient heat transfer analysis of micro-plasma arc welding process using volumetric heat source models
Artikel in diesem Heft
- Research Articles
- First-principles investigation of phase stability and elastic properties of Laves phase TaCr2 by ruthenium alloying
- Improvement and prediction on high temperature melting characteristics of coal ash
- First-principles calculations to investigate the thermal response of the ZrC(1−x)Nx ceramics at extreme conditions
- Study on the cladding path during the solidification process of multi-layer cladding of large steel ingots
- Thermodynamic analysis of vanadium distribution behavior in blast furnaces and basic oxygen furnaces
- Comparison of data-driven prediction methods for comprehensive coke ratio of blast furnace
- Effect of different isothermal times on the microstructure and mechanical properties of high-strength rebar
- Analysis of the evolution law of oxide inclusions in U75V heavy rail steel during the LF–RH refining process
- Simultaneous extraction of uranium and niobium from a low-grade natural betafite ore
- Transfer and transformation mechanism of chromium in stainless steel slag in pedosphere
- Effect of tool traverse speed on joint line remnant and mechanical properties of friction stir welded 2195-T8 Al–Li alloy joints
- Technology and analysis of 08Cr9W3Co3VNbCuBN steel large diameter thick wall pipe welding process
- Influence of shielding gas on machining and wear aspects of AISI 310–AISI 2205 dissimilar stainless steel joints
- Effect of post-weld heat treatment on 6156 aluminum alloy joint formed by electron beam welding
- Ash melting behavior and mechanism of high-calcium bituminous coal in the process of blast furnace pulverized coal injection
- Effect of high temperature tempering on the phase composition and structure of steelmaking slag
- Numerical simulation of shrinkage porosity defect in billet continuous casting
- Influence of submerged entry nozzle on funnel mold surface velocity
- Effect of cold-rolling deformation and rare earth yttrium on microstructure and texture of oriented silicon steel
- Investigation of microstructure, machinability, and mechanical properties of new-generation hybrid lead-free brass alloys
- Soft sensor method of multimode BOF steelmaking endpoint carbon content and temperature based on vMF-WSAE dynamic deep learning
- Mechanical properties and nugget evolution in resistance spot welding of Zn–Al–Mg galvanized DC51D steel
- Research on the behaviour and mechanism of void welding based on multiple scales
- Preparation of CaO–SiO2–Al2O3 inorganic fibers from melting-separated red mud
- Study on diffusion kinetics of chromium and nickel electrochemical co-deposition in a NaCl–KCl–NaF–Cr2O3–NiO molten salt
- Enhancing the efficiency of polytetrafluoroethylene-modified silica hydrosols coated solar panels by using artificial neural network and response surface methodology
- High-temperature corrosion behaviours of nickel–iron-based alloys with different molybdenum and tungsten contents in a coal ash/flue gas environment
- Characteristics and purification of Himalayan salt by high temperature melting
- Temperature uniformity optimization with power-frequency coordinated variation in multi-source microwave based on sequential quadratic programming
- A novel method for CO2 injection direct smelting vanadium steel: Dephosphorization and vanadium retention
- A study of the void surface healing mechanism in 316LN steel
- Effect of chemical composition and heat treatment on intergranular corrosion and strength of AlMgSiCu alloys
- Soft sensor method for endpoint carbon content and temperature of BOF based on multi-cluster dynamic adaptive selection ensemble learning
- Evaluating thermal properties and activation energy of phthalonitrile using sulfur-containing curing agents
- Investigation of the liquidus temperature calculation method for medium manganese steel
- High-temperature corrosion model of Incoloy 800H alloy connected with Ni-201 in MgCl2–KCl heat transfer fluid
- Investigation of the microstructure and mechanical properties of Mg–Al–Zn alloy joints formed by different laser welding processes
- Effect of refining slag compositions on its melting property and desulphurization
- Effect of P and Ti on the agglomeration behavior of Al2O3 inclusions in Fe–P–Ti alloys
- Cation-doping effects on the conductivities of the mayenite Ca12Al14O33
- Modification of Al2O3 inclusions in SWRH82B steel by La/Y rare-earth element treatment
- Possibility of metallic cobalt formation in the oxide scale during high-temperature oxidation of Co-27Cr-6Mo alloy in air
- Multi-source microwave heating temperature uniformity study based on adaptive dynamic programming
- Round-robin measurement of surface tension of high-temperature liquid platinum free of oxygen adsorption by oscillating droplet method using levitation techniques
- High-temperature production of AlN in Mg alloys with ammonia gas
- Review Article
- Advances in ultrasonic welding of lightweight alloys: A review
- Topical Issue on High-temperature Phase Change Materials for Energy Storage
- Compositional and thermophysical study of Al–Si- and Zn–Al–Mg-based eutectic alloys for latent heat storage
- Corrosion behavior of a Co−Cr−Mo−Si alloy in pure Al and Al−Si melt
- Al–Si–Fe alloy-based phase change material for high-temperature thermal energy storage
- Density and surface tension measurements of molten Al–Si based alloys
- Graphite crucible interaction with Fe–Si–B phase change material in pilot-scale experiments
- Topical Issue on Nuclear Energy Application Materials
- Dry synthesis of brannerite (UTi2O6) by mechanochemical treatment
- Special Issue on Polymer and Composite Materials (PCM) and Graphene and Novel Nanomaterials - Part I
- Heat management of LED-based Cu2O deposits on the optimal structure of heat sink
- Special Issue on Recent Developments in 3D Printed Carbon Materials - Part I
- Porous metal foam flow field and heat evaluation in PEMFC: A review
- Special Issue on Advancements in Solar Energy Technologies and Systems
- Research on electric energy measurement system based on intelligent sensor data in artificial intelligence environment
- Study of photovoltaic integrated prefabricated components for assembled buildings based on sensing technology supported by solar energy
- Topical Issue on Focus of Hot Deformation of Metaland High Entropy Alloys - Part I
- Performance optimization and investigation of metal-cored filler wires for high-strength steel during gas metal arc welding
- Three-dimensional transient heat transfer analysis of micro-plasma arc welding process using volumetric heat source models

