Abstract
Blast furnace slag and steelmaking slag, as the main accessory products of iron and steel smelting, are piled up in large quantities due to their huge output, high treatment difficulty and low comprehensive utilization rate, which has a serious impact on the land and environment. In order to improve the comprehensive utilization of steelmaking slag, low basicity blast furnace slag was added to the existing steel slag for quenching and tempering. The influence of basicity on the chemical composition and phase precipitation of mixed slag was analyzed. In the research process, the phase composition and morphology of blast furnace slag and steel slag of Baotou Steel were analyzed using FactSage7.1 thermodynamic calculation software, ZEISS high-resolution scanning electron microscope (SEM), modern fast high-resolution Bruker energy dispersive spectrometer and AMICS-Mining automatic mineral analysis software. The results show that the mineral phase composition of blast furnace slag is mainly calcium aluminum yellow feldspar and that of steelmaking slag is mainly dicalcium silicate(C2S), magnesium-iron phase solid solution, rose pyroxene and calcium iron aluminate. When the basicity of the mixed slag is 2.0, it can effectively inhibit the formation of non-cementitious mineral anorthite and promote the formation of better cementitious mineral C2S. At the same time, it is found that the melting temperature of mixed slag decreases with the increase in Al2O3 content.
1 Introduction
Steelmaking slag, as a subsidiary product of steelmaking, is an aggregate composed of various minerals and glassy substances [1]. Steelmaking slag shows different shapes due to different cool modes, low basicity steelmaking slag is gray, and high basicity steelmaking slag is brownish gray or off-white [2,3,4]. The structure of slag lump and slag shell is compact, the interface is clear, and the port is neat because of the serious vitrification of the surface layer of the quenched steelmaking slag [5]. Aging treatment [6,7,8,9,10] can effectively improve the stability of steelmaking slag and reduce the content of f-CaO in steelmaking slag. The chemical composition of steelmaking slag and Portland cement clinker is very similar to that of Portland cement clinker, and the main chemical compositions are CaO, SiO2, MgO, Fe2O3, MnO, Al2O3, and P2O5 [11,12,13]. In addition, steelmaking slag contains a small amount of sulfide and oxide [14]. CaO is one of the main components of steelmaking slag. The quantity of calcium silicate mineral is mainly determined by SiO2, and the activity of steelmaking slag is mainly determined by the content of Al2O3 [15], which mainly forms calcium aluminate or calcium aluminosilicate glass. There are three main forms of MgO [16,17], namely calcium magnesium olivine, magnesium rose pyroxene and other combined forms, RO phase solid solution, and free state magnesite, of which the combined form has good stability [18,19]. P2O5 has dual properties. When its content is low, it can promote the formation of silicate minerals [20]. When the content is high, P2O5 reacts with CaO and SiO2 in the steel slag to form sodium masmitite (7CaOP2O5·2SiO2 and 2C2S-C3P), which inhibits the formation of cementitious minerals C3S and C2S. The main mineral phases of steelmaking slag are C3S, C2S and RO. The mineral composition mainly depends on chemical composition and basicity. Steelmaking slag has good cementitious properties. Many researchers have done a lot of research on the structure and composition of steelmaking slag and the comprehensive utilization of steelmaking slag in cement and concrete. Rao [21] adjusted the basicity (2.0–3.0) and Fe2O3 (about 20%) of the steelmaking slag with quartz sand and coal dust. The results showed that the modified steelmaking slag f-CaO mass fraction was reduced by 39.6%, the ease of grinding index was increased by 11%, and the 7 and 28 days activity indices were increased by 3 and 4.8%, respectively. Zhang et al. [22] investigated the effect of iron tailings on the properties of high-temperature steelmaking slag. The results show that iron tailings can promote the improvement of steelmaking slag cementation, while the f-CaO content in steelmaking slag is reduced and the stability of steelmaking slag is improved. Liu et al. [23] studied the effect of blast furnace slag on the physical phase of steelmaking slag. The results showed that at 1,550°C, 10% blast furnace slag modified steelmaking slag, the mass fraction of C2S and C3S in slag increased significantly, f-CaO decreased to 1.64%, and the stability of steelmaking slag improved; in addition, coke reduced the iron in slag and improved the ease of grinding of steelmaking slag. Xiang [24] studied the preparation and foaming modification of reconstructed steelmaking slag powder with medium and high activity. The results showed that 75% converter steelmaking slag, 4% bauxite and 21% lime, fired at 1,290°C for 90 min, had the highest C2S and C4AF generation after air-cooled rapid cooling, increased water activity to 90.4%, reduced f-CaO mass fraction by 2.03%, dissipated RO phase, and increased slag ease of grinding. It was also demonstrated that the best performance of porous reconstituted steelmaking slag was achieved at a high-temperature foaming agent SiC doping of 1.6%. The activity index of the modified steelmaking slag can be increased to 98.2%, and the compressive strength of the composite cement mortar can reach 44.8 MPa. Wang et al. [25] analyzed the main components of fly ash and their role in steelmaking slag, and the results showed that SiO2 and Al2O3 in fly ash react with f-CaO to generate stable phases, which improve the stability of steelmaking slag, while generating calcium silicate and calcium aluminate. Lei et al. [26] studied the effect of fly ash on steelmaking slag in f-CaO. The results show that the admixture of fly ash can reduce the f-CaO content in steelmaking slag. Li et al. [27] analyzed the effect of electric furnace slag on the properties of steelmaking slag. The results showed that the electric furnace slag can promote the improvement of steelmaking slag coagulation. Zhang [28] studied the carbonation mechanism of zeolite-modified steelmaking slag products. The results showed that after pre-hydration curing for 1 day and carbonation for 2 h, the steelmaking slag test block with 5% zeolite (CSZ5-1 d) had the best compressive strength, and the steelmaking slag test block with 15% zeolite (CSZ15-1 d) had the best carbonation rate, which increased by 14 and 10.2% respectively compared with the pure steelmaking slag test block.
Most of the above research is to pure reagents or reducing agents to steelmaking slag reduction modification treatment, although the effect is obvious, most of the treatment methods are of high cost and cannot reach a large number of production practice applications. The blast furnace slag [29,30] is also the most solid waste resource; its basicity is around 1.0 and contains high content of Al2O3, which can be used as an effective modifier to effectively reduce the basicity of steelmaking slag, stimulate the activity of steelmaking slag and achieve the goal of “treating waste with waste.” However, the experimental study on the modification of steelmaking slag by high-temperature melting of blast furnace slag is relatively few, and the phase composition and structure of steelmaking slag modified by blast furnace slag are not clear. In this article, under the premise of “comprehensive utilization of solid waste,” we propose the use of water-quenched blast furnace slag to modify steelmaking slag, adjust the basicity and physical composition of steelmaking slag, eliminate the unstable factor of steelmaking slag and improve the gelation activity. It provides theoretical basis and experimental data support for the modification of steelmaking slag by blast furnace slag.
2 Experimental materials and research methods
2.1 Experimental material
The steelmaking slag and blast furnace slag used in the test were taken from Baogang Steel, and their chemical composition was tested and analyzed using Physical and Chemical Testing Center of Baogang Rare Earth Research Institute. The results are shown in Tables 1 and 2. The content of SiO2 and Al2O3 in the blast furnace slag is relatively high, and a small amount of CaF2 and REO are contained. The content of TFe and P2O5 in steelmaking slag is high, and the content of SiO2 is low.
Main chemical composition of Baogang blast furnace slag wt%
| TFe | FeO | SiO2 | CaF2 | S | K2O | Na2O | CaO | MgO | MnO | Al2O3 | TiO2 | REO |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.36 | 0.69 | 31.58 | 0.94 | 1.27 | 0.35 | 0.46 | 36.72 | 9.28 | 0.44 | 14.79 | <0.05 | 0.66 |
Main chemical composition of Baogang steelmaking slag wt%
| TFe | FeO | CaO | SiO2 | MgO | P2O5 | S | Al2O3 | MnO |
|---|---|---|---|---|---|---|---|---|
| 27.32 | 26.02 | 33.67 | 13.49 | 7.36 | 1.95 | 0.09 | 1.86 | 4.92 |
The important phases of blast furnace slag and steelmaking slag were analyzed using XRD, and the detection results are shown in Figure 1. It can be seen from Figure 1(a) and Table 1 that the main phase in the blast furnace slag is calcium aluminum melilite. According to the analysis of Table 2 and Figure 1(b), in the X-ray diffraction pattern of steelmaking slag of Baogang Steel, the diffraction angles 2θ are 32.420 and 33.067°, corresponding to the high diffraction peaks of crystal plane spacing d value of 0.276 nm and 0.271 nm. Compared with C2S standard ICSD card #86-0399, it is believed that the main mineral in the steelmaking slag of Baogang Steel is dicalcium silicate(C2S). The diffraction angles 2θ in XRD are 36.048, 41.904, 60.844°, and the corresponding interplanar spacings d values are 0.245, 0.215, 0.152 nm; the peak surface is wider, and there are strong characteristic peaks of RO phase. Compared with the standard card (ICSD card #89-0689), it is considered that the RO phase is mainly MgO·2FeO. The diffraction angles 2θ are 33.307 and 46.512°, corresponding to low-intensity diffraction peaks with crystal plane spacing of 0.269 and 0.195 nm. Compared with the standard card (ICSD card #70-3651), the diffraction peak is considered to be Ca2(AlFe)2O5, indicating that there is a small amount of calcium ferroaluminate in the steelmaking slag. Combined with the analysis of the chemical composition of the steelmaking slag, the content of Al2O3 in the steelmaking slag is low, and the analysis shows that the activity of the steelmaking slag is relatively low. The diffraction angles 2θ are 29.932, 35.238, and 62.358°, and the corresponding interplanar spacings d values are 0.298, 0.255, and 0.149 nm, respectively. Compared with the standard card (ICSD card #19-0629), the diffraction peak is Fe3O4. Other diffraction peaks have lower intensities and narrower peak areas, such as Ca3MgSi2O8(C3MS2) and Ca3SiO5(C3S).

X-ray diffraction pattern: (a) Baogang high furnace slag; (b) Baogang steelmaking slag.
At the same time, the phase composition and morphology of Baogang steelmaking slag were analyzed by ZEISS high-resolution SEM, modern fast high-resolution Bruker energy spectrometer, and AMICS Mining automatic mineral analysis software. The phase composition and content of Baogang steelmaking slag are shown in Table 3.
Phase composition of Steelmaking slag of Baogang Steel wt%
| Fe | Fe2O3 | Ca3MgSi2O8 | MgO·2FeO | Ca2SiO4 | Ca2(Al,Fe)2O5 | Ca2Al2SiO7 | Other |
|---|---|---|---|---|---|---|---|
| 14.77 | 1.56 | 24.09 | 20.60 | 23.57 | 7.94 | 0.76 | 6.71 |
The microstructure, content and distribution of various elements of the Baogang Steelmaking slag sample are shown in Figure 2, and its chemical composition is shown in Table 4. According to energy dispersive X-ray spectroscopy (EDS) analysis, the steelmaking slag of Baogang Steel mainly has four different color phases: dark gray phase, light gray phase, gray phase and white phase. It can be seen from Figure 2 and Table 4 that the phosphorus element in the steelmaking slag is enriched in the mineral phase C2S where point B is located, and point A is RO phase with FeO as matrix and MgO and MnO dissolved in solid solution. Points B1–B3 are dark gray phases, distributed in the steelmaking slag, and part of them are irregular. It is judged that they are dicalcium silicate (C2S) phase with a small amount of tricalcium phosphate (Ca3(PO4)2) dissolved. Points A1–A3 are gray oxide continuous solid solution (RO phase), containing Fe, Mg, Mn and other elements, continuously extending in dark gray phase and black phase, showing irregular shape. At point C is a gray phase yellow feldspar containing Ca, Si, O, Mg, Fe and other elements.

Morphology and EDS analysis of slag backscatter of Baogang Steel (A – MgO·2FeO; B – Ca2SiO4; A1–A3 – MgO·2FeO; B1–B3 – Ca2SiO3; C – Ca2Al2SiO7).
Each phase and element content of Baogang steelmaking slag wt%
| Serial number | Ca | Si | O | P | Fe | Mn | Mg | Al | Phase |
|---|---|---|---|---|---|---|---|---|---|
| A | 4.31 | 0.30 | 27.52 | 0.08 | 53.49 | 7.67 | 5.00 | 1.26 | MgO·2FeO |
| B | 48.65 | 10.98 | 35.83 | 2.85 | 0.78 | 0.14 | 0.09 | 0.08 | Ca2SiO4 |
| A1 | 1.65 | 0.01 | 22.82 | 0.04 | 55.18 | 7.80 | 11.27 | 0 | MgO·2FeO |
| A2 | 1.67 | 0.04 | 22.75 | 0 | 51.81 | 8.01 | 14.49 | 0 | MgO·2FeO |
| A3 | 1.23 | 0.10 | 27.85 | 0.13 | 29.82 | 6.93 | 32.29 | 0 | MgO·2FeO |
| B1 | 48.30 | 13.36 | 32.02 | 2.83 | 1.55 | 0.53 | 0.72 | 0 | Ca2SiO4 |
| B2 | 51.85 | 12.17 | 30.78 | 1.98 | 0.97 | 0.26 | 0.36 | 0.16 | Ca2SiO4 |
| B3 | 46.81 | 12.08 | 35.77 | 2.77 | 1.17 | 0.24 | 0.28 | 0.13 | Ca2SiO4 |
| C | 28.48 | 5.27 | 28.21 | 1.03 | 26.84 | 3.85 | 3.17 | 1.26 | Ca2Al2SiO7 |
2.2 Methodology
Steelmaking slag and blast furnace slag of Baogang steel were crushed and screened repeatedly to obtain powder materials (less than 0.074 mm), drying the raw materials in an electrothermal constant -temperature blast drying oven at 105°C for 2 h. The prepared raw materials were placed into a ball mill tank, mixed on the ball mill for 2 h, and then pressed into a cylinder with a diameter of d15 mm × h6 mm by a tablet press under a pressure of 6 MPa. Placing into a corundum crucible and a KTF-1700-VT high-temperature vertical furnace, heating from room temperature to 1,000°C at a heating rate of 5°C·min−1, heating from 1,000 to 1,450°C with a heating system of 8°C·min−1, keeping the temperature for 1 h, and naturally cooling along with the furnace. The detection method is as follows: first, the apparent morphology of the samples after natural cooling is photographed to evaluate the self-pulverization of the samples after roasting and cooling in the furnace. Second, D8-Advanced X-ray diffractometer(Germany Brock, Germany) was used for the XRD test. Cu-kα target was used. Scanning type is follows: emitter–detection linkage, scanning voltage of 20 kV, and scanning current of 5 mA. The scanning range was 20–80°, and the scanning speed was 2°·min−1.
The blast furnace slag, steelmaking slag and MgO reagent of the Baogang Steel group were batched according to Table 5, and their chemical composition was shown in Table 6. With the help of Factsge7.1 thermodynamic software, the variation laws of crystallization temperature, precipitated phase and phase content of mixed slag system during the cooling process at 1,600°C cooling are simulated and calculated. The mixed slag samples were prepared, roasted and cooled. The changes of the appearance of the mixed slag samples after roasting were analyzed under the conditions of different basicities and different MgO contents. The mineral phase composition and structure of the slag samples were analyzed.
Ratio of mixed slag to batching
| Serial number | Basicity (R) | Steelmaking slag (wt%) | Blast furnace slag (wt%) |
|---|---|---|---|
| 1 | 1.6 | 53.33 | 46.67 |
| 2 | 1.8 | 68.19 | 31.81 |
| 3 | 2.0 | 79.80 | 20.20 |
| 4 | 2.2 | 89.17 | 10.86 |
| 5 | 2.4 | 96.79 | 3.21 |
Main chemical composition of mixed slag wt/%
| Basicity (R) | FeO | CaO | SiO2 | MgO | Al2O3 | Fe2O3 | P2O5 |
|---|---|---|---|---|---|---|---|
| 1.6 | 14.43 | 35.66 | 22.28 | 8.39 | 8.39 | 8.39 | 1.06 |
| 1.8 | 18.19 | 35.08 | 19.49 | 8.07 | 6.05 | 6.98 | 1.35 |
| 2.0 | 21.11 | 34.63 | 17.31 | 7.82 | 4.52 | 8.15 | 1.57 |
| 2.2 | 23.46 | 34.27 | 15.57 | 7.63 | 3.29 | 9.09 | 1.75 |
| 2.4 | 25.36 | 33.97 | 14.16 | 7.47 | 2.29 | 9.84 | 1.90 |
3 Results and analysis
3.1 Influence of basicity on microstructure of mixed slag
The X-ray diffraction pattern of the molten mixed slag of blast furnace slag and steelmaking slag after being roasted and cooled 1,450°C is shown in Figure 3. It can be seen from the figure that the main phase composition of the mixed slag is Ca2Al2SiO7, MgFe2O4, MgFeAlO4 and Ca2SiO4. The intensity of the diffraction peak will change after the mixed slag with different basicities is roasted and cooled. The main peak of the X-ray diffraction pattern of mixed slag (R = 1.6–2.4) after roasting and cooling is non-gel phase gehlenite (Ca2Al2SiO7 and C2AS), which is a kind of stone group mineral and belongs to plagioclase. Comparing the diffraction angles of XRD patterns of five different basicities, it is found that the diffraction peak intensity of gehlenite decreases gradually with the increase of basicity.

XRD Patterns of mixed slag with different basicities.
When the basicity of mixed slag is 1.6–2.0 and 2θ = 33.034°, the diffraction peak intensity of Ca2SiO4 (C2S) changes from weak to strong with the increase of basicity. When the basicity of mixed slag is between 2.0–2.4 and 2θ = 33.034°, the diffraction peak intensity of C2S changes from strong to weak with the increase of basicity. There are a few diffraction peaks of MgFe2O4 and spinel MgFeAlO4 in the XRD, but the intensity of diffraction peaks does not change significantly with the change of basicity.
There are some differences between XRD mineral composition of mixed slag and thermodynamic simulation results under an equilibrium state. The reason is that the cooling conditions are different. Thermodynamic calculation can accurately calculate the cooling rate and the phase composition of the corresponding temperature equilibrium state, while the experimental study corresponds to the non-equilibrium state with furnace cooling. Comparative analysis shows that controlling the cooling rate is beneficial to the crystallization of solid solution (including spinel), α′-C2S and C2SP. Natural cooling is beneficial to the phase crystallization of calcium aluminite, magnesium ferrite and spinel. Therefore, it is expected to form and precipitate more α′-C2S and C2SP crystals by controlling the cooling regime.
The situation of the molten mixed slag of blast furnace slag and steelmaking slag after baking and cooling in the furnace is shown in Figure 4 (the crucible is cracked after cooling). It can be seen from the figure that when the basicity is between 1.6 and 2.4, the mixed slag after high-temperature roasting is in the form of a molten block, which is bonded with the wall of the corundum crucible without pulverization.

Roasting of mixed slag with different basicities at 1,450°C: (a) R = 1.6; (b) R = 1.8; (c) R = 2.0; (d) R = 2.2; (e) R = 2.4.
The SEM picture of the mixed slag baking cooling sample is shown in Figure 5. The composition of each phase of the mixed residue sample was analyzed using EDS analyzer, and the detection results of element content are shown in Table 7. According to Figure 5, there are five main mineral structures in the mixed slag. The white area (point A) is MgFe2O4 phase, the gray-black area (point B) is calcium-aluminite phase (C2AS), the gray area (point C) is phosphorus-rich C2S phase, and the light gray area (point D) is spinel phase and (point E) calcium ferrite phase.
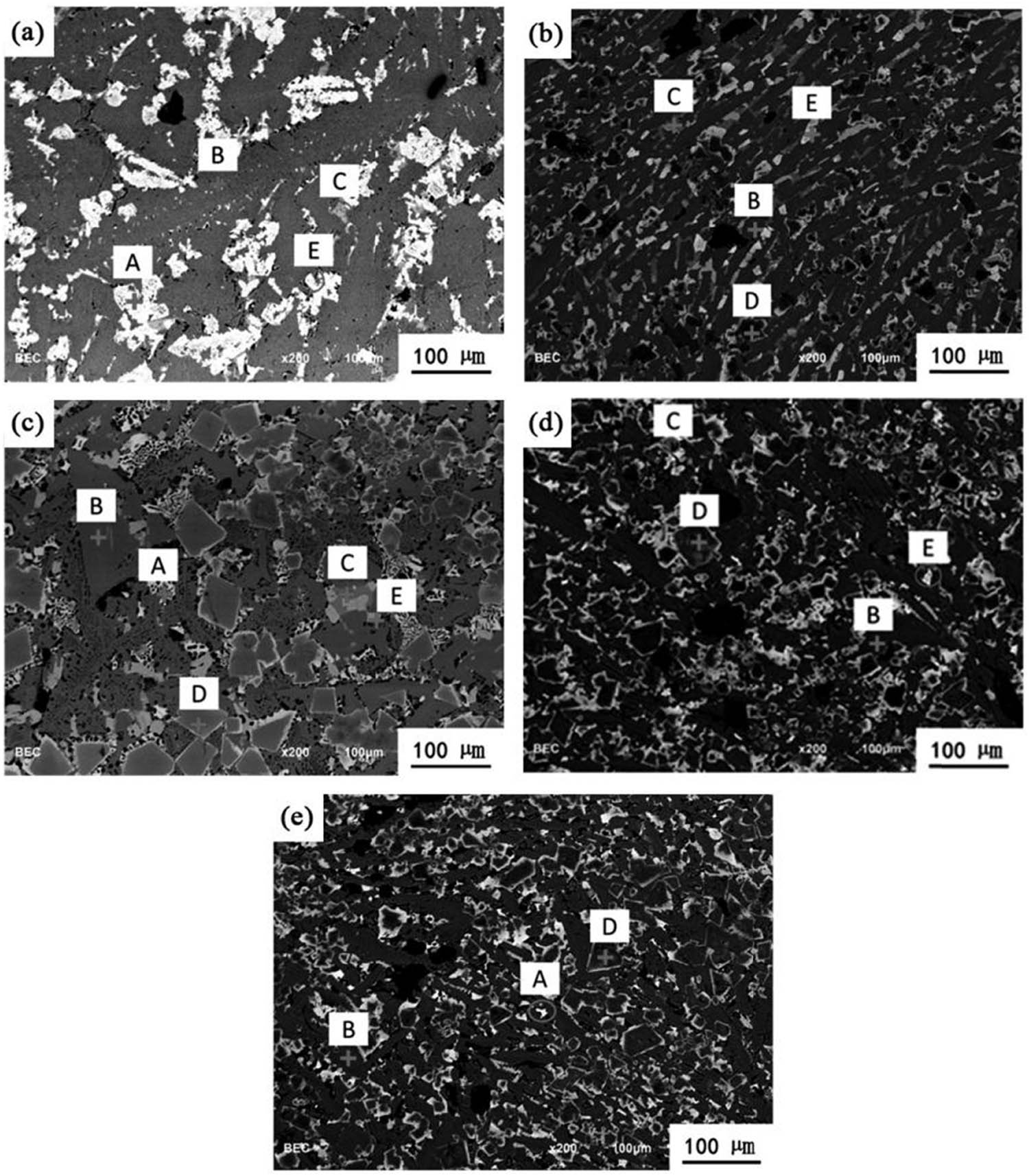
SEM mineral phase composition of mixed slag (A – MgFe2O4; B – Ca2Al2SiO7; C – Ca2SiO4-Ca3(PO4)2; D – MgFeAlO4; E – Ca2Fe2O5: (a) R = 1.6; (b) R = 1.8; (c) R = 2.0; (d) R = 2.2; (e) R = 2.4).
Element content in each phase of mixed slag with different basicity wt%
| Basicity (R) | Serial number | Ca | Si | O | P | Fe | Mn | Mg | Al |
|---|---|---|---|---|---|---|---|---|---|
| R = 1.6 | A | 2.17 | 1.22 | 24.07 | 0.36 | 52.50 | 11.48 | 5.92 | 1.30 |
| B | 29.98 | 15.18 | 28.24 | 0.41 | 5.45 | 0.25 | 1.70 | 17.74 | |
| C | 40.25 | 7.95 | 27.17 | 13.55 | 2.12 | 0.68 | 0.91 | 0.94 | |
| E | 15.86 | 4.55 | 25.98 | 1.35 | 15.87 | 1.92 | 1.61 | 1.58 | |
| R = 1.8 | B | 29.98 | 14.03 | 28.10 | 0.21 | 5.43 | 0.62 | 0.65 | 19.16 |
| C | 46.29 | 4.99 | 23.58 | 15.33 | 1.34 | 0.34 | 0.18 | 0.24 | |
| D | 0.32 | 0.04 | 28.23 | 0.31 | 25.50 | 3.57 | 15.22 | 23.62 | |
| E | 15.97 | 4.73 | 26.43 | 1.34 | 15.97 | 1.88 | 1.05 | 1.84 | |
| R = 2.0 | A | 2.21 | 1.44 | 23.60 | 0.44 | 50.54 | 11.43 | 7.65 | 1.18 |
| B | 28.82 | 13.82 | 31.01 | 0.07 | 8.54 | 0.33 | 1.28 | 15.79 | |
| C | 47.01 | 5.98 | 24.38 | 15.23 | 1.28 | 0.35 | 0.19 | 0.22 | |
| D | 0.32 | 0 | 28.86 | 0.15 | 26.34 | 3.87 | 15.11 | 23.93 | |
| E | 16.49 | 6.00 | 27.82 | 0.93 | 18.49 | 2.18 | 0.63 | 1.47 | |
| R = 2.2 | B | 29.34 | 13.78 | 29.00 | 0.26 | 7.32 | 0.19 | 0.77 | 18.99 |
| C | 46.65 | 6.06 | 23.98 | 14.88 | 1.26 | 0.34 | 0.79 | 0.24 | |
| D | 0.33 | 0.08 | 28.05 | 0.41 | 24.76 | 3.76 | 15.72 | 25.16 | |
| E | 17.15 | 2.24 | 26.84 | 0.63 | 17.48 | 6.05 | 2.67 | 1.33 | |
| R = 2.4 | A | 1.28 | 1.38 | 20.35 | 0.52 | 56.25 | 11.58 | 6.25 | 1.87 |
| B | 29.88 | 12.22 | 28.73 | 0.40 | 7.06 | 0.23 | 0.17 | 20.35 | |
| D | 0.38 | 0.05 | 28.02 | 0.38 | 25.68 | 3.42 | 15.03 | 26.18 |
The MgFe2O4 phase mainly contains iron and magnesium oxides and iron manganese oxides. The main elements of gehlenite are Ca, Al, Si and O. The phosphorus-rich phase mainly exists in the form of Ca7P2Si2O16(2C2S-C3P). In the slag, P2O5 reacts preferentially with CaO to form Ca3(PO4)2, which occurs in the C2S phase and inhibits the crystal transformation of C2S. With the increase of basicity of the mixed slag, the concentration of CaO and SiO2 changes, and the content of SiO2 and Al2O3 gradually decreases, so that the production of calcium aluminite gradually decreases. Fe2+, Fe3+ and Ca2+ produce calcium ferrite or calcium aluminate, which is gray and amorphous.
Spinel is composed of oxygen, magnesium, iron, aluminum, manganese and other elements of a mineral; its appearance is transparent color beauty and is used as a gem. In the SEM mineral phase diagram, MgFeAlO4 spinel shows massive crystals and contains trace Mn elements. Compared with the mixed slag phase diagram with a basicity of 1.6–1.8, the distribution of spinel in the mixed slag phase diagram with a basicity of 2.0–2.4 is more. It is considered that the spinel in the mixed slag phase increases with the increase in basicity.
In order to further determine the distribution of elements and mineral phases in the molten mixed slag of blast furnace slag and steelmaking slag with different basicities, the mixed slag roasted samples with basicity of 1.6 and 2.0 were scanned using SEM. The results are shown in Figure 6. According to Figure 6(1), a large amount of Mg, Fe, O and Mn elements cover the white area of point A in the mineral phase diagram of mixed slag with a basicity of 1.6, and area A is considered to be MgFe2O4 phase containing Mn element. A large amount of Ca, Al, Si and O elements are uniformly distributed in the gray and black area of point B, which is consistent with the element analysis result of point scan and is considered to be the Ca2Al2SiO7 phase. The gray area of point C contains a large number of Ca, O, and P elements and a small amount of Si elements, which is considered a P-rich phase C2S.

SEM scanning of mixed slag ((1) R = 1.6; (2) R = 2.0; (A) MgFe2O4; (B) Ca2Al2SiO7; (C) Ca2SiO4-Ca3(PO4)2; (D) MgFeAlO4; (E) Ca2Fe2O5).
Figure 6(2) shows that the light gray area of point D in the mineral phase diagram of the mixed slag with a basicity of 2.0 (lumpy crystal) contains a large amount of Mg, Fe, Al, O and trace Mn, which is considered to be MgFeAlO4, and there may be trace MnFeAlO4. The light gray area of point E is mainly distributed with Ca, Fe and O elements, mixed with trace P, Mn and Mg elements. Combined with the energy spectrum data in Table 7, point E is judged to be Ca2Fe2O5 phase. Therefore, it is believed that the qualitative results of phase are more accurate after XRD analysis, SEM point scanning and surface scanning analysis of the roasting samples with different basicities of blast furnace slag and steelmaking slag melting mixture.
Table 8 and Figure 7 are the results of composition and content calculated using binary method for roasted samples of molten slag and steelmaking slag with different basicities. Combined with the figure, it can be seen that with the increase of basicity from 1.6 to 2.4, the content of calcium-aluminite phase in the mixed slag decreases from 62.56 to 30.28%, the content of spinel phase increases from 4.82 to 27.13%. The content of the calcium ferrite phase increased from 9.35 to 14.63%, and the content of the magnesium ferrite phase increases from 5.29 to 5.91%. When the basicity increases from 1.6 to 2.0, the content of the dicalcium silicate phase increases from 12.59 to 13.67%. When the basicity increased from 2.0 to 2.4, the content of the dicalcium silicate phase decreased from 13.67 to 12.46%. It shows that the change of basicity of mixed slag will directly affect the content change of phase composition of mixed slag.
Test results of phase composition and content of mixed slag with Basicity R = 1.6–2.4 wt%
| Phase (mean) | Voids | Ca2Al2SiO7 | MgFeAlO4 | Ca2SiO4 | CaFe2O4 | MgFe2O4 |
|---|---|---|---|---|---|---|
| R = 1.6 | 5.39 | 62.56 | 4.82 | 12.59 | 9.35 | 5.29 |
| R = 1.8 | 7.20 | 57.13 | 8.29 | 12.65 | 9.42 | 5.31 |
| R = 2.0 | 10.96 | 37.16 | 23.16 | 13.67 | 9.59 | 5.46 |
| R = 2.2 | 12.72 | 33.85 | 24.28 | 12.71 | 10.80 | 5.64 |
| R = 2.4 | 9.59 | 30.28 | 27.13 | 12.46 | 14.63 | 5.91 |

Composition and content of roasting mixed slag with different basicities calculated by the binary method.
3.2 Influence of basicity on melting property of mixed slag
The equilibrium phase composition and melting temperature of mixed slag with different basicities of liquid module in FactSage7.1 were analyzed. The specific parameter Settings are shown in Table 9.
FactSage7.1 parameter settings
| The database | FToxid7.1, FactPS7.1 |
|---|---|
| Compound type | Monoxide |
| Solid solution | FToxide-SLAGA, FToxide-SPANA, FToxide-MeO-A, FToxide-cPyrA, FToxide-oPyr, FToxide-pPyrA, FToxide-LcPy, FToxide-WOLLA, FToxide-bC2S, FToxide-aC2S, FToxide-Mel, FToxide-OlivA |
3.2.1 Thermodynamic simulation of basicity on melting property of mixed slag
According to the batching ratio of mixed slags (R = 1.6–2.4) in Table 5, Factsage7.1 thermodynamic software was used to simulate the influence of different basicities on the melting temperature of mixed slags. The calculation results are shown in Table 10 and Figure 8. With the increase of basicity, the initial melting temperature shows a trend of first increasing and then decreasing. The analysis shows that the content of steelmaking slag increases with the increase in basicity. However, TFe in steelmaking slag reacts with CaO and SiO2 in slag to form calcium and iron olivine (CaO·FeO·SiO2 and CFS) phase. The melting point of CFS olivine is 1,205°C, so the initial melting temperature increases slightly when the basicity is 1.6–1.8. With the basicity increasing from 1.8 to 2.4, a low melting point silicate phase is formed in the mixed slag, which makes the initial melting temperature decrease continuously. From 1231.45 to 1152.27°C, while the liquidus temperature has been increasing, the melting temperature range has been increasing. The analysis shows that with the increase of basicity, a small amount of silicate with a low melting point is formed in the mixed slag, so that the melting temperature is lowered, while the content of Al2O3 in the mixed slag is reduced, and the formation of gehlenite with low melting point is reduced, so that the liquidus temperature of the mixed slag is increased.
Liquidus temperature of mixed slag samples of variable basicity series/°C
| Basicity (R) | 1.6 | 1.8 | 2.0 | 2.2 | 2.4 |
|---|---|---|---|---|---|
| Solidus temp | 1231.45 | 1233.91 | 1159.98 | 1156.12 | 1152.27 |
| Liquidus temperature | 1453.98 | 1532.71 | 1583.24 | 1619.17 | 1642.33 |

Melting temperature of mixed slag samples with variable basicity.
3.2.2 Experimental study on the melting performance of mixed slag by basicity
The CQKJ-II hemispherical melting point melting rate synthetic tester was used to study the influence of different basicities on the melting temperature of the molten mixture of blast furnace slag and steelmaking slag, and the results are shown in Table 11.
Test results of melting point of mixed slag with different basicity/°C
| Basicity (R) | Softening temperature | Hemisphere temperature | Flow temperature | Soft melting range |
|---|---|---|---|---|
| 1.6 | 1,316 | 1,318 | 1,332 | 16 |
| 1.8 | 1,334 | 1,341 | 1,351 | 17 |
| 2.0 | 1,344 | 1,362 | 1,368 | 24 |
| 2.2 | 1,371 | >1,373 | >1,373 | — |
| 2.4 | >1,373 | >1,373 | >1,373 | — |
Note: melting temperature = hemispherical temperature; softening interval = flow temperature – softening temperature; liquidus temperature is the temperature at which the solid phase melts completely during the heating process, which is called the complete melting temperature of the mixed slag.
The melting temperature (softening temperature, hemispheric temperature, flow temperature and liquidus temperature) of the mixed slag varies with basicity as shown in Figure 9. It can be seen from the figure that with the increase of basicity, the softening, hemispherical, flowing and liquidus temperatures of the mixed slag increase. According to thermodynamic data analysis that: mixing slag in the process of heating up, have been dissolved equilibrium phase C2AF, α′-C2S, rich phosphorus phase C2SP, melilite, magnesium rhodonite (Ca3MgSi2O8, C3MS2) and solid solution (including spinel). With the increase of basicity, the addition of steelmaking slag increases, which changes the shape of the spinel solid solution in mixed slag, and the melting temperature of the solid solution increases from 1,400 to 1,600°C. The melting temperature of mixed slag changes with the change. Due to the limitation of experimental equipment, the highest actual temperature of the melting rate tester can only reach 1,373°C, so the specific data of melting temperature with basicity between 2.2 and 2.4 cannot be obtained.

Melting properties of mixed slags with variable basicity.
When the basicity is between 1.6 and 2.0, with the increase of the basicity, the softening, hemisphere, flow and liquidus temperature of the mixed slag are greatly increased, the softening and melting temperature range is increased, the hemisphere temperature is increased from 1,318 to 1,362°C, and the liquidus temperature increases from 1453.98 to 1583.24°C, namely, the hemisphere temperature is increased by about 11°C and the liquidus temperature is increased by about 32.31°C when the basicity is increased by 0.1 on average; the soft melting range is 16 to 24°C.
4 Conclusions
According to the simulation calculation of Factsage7.1 thermodynamic software, when the basicity is between 1.6 and 2.4, the mixed slag will precipitate solid solution, α′-C2S, C2AS, C2SP and C2AF during the slow cooling process at 1,600°C. When the basicity is lower than 2.0, magnesium rose pyroxene (Ca3MgSi2O8) precipitates from the phase.
The melting mixed slag of blast furnace slag and steel slag (R = 1.6–2.4) was calcined at 1,450°C for 1 h. With the increase of basicity, the content of gehlenite decreased from 62.56 to 30.28%, the content of spinel increased from 4.82 to 27.13%, and the content of calcium ferrite increased from 9.35 to 14.63%. When the basicity of mixed slag is 2.0, the proportion of C2S formation is the highest, reaching 13.67%. Therefore, it is considered that controlling the slag basicity at about 2.0 is beneficial to reduce the content of the non-gelatinized mineral calcium alumino yellow feldspar and promote the generation of the gelatinized mineral C2S.
With the increase of basicity, the content of Al2O3 in the molten slag of blast furnace slag and steelmaking slag decreases, the content of low melting point calcium aluminum melilite generated decreases, and the melting temperature of the mixed slag increases continuously. The basicity is between 1.6 and 2.0. The hemispherical temperature increases by about 11°C and the melting range is between 16 and 24°C, when the basicity increases by 0.1. When the basicity is higher than 2.0, the melting temperature of the mixed slag rises sharply.
Acknowledgments
The project was supported by the National Key R&D Program Grant (2020YFC1909105) and the Inner Mongolia Autonomous Region Science and Technology Major Project (2021ZD0016-05-04).
-
Funding information: National Key R&D Program funded project (2020YFC1909105); Inner Mongolia Autonomous Region Science and Technology Major Special Project (2021ZD0016-05-04).
-
Author contributions: Shuai Hao: the experimental results are analyzed and a paper is written. Guo-ping Luo: The theory of the paper is guided, and the relevant content of the article has been modified. Yin-sheng Chen: provide experimental data and assist in analysis. Sheng-li An: valuable suggestions are given for experimental research. Yi-fan Chai and Wei Song: guided the whole experiment process.
-
Conflict of interest: No conflict of interest exits in the submission of this manuscript, and the manuscript is approved by all authors for publication.
-
Data availability statement: All authors can confirm that all data used in this article can be published in the Journal “High Temperature Materials and Processes.”
References
[1] Xie, T. and L. S. Taylor. Feasibility study on self-pulverization of converter Slag. Journal of Anhui University of Technology (Natural Science Edition), Vol. 33, No. 2, 2016, pp. 105–109.Search in Google Scholar
[2] Deng, Z. H., J. Wang, and Y. Zhou. Study on mineral facies of converter slag. Journal of Anhui University of Technology (Natural Science Edition), Vol. 28, No. 3, 2011, pp. 201–204.Search in Google Scholar
[3] Lin, C. Basic research on self-pulverization and Vanadium extraction from steelmaking slag of stone coal converter. Master’s Thesis. Anhui University of Technology, China, 2018.Search in Google Scholar
[4] Zhang, Y. Z. and Y. B. Lei. Analysis of mineral phase composition and Micromorphology of Steelmaking slag. Metallurgical Analysis, Vol. 31, No. 9, 2011, pp. 11–17.Search in Google Scholar
[5] Feng, S. K., C. J. Liu, and M. F. Jiang. Analysis of phase composition of chromium bearing steelmaking slag under different cooling conditions. Proceedings of the 18th (2014) National Steelmaking Academic Conference -S09: Environmental Protection, 2014-48-52.Search in Google Scholar
[6] Zhu, G. H., Y. S. Qian, and L. G. Jin. Volume stability of steelmaking slag and development and utilization of modified steelmaking slag powder. China Cement, 2022, No. 3, pp. 85–89.Search in Google Scholar
[7] Zhao, H. Study on aging treatment and properties of road steelmaking slag. Western Transportation Science and Technology, 2021, No. 4, pp. 91–94.Search in Google Scholar
[8] Zou, H. N. and Y. Feng. Analysis of soaking aging treatment time of steelmaking slag. Comprehensive Utilization of Fly Ash, 2016, No. 1, pp. 26–28.Search in Google Scholar
[9] Qin, L. Q. Effect of aging on volume and water stability of steelmaking slag and asphalt concrete. Sino-Foreign Highway, 2019, Vol. 39, No. 6, pp. 273–279.Search in Google Scholar
[10] Zou, M., Y. Shen, and J. Liu. Review on application of steelmaking slag powder in cement-based materials. Bulletin of the Chinese Ceramic Society, 2021, Vol. 40, No. 9, pp. 2964–2977.Search in Google Scholar
[11] Guo, J. L., J. X. Zhao, and M. Huang. Review and suggestion of comprehensive utilization technology of steelmaking slag. China Metallurgy, Vol. 19, No. 2, 2009, pp. 35–38.Search in Google Scholar
[12] Li, Y. Study on preparation of solid waste based cementing materials and concrete with handan iron and steel metallurgical slag. Doctoral Thesis. University of Science and Technology Beijing, China, 2021.Search in Google Scholar
[13] Wang, Z. Y. and M. Wang. Research progress of dicalcium silicate and its Main mineral low calcium cement. Materials Review, Vol. 30, No. 1, 2016, pp. 73–78.Search in Google Scholar
[14] Fan, Z. M. Experimental study of steelmaking slag powder as cement mixture. China Cement, Vol. 1, 2022, pp. 92–94.Search in Google Scholar
[15] Wang, Z. Research on steelmaking slag as cement mixture, Hebei University of Engineering, Handan, 2020.Search in Google Scholar
[16] Gencel, O., O. Karadag, O. H. Oren, and T. Bilir. Steelmaking slag and its applications in cement and concrete technology: A review. Construction and Building Materials, Vol. 283, 2021, id. 122783.10.1016/j.conbuildmat.2021.122783Search in Google Scholar
[17] Xu, Y., Q. L. Wang, C. G. Hu, and Z. Z. Zhang Study on preparation of High Activity and High stability Steelmaking slag. Comprehensive Utilization of Mineral Resources, Vol. 4, 2019, pp. 106–110.Search in Google Scholar
[18] Deng, Z. H., J. Wang, H. C. Wang, Y. Zhou, B. G. Wu, and Y. C. Dong. Effect of P2O5 on mineral structure of converter Steelmaking slag. Journal of Anhui University of Technology (Natural Science Edition), Vol. 31, No. 4, 2014, pp. 340–344.Search in Google Scholar
[19] Zhang, Z. L. and R. Chen. Phase composition and microstructure of converter steelmaking slag. Journal of Materials and Metallurgy, Vol. 18, No. 1, 2019, pp. 37–40.Search in Google Scholar
[20] Xiao, Y. L., Y. D. Li, J. Y. Xiang, X. W. Lv, and H. L. Wang. Effect of cooling rate on phase structure and stability of steelmaking slag. Steel Making, Vol. 37, No. 6, pp. 76–81.Search in Google Scholar
[21] Rao, L. Study on internal law of composition, structure and properties of converter steelmaking slag and its application. Doctoral Thesis. University of Science and Technology Beijing, China, Beijing, 2020.Search in Google Scholar
[22] Zhang, Z. S., F. Lian, H. Q. Liao, Q. Yang, and W. B. Cao. Properties of steelmaking slag modified by iron tailings at high temperature. Journal of University of Science and Technology Beijing, Vol. 34, 2012, pp. 1379–1384.Search in Google Scholar
[23] Liu, S. Y., Z. J. Wang, B. Peng, C. S. Yue, M. Guo, and M. Zhang. Physical and chemical basis of steelmaking slag modified by blast furnace slag. Journal of Engineering Science, Vol. 40, 2018, pp. 557–564.Search in Google Scholar
[24] Xiang, R. H. Study on preparation and foaming modification of reconstituted steelmaking slag powder with medium and high activity. Master’s Thesis. Guilin University of Technology, China, 2021.Search in Google Scholar
[25] Wang, H. G., B. Peng, C. S. Yue, L. Wu, G. B. Qiu, and Z. T. Bai. Research progress and prospect of steel slag modification. Environmental Engineering, Vol. 38, No. 5, 2020, pp. 133–137, 106.Search in Google Scholar
[26] Lei, Y. B., Y. Z. Zhang, H. W. Xing, Y. Long, and T. L. Tian. High temperature Melting and digestion of free CaO from converter slag mixed with fly ash. Hebei Metallurgy, Vol. 4, 2011, pp. 11–14.Search in Google Scholar
[27] Li, Z., S. Zhao, X. Zhao, and T. He. Cementitious property modification of basic oxygen furnace steelmaking slag. Construction and Building Materials, Vol. 48, 2013, pp. 575–579.10.1016/j.conbuildmat.2013.07.068Search in Google Scholar
[28] Zhang, X. Carbonation mechanism of steelmaking slag products modified by zeolite. Master’s Thesis. Dalian University of Technology, China, 2021.Search in Google Scholar
[29] Liu, Z., Y. C. Wang, F. G. Zhao, G. P. Luo, and B. Zhao. Influence of binary basicity on physical properties of blast furnace slag of Baogang Group. Journal of Iron and Steel Research, Vol. 31, No. 8, 2019, pp. 696–701.Search in Google Scholar
[30] G. L. Zhao, D. Q. Cang, and Y. H. Lin. Preparation of high acidity coefficient mineral wool by modified molten steelmaking slag. Journal of Iron and Steel Research, 2022, Vol. 34, No. 2, pp. 142–149.Search in Google Scholar
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- First-principles investigation of phase stability and elastic properties of Laves phase TaCr2 by ruthenium alloying
- Improvement and prediction on high temperature melting characteristics of coal ash
- First-principles calculations to investigate the thermal response of the ZrC(1−x)Nx ceramics at extreme conditions
- Study on the cladding path during the solidification process of multi-layer cladding of large steel ingots
- Thermodynamic analysis of vanadium distribution behavior in blast furnaces and basic oxygen furnaces
- Comparison of data-driven prediction methods for comprehensive coke ratio of blast furnace
- Effect of different isothermal times on the microstructure and mechanical properties of high-strength rebar
- Analysis of the evolution law of oxide inclusions in U75V heavy rail steel during the LF–RH refining process
- Simultaneous extraction of uranium and niobium from a low-grade natural betafite ore
- Transfer and transformation mechanism of chromium in stainless steel slag in pedosphere
- Effect of tool traverse speed on joint line remnant and mechanical properties of friction stir welded 2195-T8 Al–Li alloy joints
- Technology and analysis of 08Cr9W3Co3VNbCuBN steel large diameter thick wall pipe welding process
- Influence of shielding gas on machining and wear aspects of AISI 310–AISI 2205 dissimilar stainless steel joints
- Effect of post-weld heat treatment on 6156 aluminum alloy joint formed by electron beam welding
- Ash melting behavior and mechanism of high-calcium bituminous coal in the process of blast furnace pulverized coal injection
- Effect of high temperature tempering on the phase composition and structure of steelmaking slag
- Numerical simulation of shrinkage porosity defect in billet continuous casting
- Influence of submerged entry nozzle on funnel mold surface velocity
- Effect of cold-rolling deformation and rare earth yttrium on microstructure and texture of oriented silicon steel
- Investigation of microstructure, machinability, and mechanical properties of new-generation hybrid lead-free brass alloys
- Soft sensor method of multimode BOF steelmaking endpoint carbon content and temperature based on vMF-WSAE dynamic deep learning
- Mechanical properties and nugget evolution in resistance spot welding of Zn–Al–Mg galvanized DC51D steel
- Research on the behaviour and mechanism of void welding based on multiple scales
- Preparation of CaO–SiO2–Al2O3 inorganic fibers from melting-separated red mud
- Study on diffusion kinetics of chromium and nickel electrochemical co-deposition in a NaCl–KCl–NaF–Cr2O3–NiO molten salt
- Enhancing the efficiency of polytetrafluoroethylene-modified silica hydrosols coated solar panels by using artificial neural network and response surface methodology
- High-temperature corrosion behaviours of nickel–iron-based alloys with different molybdenum and tungsten contents in a coal ash/flue gas environment
- Characteristics and purification of Himalayan salt by high temperature melting
- Temperature uniformity optimization with power-frequency coordinated variation in multi-source microwave based on sequential quadratic programming
- A novel method for CO2 injection direct smelting vanadium steel: Dephosphorization and vanadium retention
- A study of the void surface healing mechanism in 316LN steel
- Effect of chemical composition and heat treatment on intergranular corrosion and strength of AlMgSiCu alloys
- Soft sensor method for endpoint carbon content and temperature of BOF based on multi-cluster dynamic adaptive selection ensemble learning
- Evaluating thermal properties and activation energy of phthalonitrile using sulfur-containing curing agents
- Investigation of the liquidus temperature calculation method for medium manganese steel
- High-temperature corrosion model of Incoloy 800H alloy connected with Ni-201 in MgCl2–KCl heat transfer fluid
- Investigation of the microstructure and mechanical properties of Mg–Al–Zn alloy joints formed by different laser welding processes
- Effect of refining slag compositions on its melting property and desulphurization
- Effect of P and Ti on the agglomeration behavior of Al2O3 inclusions in Fe–P–Ti alloys
- Cation-doping effects on the conductivities of the mayenite Ca12Al14O33
- Modification of Al2O3 inclusions in SWRH82B steel by La/Y rare-earth element treatment
- Possibility of metallic cobalt formation in the oxide scale during high-temperature oxidation of Co-27Cr-6Mo alloy in air
- Multi-source microwave heating temperature uniformity study based on adaptive dynamic programming
- Round-robin measurement of surface tension of high-temperature liquid platinum free of oxygen adsorption by oscillating droplet method using levitation techniques
- High-temperature production of AlN in Mg alloys with ammonia gas
- Review Article
- Advances in ultrasonic welding of lightweight alloys: A review
- Topical Issue on High-temperature Phase Change Materials for Energy Storage
- Compositional and thermophysical study of Al–Si- and Zn–Al–Mg-based eutectic alloys for latent heat storage
- Corrosion behavior of a Co−Cr−Mo−Si alloy in pure Al and Al−Si melt
- Al–Si–Fe alloy-based phase change material for high-temperature thermal energy storage
- Density and surface tension measurements of molten Al–Si based alloys
- Graphite crucible interaction with Fe–Si–B phase change material in pilot-scale experiments
- Topical Issue on Nuclear Energy Application Materials
- Dry synthesis of brannerite (UTi2O6) by mechanochemical treatment
- Special Issue on Polymer and Composite Materials (PCM) and Graphene and Novel Nanomaterials - Part I
- Heat management of LED-based Cu2O deposits on the optimal structure of heat sink
- Special Issue on Recent Developments in 3D Printed Carbon Materials - Part I
- Porous metal foam flow field and heat evaluation in PEMFC: A review
- Special Issue on Advancements in Solar Energy Technologies and Systems
- Research on electric energy measurement system based on intelligent sensor data in artificial intelligence environment
- Study of photovoltaic integrated prefabricated components for assembled buildings based on sensing technology supported by solar energy
- Topical Issue on Focus of Hot Deformation of Metaland High Entropy Alloys - Part I
- Performance optimization and investigation of metal-cored filler wires for high-strength steel during gas metal arc welding
- Three-dimensional transient heat transfer analysis of micro-plasma arc welding process using volumetric heat source models
Articles in the same Issue
- Research Articles
- First-principles investigation of phase stability and elastic properties of Laves phase TaCr2 by ruthenium alloying
- Improvement and prediction on high temperature melting characteristics of coal ash
- First-principles calculations to investigate the thermal response of the ZrC(1−x)Nx ceramics at extreme conditions
- Study on the cladding path during the solidification process of multi-layer cladding of large steel ingots
- Thermodynamic analysis of vanadium distribution behavior in blast furnaces and basic oxygen furnaces
- Comparison of data-driven prediction methods for comprehensive coke ratio of blast furnace
- Effect of different isothermal times on the microstructure and mechanical properties of high-strength rebar
- Analysis of the evolution law of oxide inclusions in U75V heavy rail steel during the LF–RH refining process
- Simultaneous extraction of uranium and niobium from a low-grade natural betafite ore
- Transfer and transformation mechanism of chromium in stainless steel slag in pedosphere
- Effect of tool traverse speed on joint line remnant and mechanical properties of friction stir welded 2195-T8 Al–Li alloy joints
- Technology and analysis of 08Cr9W3Co3VNbCuBN steel large diameter thick wall pipe welding process
- Influence of shielding gas on machining and wear aspects of AISI 310–AISI 2205 dissimilar stainless steel joints
- Effect of post-weld heat treatment on 6156 aluminum alloy joint formed by electron beam welding
- Ash melting behavior and mechanism of high-calcium bituminous coal in the process of blast furnace pulverized coal injection
- Effect of high temperature tempering on the phase composition and structure of steelmaking slag
- Numerical simulation of shrinkage porosity defect in billet continuous casting
- Influence of submerged entry nozzle on funnel mold surface velocity
- Effect of cold-rolling deformation and rare earth yttrium on microstructure and texture of oriented silicon steel
- Investigation of microstructure, machinability, and mechanical properties of new-generation hybrid lead-free brass alloys
- Soft sensor method of multimode BOF steelmaking endpoint carbon content and temperature based on vMF-WSAE dynamic deep learning
- Mechanical properties and nugget evolution in resistance spot welding of Zn–Al–Mg galvanized DC51D steel
- Research on the behaviour and mechanism of void welding based on multiple scales
- Preparation of CaO–SiO2–Al2O3 inorganic fibers from melting-separated red mud
- Study on diffusion kinetics of chromium and nickel electrochemical co-deposition in a NaCl–KCl–NaF–Cr2O3–NiO molten salt
- Enhancing the efficiency of polytetrafluoroethylene-modified silica hydrosols coated solar panels by using artificial neural network and response surface methodology
- High-temperature corrosion behaviours of nickel–iron-based alloys with different molybdenum and tungsten contents in a coal ash/flue gas environment
- Characteristics and purification of Himalayan salt by high temperature melting
- Temperature uniformity optimization with power-frequency coordinated variation in multi-source microwave based on sequential quadratic programming
- A novel method for CO2 injection direct smelting vanadium steel: Dephosphorization and vanadium retention
- A study of the void surface healing mechanism in 316LN steel
- Effect of chemical composition and heat treatment on intergranular corrosion and strength of AlMgSiCu alloys
- Soft sensor method for endpoint carbon content and temperature of BOF based on multi-cluster dynamic adaptive selection ensemble learning
- Evaluating thermal properties and activation energy of phthalonitrile using sulfur-containing curing agents
- Investigation of the liquidus temperature calculation method for medium manganese steel
- High-temperature corrosion model of Incoloy 800H alloy connected with Ni-201 in MgCl2–KCl heat transfer fluid
- Investigation of the microstructure and mechanical properties of Mg–Al–Zn alloy joints formed by different laser welding processes
- Effect of refining slag compositions on its melting property and desulphurization
- Effect of P and Ti on the agglomeration behavior of Al2O3 inclusions in Fe–P–Ti alloys
- Cation-doping effects on the conductivities of the mayenite Ca12Al14O33
- Modification of Al2O3 inclusions in SWRH82B steel by La/Y rare-earth element treatment
- Possibility of metallic cobalt formation in the oxide scale during high-temperature oxidation of Co-27Cr-6Mo alloy in air
- Multi-source microwave heating temperature uniformity study based on adaptive dynamic programming
- Round-robin measurement of surface tension of high-temperature liquid platinum free of oxygen adsorption by oscillating droplet method using levitation techniques
- High-temperature production of AlN in Mg alloys with ammonia gas
- Review Article
- Advances in ultrasonic welding of lightweight alloys: A review
- Topical Issue on High-temperature Phase Change Materials for Energy Storage
- Compositional and thermophysical study of Al–Si- and Zn–Al–Mg-based eutectic alloys for latent heat storage
- Corrosion behavior of a Co−Cr−Mo−Si alloy in pure Al and Al−Si melt
- Al–Si–Fe alloy-based phase change material for high-temperature thermal energy storage
- Density and surface tension measurements of molten Al–Si based alloys
- Graphite crucible interaction with Fe–Si–B phase change material in pilot-scale experiments
- Topical Issue on Nuclear Energy Application Materials
- Dry synthesis of brannerite (UTi2O6) by mechanochemical treatment
- Special Issue on Polymer and Composite Materials (PCM) and Graphene and Novel Nanomaterials - Part I
- Heat management of LED-based Cu2O deposits on the optimal structure of heat sink
- Special Issue on Recent Developments in 3D Printed Carbon Materials - Part I
- Porous metal foam flow field and heat evaluation in PEMFC: A review
- Special Issue on Advancements in Solar Energy Technologies and Systems
- Research on electric energy measurement system based on intelligent sensor data in artificial intelligence environment
- Study of photovoltaic integrated prefabricated components for assembled buildings based on sensing technology supported by solar energy
- Topical Issue on Focus of Hot Deformation of Metaland High Entropy Alloys - Part I
- Performance optimization and investigation of metal-cored filler wires for high-strength steel during gas metal arc welding
- Three-dimensional transient heat transfer analysis of micro-plasma arc welding process using volumetric heat source models

